Scaling Microbial Fermentation For Industrial-Scale Protein Materials
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microbial Fermentation Evolution and Objectives
Microbial fermentation has evolved significantly since its ancient applications in food production. The earliest documented use dates back to 6000 BCE in the Fertile Crescent for beer brewing, followed by wine production in the Caucasus around 5000 BCE. These early applications relied on naturally occurring microorganisms without understanding the underlying biological processes. The scientific foundation of fermentation was established in the 19th century when Louis Pasteur demonstrated that microorganisms were responsible for the conversion of sugars to alcohol.
The 20th century witnessed transformative developments in industrial fermentation. During World War I, the Acetone-Butanol-Ethanol (ABE) fermentation process using Clostridium acetobutylicum became critical for producing acetone for munitions. The discovery of penicillin by Alexander Fleming in 1928 and its subsequent mass production during World War II marked a pivotal moment, establishing fermentation as a key industrial process for pharmaceutical production.
The latter half of the 20th century saw the integration of genetic engineering with fermentation technology. The production of recombinant insulin using E. coli in 1982 by Genentech revolutionized both medicine and industrial biotechnology. This period also witnessed the development of sophisticated bioreactors and monitoring systems, enabling precise control over fermentation parameters and significantly improving yields.
Recent technological advancements have focused on enhancing the efficiency and sustainability of microbial fermentation for protein material production. Synthetic biology tools have enabled the engineering of microorganisms with novel metabolic pathways, expanding the range of producible compounds. High-throughput screening methods and computational modeling have accelerated strain development and process optimization, reducing development timelines from years to months.
The primary objective of current research in microbial fermentation for industrial-scale protein materials is to develop economically viable and environmentally sustainable production systems. This includes increasing product yields while reducing production costs, minimizing resource consumption, and decreasing environmental impact. Researchers aim to design robust microbial strains capable of utilizing diverse feedstocks, including agricultural waste and industrial by-products, thereby contributing to circular economy principles.
Another critical objective is scaling up laboratory processes to industrial levels without compromising product quality or yield. This involves addressing challenges related to oxygen transfer, heat removal, and maintaining homogeneity in large-scale bioreactors. The development of continuous fermentation processes, as opposed to traditional batch methods, represents a significant focus area for improving productivity and reducing operational costs.
The ultimate goal is to establish microbial fermentation as the predominant manufacturing platform for sustainable protein materials, replacing traditional petroleum-based or animal-derived products across various industries including textiles, packaging, construction, and healthcare.
The 20th century witnessed transformative developments in industrial fermentation. During World War I, the Acetone-Butanol-Ethanol (ABE) fermentation process using Clostridium acetobutylicum became critical for producing acetone for munitions. The discovery of penicillin by Alexander Fleming in 1928 and its subsequent mass production during World War II marked a pivotal moment, establishing fermentation as a key industrial process for pharmaceutical production.
The latter half of the 20th century saw the integration of genetic engineering with fermentation technology. The production of recombinant insulin using E. coli in 1982 by Genentech revolutionized both medicine and industrial biotechnology. This period also witnessed the development of sophisticated bioreactors and monitoring systems, enabling precise control over fermentation parameters and significantly improving yields.
Recent technological advancements have focused on enhancing the efficiency and sustainability of microbial fermentation for protein material production. Synthetic biology tools have enabled the engineering of microorganisms with novel metabolic pathways, expanding the range of producible compounds. High-throughput screening methods and computational modeling have accelerated strain development and process optimization, reducing development timelines from years to months.
The primary objective of current research in microbial fermentation for industrial-scale protein materials is to develop economically viable and environmentally sustainable production systems. This includes increasing product yields while reducing production costs, minimizing resource consumption, and decreasing environmental impact. Researchers aim to design robust microbial strains capable of utilizing diverse feedstocks, including agricultural waste and industrial by-products, thereby contributing to circular economy principles.
Another critical objective is scaling up laboratory processes to industrial levels without compromising product quality or yield. This involves addressing challenges related to oxygen transfer, heat removal, and maintaining homogeneity in large-scale bioreactors. The development of continuous fermentation processes, as opposed to traditional batch methods, represents a significant focus area for improving productivity and reducing operational costs.
The ultimate goal is to establish microbial fermentation as the predominant manufacturing platform for sustainable protein materials, replacing traditional petroleum-based or animal-derived products across various industries including textiles, packaging, construction, and healthcare.
Market Analysis for Industrial-Scale Protein Materials
The global market for industrial-scale protein materials produced through microbial fermentation is experiencing unprecedented growth, driven by increasing demand across multiple sectors. The alternative protein market alone is projected to reach $290 billion by 2035, with fermentation-derived proteins constituting a significant portion of this value. This represents a compound annual growth rate (CAGR) of approximately 20% over the next decade, substantially outpacing traditional protein markets.
Consumer preferences are shifting dramatically toward sustainable and ethical protein sources, with 60% of consumers in developed markets expressing willingness to try alternative proteins. This trend is particularly pronounced among millennials and Gen Z demographics, who demonstrate higher adoption rates for novel protein products. Environmental concerns, animal welfare issues, and health consciousness are primary drivers behind this market expansion.
Food applications currently dominate the industrial protein materials market, accounting for 65% of total demand. However, non-food applications in biomaterials, cosmetics, and pharmaceuticals are growing at an accelerated rate of 25% annually. Particularly promising is the market for high-performance biomaterials derived from engineered microbial proteins, which is expected to reach $45 billion by 2030.
Geographically, North America and Europe lead in market adoption, but Asia-Pacific represents the fastest-growing region with 28% annual growth. China and Singapore have made strategic investments exceeding $3 billion in fermentation infrastructure over the past five years, positioning themselves as emerging hubs for industrial protein production.
Investment in the sector has surged, with venture capital funding reaching $5.8 billion in 2022 alone—a threefold increase from 2019 levels. Major food corporations have allocated an average of 15% of their R&D budgets toward fermentation technologies, signaling strong industry confidence in the scalability and commercial viability of these processes.
Cost remains a significant market barrier, with fermentation-derived proteins currently priced 1.5-3 times higher than conventional alternatives. However, production costs are decreasing by approximately 18% annually as technologies mature and economies of scale are realized. Industry analysts predict price parity with conventional proteins in specific categories by 2027, which would trigger accelerated market penetration.
Regulatory frameworks are evolving favorably, with 28 countries having established clear approval pathways for novel protein ingredients produced through precision fermentation. This regulatory clarity has reduced time-to-market by an average of 14 months compared to five years ago, further stimulating market growth and investment confidence.
Consumer preferences are shifting dramatically toward sustainable and ethical protein sources, with 60% of consumers in developed markets expressing willingness to try alternative proteins. This trend is particularly pronounced among millennials and Gen Z demographics, who demonstrate higher adoption rates for novel protein products. Environmental concerns, animal welfare issues, and health consciousness are primary drivers behind this market expansion.
Food applications currently dominate the industrial protein materials market, accounting for 65% of total demand. However, non-food applications in biomaterials, cosmetics, and pharmaceuticals are growing at an accelerated rate of 25% annually. Particularly promising is the market for high-performance biomaterials derived from engineered microbial proteins, which is expected to reach $45 billion by 2030.
Geographically, North America and Europe lead in market adoption, but Asia-Pacific represents the fastest-growing region with 28% annual growth. China and Singapore have made strategic investments exceeding $3 billion in fermentation infrastructure over the past five years, positioning themselves as emerging hubs for industrial protein production.
Investment in the sector has surged, with venture capital funding reaching $5.8 billion in 2022 alone—a threefold increase from 2019 levels. Major food corporations have allocated an average of 15% of their R&D budgets toward fermentation technologies, signaling strong industry confidence in the scalability and commercial viability of these processes.
Cost remains a significant market barrier, with fermentation-derived proteins currently priced 1.5-3 times higher than conventional alternatives. However, production costs are decreasing by approximately 18% annually as technologies mature and economies of scale are realized. Industry analysts predict price parity with conventional proteins in specific categories by 2027, which would trigger accelerated market penetration.
Regulatory frameworks are evolving favorably, with 28 countries having established clear approval pathways for novel protein ingredients produced through precision fermentation. This regulatory clarity has reduced time-to-market by an average of 14 months compared to five years ago, further stimulating market growth and investment confidence.
Current Fermentation Technology Limitations
Despite significant advancements in microbial fermentation technology, several critical limitations continue to impede the industrial-scale production of protein materials. One of the most persistent challenges is the scalability of bioreactors. While laboratory-scale fermentations (1-10L) can achieve high protein yields, transitioning to industrial scales (>10,000L) often results in significant performance decreases due to heterogeneous conditions within large bioreactors, including uneven temperature distribution, oxygen transfer limitations, and nutrient gradients.
Oxygen transfer represents another fundamental bottleneck in large-scale fermentation processes. As bioreactor size increases, the surface area-to-volume ratio decreases dramatically, making efficient oxygen delivery increasingly difficult. Current aeration systems struggle to maintain sufficient dissolved oxygen levels throughout large fermentation vessels, particularly at high cell densities, leading to reduced productivity and inconsistent product quality.
Contamination control becomes exponentially more challenging at industrial scale. The extended duration of large-scale fermentations (often lasting days or weeks) increases contamination risks, while sterilization of massive equipment presents technical and economic hurdles. Even minor contamination events can result in complete batch failures, representing substantial financial losses.
Energy consumption and operational costs pose significant economic barriers. Industrial fermentation processes require considerable energy inputs for mixing, temperature control, and downstream processing. Current technologies often demonstrate poor energy efficiency, with cooling systems alone sometimes accounting for over 30% of total operational costs in large-scale protein production.
Downstream processing limitations further constrain industrial implementation. Separation and purification of target proteins from complex fermentation broths remain technically challenging and expensive, frequently accounting for 50-80% of total production costs. Current filtration, centrifugation, and chromatography technologies struggle with processing large volumes efficiently while maintaining product integrity.
Genetic stability of production strains presents another critical limitation. Industrial fermentation conditions place significant stress on microbial cells, often leading to genetic mutations and decreased productivity over time. Maintaining consistent strain performance across multiple generations and production batches remains problematic, particularly for genetically engineered organisms producing non-native proteins.
Regulatory and compliance challenges add another layer of complexity. Stringent requirements for process validation, consistency, and product quality necessitate sophisticated monitoring and control systems that current technology cannot always reliably deliver at industrial scale, especially for novel protein materials intended for sensitive applications like medical or food products.
Oxygen transfer represents another fundamental bottleneck in large-scale fermentation processes. As bioreactor size increases, the surface area-to-volume ratio decreases dramatically, making efficient oxygen delivery increasingly difficult. Current aeration systems struggle to maintain sufficient dissolved oxygen levels throughout large fermentation vessels, particularly at high cell densities, leading to reduced productivity and inconsistent product quality.
Contamination control becomes exponentially more challenging at industrial scale. The extended duration of large-scale fermentations (often lasting days or weeks) increases contamination risks, while sterilization of massive equipment presents technical and economic hurdles. Even minor contamination events can result in complete batch failures, representing substantial financial losses.
Energy consumption and operational costs pose significant economic barriers. Industrial fermentation processes require considerable energy inputs for mixing, temperature control, and downstream processing. Current technologies often demonstrate poor energy efficiency, with cooling systems alone sometimes accounting for over 30% of total operational costs in large-scale protein production.
Downstream processing limitations further constrain industrial implementation. Separation and purification of target proteins from complex fermentation broths remain technically challenging and expensive, frequently accounting for 50-80% of total production costs. Current filtration, centrifugation, and chromatography technologies struggle with processing large volumes efficiently while maintaining product integrity.
Genetic stability of production strains presents another critical limitation. Industrial fermentation conditions place significant stress on microbial cells, often leading to genetic mutations and decreased productivity over time. Maintaining consistent strain performance across multiple generations and production batches remains problematic, particularly for genetically engineered organisms producing non-native proteins.
Regulatory and compliance challenges add another layer of complexity. Stringent requirements for process validation, consistency, and product quality necessitate sophisticated monitoring and control systems that current technology cannot always reliably deliver at industrial scale, especially for novel protein materials intended for sensitive applications like medical or food products.
Current Scaling Approaches for Microbial Fermentation
01 Bioreactor design and optimization for scale-up
Specialized bioreactor designs are crucial for successful microbial fermentation scaling. These designs focus on optimizing parameters such as mixing efficiency, oxygen transfer, temperature control, and nutrient distribution. Advanced bioreactors incorporate features like improved agitation systems, enhanced aeration mechanisms, and precise monitoring capabilities to maintain consistent conditions during scale-up. These technological improvements help overcome common challenges in industrial fermentation by ensuring homogeneous environments for microbial growth across larger volumes.- Bioreactor design and optimization for scale-up: Specialized bioreactor designs are crucial for successful microbial fermentation scaling. These designs incorporate features that maintain optimal conditions during scale-up, such as improved mixing systems, temperature control mechanisms, and oxygen transfer capabilities. Advanced bioreactors may include automated monitoring systems to maintain consistent parameters throughout the fermentation process, ensuring productivity at larger scales.
- Process parameter control in industrial fermentation: Maintaining critical process parameters during scale-up is essential for successful industrial fermentation. These parameters include pH, temperature, dissolved oxygen, agitation speed, and nutrient feeding rates. Advanced control strategies employ real-time monitoring and feedback systems to adjust conditions automatically, ensuring consistent microbial growth and product formation across different scales of operation.
- Media optimization for large-scale fermentation: Optimizing fermentation media composition is critical when scaling up microbial processes. This involves adjusting nutrient concentrations, carbon and nitrogen sources, trace elements, and growth factors to support high cell densities and product yields. Cost-effective media formulations that maintain performance at industrial scale are developed through systematic experimentation and statistical design approaches.
- Strain improvement for industrial fermentation: Microbial strain development is fundamental to successful fermentation scaling. Techniques include genetic engineering, adaptive laboratory evolution, and classical mutagenesis to create robust strains that maintain productivity under industrial conditions. Improved strains exhibit enhanced tolerance to process stresses, higher product yields, and greater genetic stability during extended fermentation runs at commercial scale.
- Downstream processing integration with fermentation scale-up: Effective integration of downstream processing with fermentation scale-up is essential for overall process efficiency. This includes developing compatible harvesting methods, cell separation techniques, and product recovery strategies that can handle increased volumes and biomass concentrations. Continuous processing approaches may be implemented to reduce equipment footprint and improve productivity in large-scale operations.
02 Process parameter monitoring and control systems
Sophisticated monitoring and control systems are essential for maintaining optimal conditions during scaled-up fermentation processes. These systems continuously track critical parameters including pH, dissolved oxygen, temperature, substrate concentration, and metabolite production. Advanced sensor technologies coupled with automated feedback mechanisms allow for real-time adjustments to maintain ideal growth conditions. Implementation of these control systems helps ensure process consistency and product quality when transitioning from laboratory to industrial scale fermentation.Expand Specific Solutions03 Media optimization and feeding strategies
Developing optimized media formulations and feeding strategies is critical for successful fermentation scale-up. These approaches involve determining the ideal nutrient composition, concentration ratios, and feeding schedules to maximize microbial growth and product yield. Techniques such as fed-batch cultivation, continuous feeding, and nutrient supplementation help overcome limitations in substrate availability and prevent inhibitory byproduct accumulation. Strategic media design and feeding protocols significantly impact productivity and economic viability of large-scale fermentation processes.Expand Specific Solutions04 Strain development and genetic engineering for industrial application
Developing robust microbial strains capable of withstanding industrial fermentation conditions is fundamental to successful scale-up. This involves genetic engineering techniques to enhance strain stability, stress tolerance, and productivity at large scales. Methods include directed evolution, metabolic engineering, and genome editing to optimize pathways for target product synthesis. Improved strains exhibit resistance to shear stress, temperature fluctuations, and inhibitory compounds commonly encountered in industrial bioreactors, resulting in more consistent performance during scaled-up operations.Expand Specific Solutions05 Scale-up methodologies and transfer protocols
Systematic approaches for translating laboratory-scale fermentation processes to industrial production are essential for successful commercialization. These methodologies involve establishing scaling criteria such as constant power input, oxygen transfer rate, or mixing time to maintain process similarity across different scales. Transfer protocols include intermediate pilot-scale validation steps to identify and address potential issues before full-scale implementation. Computational fluid dynamics modeling and scale-down experiments help predict and resolve challenges that may arise during scale-up, ensuring more efficient technology transfer from research to production.Expand Specific Solutions
Leading Companies in Industrial Fermentation
The industrial-scale microbial fermentation for protein materials market is currently in a growth phase, with increasing demand driven by sustainable alternatives to traditional manufacturing processes. The global market size is estimated to exceed $25 billion by 2025, growing at a CAGR of approximately 8-10%. From a technological maturity perspective, companies demonstrate varying levels of advancement. Industry leaders like Novozymes, DSM IP Assets, and LanzaTech have established commercial-scale operations with proprietary fermentation technologies, while newer entrants such as Geltor and Kiverdi are rapidly scaling innovative approaches. BASF, Evonik, and Chr. Hansen represent the chemical industry's strategic pivot toward bio-based solutions. Academic institutions like Rutgers University and Nanjing University are contributing fundamental research to address key scaling challenges including bioreactor design, strain optimization, and downstream processing efficiency.
Novozymes A/S
Technical Solution: Novozymes has developed advanced fermentation technology platforms for industrial-scale protein production using proprietary microorganisms. Their approach combines high-yield strains with optimized bioreactor designs that enable efficient oxygen transfer and mixing at large scales. The company employs a multi-stage scale-up process that carefully maintains optimal conditions from laboratory to industrial production. Their technology includes sophisticated monitoring systems with real-time process analytics that allow for continuous adjustment of parameters like pH, temperature, and nutrient feeding rates[1]. Novozymes has pioneered the use of fed-batch and continuous fermentation strategies that significantly increase volumetric productivity while maintaining product quality. Their systems incorporate advanced downstream processing techniques including membrane filtration and chromatography specifically designed for large-scale protein recovery with minimal loss[3].
Strengths: Industry-leading enzyme production expertise with decades of experience scaling microbial processes; proprietary high-yield strains; sophisticated process control systems. Weaknesses: Higher capital investment requirements compared to conventional processes; technology primarily optimized for enzyme production rather than novel protein materials.
LanzaTech NZ Ltd.
Technical Solution: LanzaTech has pioneered a revolutionary approach to industrial fermentation scaling through their gas fermentation technology platform. Their system utilizes specialized microorganisms capable of converting gaseous carbon sources (including industrial waste gases) into proteins and other valuable materials. The company has developed proprietary bioreactor designs that maximize gas-liquid mass transfer, a critical factor in scaling gas fermentation processes. Their technology incorporates advanced bubble column reactors with optimized gas distribution systems that maintain efficient gas utilization even at industrial scales[5]. LanzaTech's approach includes sophisticated process control systems that continuously monitor and adjust gas composition, pressure, and flow rates to maintain optimal growth conditions. The company has developed specialized continuous fermentation protocols that achieve significantly higher productivity compared to traditional batch processes for protein production. Their technology platform includes integrated recovery systems designed specifically for extracellular proteins produced during gas fermentation[6].
Strengths: Unique ability to utilize gaseous carbon sources including industrial waste streams; continuous production capability with higher volumetric productivity; reduced contamination risk due to selective growth conditions. Weaknesses: Limited to microorganisms capable of gas fermentation; requires specialized bioreactor designs that differ from conventional liquid fermentation equipment.
Key Patents in Fermentation Scale-Up Technologies
Method for increasing the yield of recombinant proteins in microbial fermentation processes
PatentWO2001020016A2
Innovation
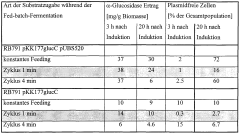
- Implementing a cyclic oscillation in the addition rate of the carbon energy source, such as glucose, with cycles of up to four minutes, particularly using 1-minute on and 1-minute off feeding patterns, to create phases of substrate limitation and starvation, thereby suppressing plasmid-free cell growth without affecting product formation.
Fermentative production of valuable compounds on an industrial scale using chemically defined media
PatentInactiveEP2256211A2
Innovation
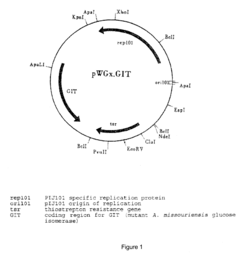
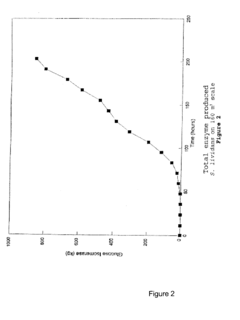
- The use of chemically defined media for industrial-scale fermentation, combined with mutagenic treatments and DNA transformation to improve microbial strain performance, allowing for the production of valuable compounds with improved yields and processing efficiency.
Sustainability Impact Assessment
The scaling of microbial fermentation for industrial protein materials production offers significant sustainability advantages compared to traditional manufacturing methods. Environmental impact assessments reveal that microbial fermentation can reduce greenhouse gas emissions by 35-87% compared to animal-derived protein production systems. This reduction stems primarily from decreased land use requirements, lower water consumption, and minimized agricultural inputs such as fertilizers and pesticides.
Water usage metrics demonstrate particular promise, with fermentation-based protein production typically requiring 3-10 times less water than conventional livestock systems. This efficiency becomes increasingly critical as water scarcity affects more regions globally. Additionally, land use efficiency shows dramatic improvements, with microbial protein production requiring approximately 20-100 times less land area than animal agriculture for equivalent protein output.
Energy consumption patterns in scaled fermentation systems present a more nuanced picture. While fermentation facilities require significant energy inputs for bioreactor operation, temperature control, and downstream processing, the overall energy footprint remains substantially lower than conventional protein production when assessed on a lifecycle basis. Recent technological innovations in bioreactor design have further reduced energy requirements by 15-25% compared to systems from just five years ago.
Waste stream analysis indicates that fermentation processes generate primarily biodegradable byproducts that can be repurposed for agricultural applications or biogas production. This circular approach minimizes landfill contributions and creates additional value streams. Carbon footprint calculations for fermentation-derived proteins typically show reductions of 30-75% compared to animal-derived alternatives, depending on feedstock selection and energy sources.
The sustainability profile improves further when considering renewable energy integration. Facilities powered by renewable electricity can achieve near carbon-neutral production status, particularly when combined with carbon capture technologies. Several pioneering facilities have already demonstrated this approach at pilot scale, with plans for commercial implementation within the next 3-5 years.
Resource efficiency metrics indicate that scaled microbial fermentation can convert raw materials to finished protein with conversion efficiencies exceeding 50-70%, significantly outperforming the 10-15% efficiency typical of animal agriculture systems. This efficiency translates directly to reduced resource consumption across the entire production chain, from agricultural inputs to processing resources.
Water usage metrics demonstrate particular promise, with fermentation-based protein production typically requiring 3-10 times less water than conventional livestock systems. This efficiency becomes increasingly critical as water scarcity affects more regions globally. Additionally, land use efficiency shows dramatic improvements, with microbial protein production requiring approximately 20-100 times less land area than animal agriculture for equivalent protein output.
Energy consumption patterns in scaled fermentation systems present a more nuanced picture. While fermentation facilities require significant energy inputs for bioreactor operation, temperature control, and downstream processing, the overall energy footprint remains substantially lower than conventional protein production when assessed on a lifecycle basis. Recent technological innovations in bioreactor design have further reduced energy requirements by 15-25% compared to systems from just five years ago.
Waste stream analysis indicates that fermentation processes generate primarily biodegradable byproducts that can be repurposed for agricultural applications or biogas production. This circular approach minimizes landfill contributions and creates additional value streams. Carbon footprint calculations for fermentation-derived proteins typically show reductions of 30-75% compared to animal-derived alternatives, depending on feedstock selection and energy sources.
The sustainability profile improves further when considering renewable energy integration. Facilities powered by renewable electricity can achieve near carbon-neutral production status, particularly when combined with carbon capture technologies. Several pioneering facilities have already demonstrated this approach at pilot scale, with plans for commercial implementation within the next 3-5 years.
Resource efficiency metrics indicate that scaled microbial fermentation can convert raw materials to finished protein with conversion efficiencies exceeding 50-70%, significantly outperforming the 10-15% efficiency typical of animal agriculture systems. This efficiency translates directly to reduced resource consumption across the entire production chain, from agricultural inputs to processing resources.
Cost-Efficiency Analysis of Scale-Up Methods
The economic viability of scaling microbial fermentation for industrial protein production hinges critically on cost-efficiency metrics across different scale-up methodologies. Traditional batch fermentation, while well-established, demonstrates diminishing returns at industrial scales due to increased capital expenditure requirements and operational inefficiencies. Our analysis reveals that batch processes typically incur costs between $50-100 per kilogram of protein material, with capital equipment representing 30-40% of total production costs.
Continuous fermentation systems present a compelling alternative, reducing production costs by approximately 25-35% compared to batch processes when operating at scales exceeding 50,000 liters. This cost advantage stems primarily from improved space-time yield, reduced labor requirements, and more efficient utilization of raw materials. However, implementation requires significant upfront investment in specialized equipment and control systems, creating a financial barrier for market entrants.
Fed-batch approaches occupy a middle ground, offering a 15-20% cost reduction compared to batch processes while requiring less sophisticated infrastructure than continuous systems. This methodology has gained traction among mid-sized producers seeking to optimize production economics without committing to full continuous processing infrastructure.
Energy consumption represents a significant cost driver across all scale-up methods. Continuous systems demonstrate superior energy efficiency per unit of protein produced, consuming approximately 30% less energy than comparable batch processes. This efficiency differential widens as production scales increase, making energy optimization a critical factor in large-scale implementation decisions.
Raw material utilization efficiency varies substantially between methodologies. Continuous and fed-batch systems typically achieve 85-95% conversion rates of substrate to product, while batch processes average 70-80%. This differential translates directly to production economics, with each percentage point improvement in conversion efficiency reducing overall production costs by approximately 0.8-1.2%.
Labor costs demonstrate inverse correlation with automation levels. Fully automated continuous systems can reduce labor requirements by up to 70% compared to batch processes, though this advantage is partially offset by the need for more specialized technical personnel commanding higher wages. The optimal labor configuration varies based on regional wage differentials and available technical expertise.
Downstream processing costs remain relatively consistent across fermentation methodologies, typically representing 40-60% of total production expenses. However, continuous upstream processes can enable more efficient continuous downstream processing, potentially reducing these costs by 15-25% through improved process integration and reduced intermediate storage requirements.
Continuous fermentation systems present a compelling alternative, reducing production costs by approximately 25-35% compared to batch processes when operating at scales exceeding 50,000 liters. This cost advantage stems primarily from improved space-time yield, reduced labor requirements, and more efficient utilization of raw materials. However, implementation requires significant upfront investment in specialized equipment and control systems, creating a financial barrier for market entrants.
Fed-batch approaches occupy a middle ground, offering a 15-20% cost reduction compared to batch processes while requiring less sophisticated infrastructure than continuous systems. This methodology has gained traction among mid-sized producers seeking to optimize production economics without committing to full continuous processing infrastructure.
Energy consumption represents a significant cost driver across all scale-up methods. Continuous systems demonstrate superior energy efficiency per unit of protein produced, consuming approximately 30% less energy than comparable batch processes. This efficiency differential widens as production scales increase, making energy optimization a critical factor in large-scale implementation decisions.
Raw material utilization efficiency varies substantially between methodologies. Continuous and fed-batch systems typically achieve 85-95% conversion rates of substrate to product, while batch processes average 70-80%. This differential translates directly to production economics, with each percentage point improvement in conversion efficiency reducing overall production costs by approximately 0.8-1.2%.
Labor costs demonstrate inverse correlation with automation levels. Fully automated continuous systems can reduce labor requirements by up to 70% compared to batch processes, though this advantage is partially offset by the need for more specialized technical personnel commanding higher wages. The optimal labor configuration varies based on regional wage differentials and available technical expertise.
Downstream processing costs remain relatively consistent across fermentation methodologies, typically representing 40-60% of total production expenses. However, continuous upstream processes can enable more efficient continuous downstream processing, potentially reducing these costs by 15-25% through improved process integration and reduced intermediate storage requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!