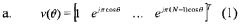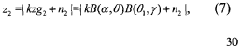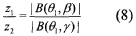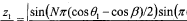Broadening the Scope of mmWave in Digital Health Advancements
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mmWave Technology Evolution and Healthcare Objectives
Millimeter wave (mmWave) technology has evolved significantly since its initial development in the mid-20th century for military and telecommunications applications. Operating in the frequency range of 30-300 GHz, mmWave technology offers unique capabilities including high bandwidth, fine spatial resolution, and the ability to penetrate certain materials while being reflected by others. These characteristics have positioned mmWave as a transformative technology across multiple industries, with healthcare emerging as a particularly promising frontier for innovation.
The evolution of mmWave in healthcare applications can be traced through several key developmental phases. Initially limited by hardware constraints and high implementation costs, early medical applications primarily focused on experimental imaging. The miniaturization of components and advancements in semiconductor technology during the 1990s and early 2000s enabled more practical implementations, though still primarily in research settings. The past decade has witnessed accelerated development, with mmWave technology becoming increasingly integrated into commercial healthcare solutions.
Current technological trends indicate a convergence of mmWave capabilities with artificial intelligence, cloud computing, and IoT frameworks, creating unprecedented opportunities for digital health applications. The non-ionizing nature of mmWave radiation presents a safer alternative to X-rays for certain diagnostic procedures, while its high-resolution capabilities enable detailed imaging of biological tissues and physiological processes without invasive procedures.
The primary objectives for broadening mmWave applications in digital health encompass several dimensions. From a technical perspective, goals include improving signal processing algorithms for biological data interpretation, enhancing sensor miniaturization for wearable applications, and developing more energy-efficient systems for continuous monitoring. Clinical objectives focus on validating mmWave technology for vital sign monitoring, sleep analysis, gait assessment, and early detection of conditions ranging from respiratory disorders to cardiovascular diseases.
Regulatory and standardization objectives represent another critical dimension, as the integration of mmWave technology into healthcare requires navigating complex approval processes across different jurisdictions. Establishing standardized protocols for data collection, processing, and interpretation will be essential for widespread clinical adoption and interoperability with existing healthcare systems.
The long-term vision for mmWave in digital health extends beyond current applications to encompass continuous health monitoring, predictive diagnostics, and personalized medicine. As the technology matures, the potential for mmWave to enable early disease detection, remote patient monitoring, and non-invasive treatment monitoring could fundamentally transform healthcare delivery models, particularly for aging populations and in regions with limited access to traditional healthcare infrastructure.
The evolution of mmWave in healthcare applications can be traced through several key developmental phases. Initially limited by hardware constraints and high implementation costs, early medical applications primarily focused on experimental imaging. The miniaturization of components and advancements in semiconductor technology during the 1990s and early 2000s enabled more practical implementations, though still primarily in research settings. The past decade has witnessed accelerated development, with mmWave technology becoming increasingly integrated into commercial healthcare solutions.
Current technological trends indicate a convergence of mmWave capabilities with artificial intelligence, cloud computing, and IoT frameworks, creating unprecedented opportunities for digital health applications. The non-ionizing nature of mmWave radiation presents a safer alternative to X-rays for certain diagnostic procedures, while its high-resolution capabilities enable detailed imaging of biological tissues and physiological processes without invasive procedures.
The primary objectives for broadening mmWave applications in digital health encompass several dimensions. From a technical perspective, goals include improving signal processing algorithms for biological data interpretation, enhancing sensor miniaturization for wearable applications, and developing more energy-efficient systems for continuous monitoring. Clinical objectives focus on validating mmWave technology for vital sign monitoring, sleep analysis, gait assessment, and early detection of conditions ranging from respiratory disorders to cardiovascular diseases.
Regulatory and standardization objectives represent another critical dimension, as the integration of mmWave technology into healthcare requires navigating complex approval processes across different jurisdictions. Establishing standardized protocols for data collection, processing, and interpretation will be essential for widespread clinical adoption and interoperability with existing healthcare systems.
The long-term vision for mmWave in digital health extends beyond current applications to encompass continuous health monitoring, predictive diagnostics, and personalized medicine. As the technology matures, the potential for mmWave to enable early disease detection, remote patient monitoring, and non-invasive treatment monitoring could fundamentally transform healthcare delivery models, particularly for aging populations and in regions with limited access to traditional healthcare infrastructure.
Digital Health Market Demand for mmWave Applications
The digital health market is experiencing unprecedented growth, with the global market value projected to reach $660 billion by 2025, growing at a CAGR of approximately 25%. Within this expanding ecosystem, millimeter wave (mmWave) technology is emerging as a critical enabler for next-generation healthcare applications, driven by its unique capabilities in non-invasive sensing and monitoring.
Remote patient monitoring represents one of the most significant market opportunities for mmWave applications, valued at $117 billion globally. The COVID-19 pandemic has accelerated this trend, with 71% of healthcare providers now viewing remote monitoring as a necessity rather than an option. mmWave technology's ability to detect vital signs without physical contact addresses the growing demand for continuous health monitoring solutions that minimize infection risks while maintaining clinical accuracy.
The aging population demographic is creating substantial market pull for mmWave-enabled health solutions. With the global population over 65 expected to double by 2050, there is increasing demand for technologies that can support aging-in-place and reduce hospitalization rates. mmWave sensors can detect falls, monitor respiratory patterns during sleep, and track mobility changes—all critical parameters for elderly care that currently lack reliable non-invasive monitoring solutions.
Chronic disease management represents another significant market segment, with diabetes, cardiovascular diseases, and respiratory conditions affecting over 1.7 billion people worldwide. The economic burden of these conditions exceeds $3.7 trillion annually. mmWave technology offers promising applications in glucose monitoring, cardiovascular assessment, and pulmonary function evaluation without breaking the skin barrier, addressing a critical need for painless, continuous monitoring solutions.
Mental health applications are emerging as a frontier market for mmWave technology. With mental health disorders affecting nearly 1 billion people globally, there is growing interest in technologies that can objectively measure stress, anxiety, and sleep quality. mmWave's ability to detect subtle physiological changes associated with emotional states could transform mental health monitoring from subjective self-reporting to objective measurement.
The preventive healthcare segment is projected to grow at 29% annually through 2028, with consumers increasingly investing in technologies that help prevent disease rather than treat it. mmWave-enabled wearables and home monitoring systems align perfectly with this shift toward proactive health management, offering early detection capabilities for conditions ranging from arrhythmias to early-stage respiratory infections.
Remote patient monitoring represents one of the most significant market opportunities for mmWave applications, valued at $117 billion globally. The COVID-19 pandemic has accelerated this trend, with 71% of healthcare providers now viewing remote monitoring as a necessity rather than an option. mmWave technology's ability to detect vital signs without physical contact addresses the growing demand for continuous health monitoring solutions that minimize infection risks while maintaining clinical accuracy.
The aging population demographic is creating substantial market pull for mmWave-enabled health solutions. With the global population over 65 expected to double by 2050, there is increasing demand for technologies that can support aging-in-place and reduce hospitalization rates. mmWave sensors can detect falls, monitor respiratory patterns during sleep, and track mobility changes—all critical parameters for elderly care that currently lack reliable non-invasive monitoring solutions.
Chronic disease management represents another significant market segment, with diabetes, cardiovascular diseases, and respiratory conditions affecting over 1.7 billion people worldwide. The economic burden of these conditions exceeds $3.7 trillion annually. mmWave technology offers promising applications in glucose monitoring, cardiovascular assessment, and pulmonary function evaluation without breaking the skin barrier, addressing a critical need for painless, continuous monitoring solutions.
Mental health applications are emerging as a frontier market for mmWave technology. With mental health disorders affecting nearly 1 billion people globally, there is growing interest in technologies that can objectively measure stress, anxiety, and sleep quality. mmWave's ability to detect subtle physiological changes associated with emotional states could transform mental health monitoring from subjective self-reporting to objective measurement.
The preventive healthcare segment is projected to grow at 29% annually through 2028, with consumers increasingly investing in technologies that help prevent disease rather than treat it. mmWave-enabled wearables and home monitoring systems align perfectly with this shift toward proactive health management, offering early detection capabilities for conditions ranging from arrhythmias to early-stage respiratory infections.
Current mmWave Implementation Challenges in Healthcare
Despite the promising potential of millimeter wave (mmWave) technology in healthcare applications, several significant implementation challenges currently limit its widespread adoption. The primary technical hurdle involves signal penetration limitations, as mmWave frequencies (30-300 GHz) experience substantial attenuation when interacting with human tissue, reducing effective sensing depth for internal physiological monitoring. This characteristic restricts many applications to surface-level measurements or requires complex signal processing algorithms to extract meaningful data from attenuated signals.
Hardware miniaturization presents another substantial challenge. Current mmWave systems for healthcare typically require bulky equipment including specialized antennas, transmitters, and receivers that are difficult to integrate into portable or wearable medical devices. The power consumption of these systems further complicates matters, as high-frequency operation demands significant energy, creating barriers for battery-powered or long-term monitoring applications.
Signal processing complexity represents a major technical obstacle. The high-frequency nature of mmWave signals generates enormous data volumes requiring sophisticated real-time processing capabilities. Additionally, biological signals often present as subtle variations within noisy environments, necessitating advanced algorithms for accurate feature extraction and interpretation. These computational demands frequently exceed the capabilities of compact, low-power devices suitable for healthcare settings.
Regulatory compliance and safety considerations significantly impact implementation timelines. The use of mmWave technology in healthcare applications must adhere to strict medical device regulations and electromagnetic exposure standards. Obtaining necessary approvals requires extensive testing and validation, particularly for novel applications where safety protocols may not be well-established.
Cost factors further constrain adoption, as specialized mmWave components remain expensive compared to technologies operating at lower frequencies. The precision manufacturing required for mmWave circuits and antennas contributes to higher production costs, while specialized expertise needed for system design and implementation adds to development expenses.
Interoperability challenges also exist between mmWave systems and established healthcare IT infrastructure. The integration of mmWave-based diagnostic or monitoring tools with electronic health records and other clinical systems requires standardized data formats and communication protocols that are still evolving for these applications.
Environmental interference presents practical deployment challenges in clinical settings. Medical facilities contain numerous electronic devices and metallic objects that can cause signal reflections, multipath effects, and electromagnetic interference, potentially compromising measurement accuracy and reliability of mmWave systems.
Hardware miniaturization presents another substantial challenge. Current mmWave systems for healthcare typically require bulky equipment including specialized antennas, transmitters, and receivers that are difficult to integrate into portable or wearable medical devices. The power consumption of these systems further complicates matters, as high-frequency operation demands significant energy, creating barriers for battery-powered or long-term monitoring applications.
Signal processing complexity represents a major technical obstacle. The high-frequency nature of mmWave signals generates enormous data volumes requiring sophisticated real-time processing capabilities. Additionally, biological signals often present as subtle variations within noisy environments, necessitating advanced algorithms for accurate feature extraction and interpretation. These computational demands frequently exceed the capabilities of compact, low-power devices suitable for healthcare settings.
Regulatory compliance and safety considerations significantly impact implementation timelines. The use of mmWave technology in healthcare applications must adhere to strict medical device regulations and electromagnetic exposure standards. Obtaining necessary approvals requires extensive testing and validation, particularly for novel applications where safety protocols may not be well-established.
Cost factors further constrain adoption, as specialized mmWave components remain expensive compared to technologies operating at lower frequencies. The precision manufacturing required for mmWave circuits and antennas contributes to higher production costs, while specialized expertise needed for system design and implementation adds to development expenses.
Interoperability challenges also exist between mmWave systems and established healthcare IT infrastructure. The integration of mmWave-based diagnostic or monitoring tools with electronic health records and other clinical systems requires standardized data formats and communication protocols that are still evolving for these applications.
Environmental interference presents practical deployment challenges in clinical settings. Medical facilities contain numerous electronic devices and metallic objects that can cause signal reflections, multipath effects, and electromagnetic interference, potentially compromising measurement accuracy and reliability of mmWave systems.
Existing mmWave Digital Health Monitoring Systems
01 mmWave technology in communication systems
Millimeter wave technology is being increasingly applied in communication systems to enable high-speed data transmission and network performance. These systems utilize the high frequency spectrum (30-300 GHz) to provide greater bandwidth capacity for wireless communications. The technology supports advanced networking capabilities including improved signal processing, network management, and integration with existing communication infrastructures.- mmWave technology in communication systems: Millimeter wave technology is being increasingly applied in communication systems to enable high-speed data transmission and network performance. These systems utilize the high frequency spectrum (typically 30-300 GHz) to provide greater bandwidth capacity for applications like 5G networks. The technology allows for improved network management, reduced latency, and enhanced connectivity in various environments, supporting the growing demands of modern telecommunications infrastructure.
- mmWave applications in sensing and imaging: Millimeter wave technology is expanding into sensing and imaging applications, leveraging its unique properties to develop advanced detection systems. These applications include security scanning, medical imaging, automotive radar, and environmental monitoring. The high-frequency waves can penetrate certain materials while reflecting off others, enabling detailed imaging capabilities that conventional technologies cannot achieve, thus broadening the scope of non-invasive detection methods.
- Integration of mmWave with computing platforms: The integration of millimeter wave technology with computing platforms represents a significant expansion of its application scope. This includes embedding mmWave capabilities in software systems, cloud infrastructures, and edge computing devices. Such integration enables real-time data processing, enhanced system management, and improved resource allocation, creating more efficient and responsive technological ecosystems that can handle complex computational tasks while maintaining high-speed connectivity.
- mmWave technology in healthcare and medical devices: Millimeter wave technology is finding increasing applications in healthcare and medical devices, broadening its scope beyond traditional communications. These applications include non-invasive diagnostic tools, therapeutic devices, patient monitoring systems, and medical imaging equipment. The technology's ability to provide high-resolution imaging and precise sensing capabilities makes it valuable for detecting physiological changes and supporting advanced medical interventions without the risks associated with ionizing radiation.
- mmWave in smart infrastructure and IoT: The application of millimeter wave technology is expanding to smart infrastructure and Internet of Things (IoT) deployments. This includes smart buildings, cities, transportation systems, and industrial environments where mmWave technology enables high-bandwidth connectivity for numerous devices. The technology supports advanced monitoring, automation, and control systems that require reliable and high-speed data transmission, contributing to more efficient resource management and enhanced operational capabilities in complex interconnected environments.
02 mmWave applications in sensing and imaging
Millimeter wave technology is expanding into sensing and imaging applications, leveraging its unique properties for high-resolution detection and visualization. These systems can penetrate certain materials while providing detailed imaging capabilities, making them suitable for security screening, medical diagnostics, and industrial inspection. The technology enables non-invasive scanning with improved accuracy and detail compared to conventional methods.Expand Specific Solutions03 Integration of mmWave in computing and software systems
The integration of millimeter wave technology with computing platforms and software systems is creating new capabilities for data processing and system management. This includes specialized operating systems, virtualization technologies, and software frameworks designed to handle the unique requirements of mmWave applications. These integrations enable more efficient resource allocation, improved system performance, and enhanced user experiences.Expand Specific Solutions04 mmWave technology in navigation and positioning systems
Millimeter wave technology is being applied to enhance navigation and positioning systems with greater precision and reliability. These applications utilize the high-frequency characteristics of mmWave to provide accurate distance measurements, object detection, and spatial mapping. The technology supports advanced guidance systems, autonomous navigation, and location-based services in various environments and conditions.Expand Specific Solutions05 mmWave in IoT and smart device applications
The expansion of millimeter wave technology into Internet of Things (IoT) and smart device applications is enabling new functionalities and use cases. These implementations leverage mmWave's high bandwidth and low latency characteristics to support device connectivity, data exchange, and remote sensing capabilities. The technology facilitates advanced automation, monitoring systems, and interactive smart environments across consumer, industrial, and commercial sectors.Expand Specific Solutions
Key Industry Players in mmWave Healthcare Solutions
The millimeter wave (mmWave) technology in digital health is currently in an early growth phase, with the market expected to expand significantly as applications mature. The global market size for mmWave in healthcare is projected to reach substantial growth in the coming years, driven by increasing demand for non-invasive monitoring solutions. Leading players like Samsung Electronics, Philips, and Intel are advancing the technology's maturity through significant R&D investments, while specialized companies such as Peltbeam are developing innovative signal enhancement solutions. Academic institutions including NYU and IIT Madras are collaborating with industry leaders like Qualcomm and Huawei to overcome technical challenges related to signal penetration and range limitations. The competitive landscape is diverse, with telecommunications companies (NTT, British Telecom) and medical technology firms (Zhongchengkangfu Technology) exploring different applications across remote patient monitoring, diagnostic imaging, and therapeutic interventions.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed advanced mmWave technology for digital health applications focusing on non-invasive vital sign monitoring systems. Their solution integrates 60GHz mmWave radar sensors with proprietary signal processing algorithms to detect micro-movements caused by heartbeats and respiration. The technology enables contactless monitoring of heart rate, respiratory rate, and sleep patterns with clinical-grade accuracy. Samsung's implementation includes specialized antenna arrays that improve signal penetration through clothing while maintaining privacy. Their system-on-chip designs specifically optimize power consumption for continuous health monitoring, allowing integration into smartphones and wearable devices while maintaining battery efficiency.
Strengths: Extensive consumer electronics ecosystem allowing seamless integration across devices; strong manufacturing capabilities enabling cost-effective production. Weaknesses: Relatively new entrant to medical-grade monitoring compared to established healthcare companies; regulatory approval processes may slow market penetration.
Koninklijke Philips NV
Technical Solution: Philips has pioneered mmWave technology in digital health through their Vital Signs Monitoring (VSM) platform. Their solution employs 77GHz frequency-modulated continuous wave (FMCW) radar technology to enable contactless patient monitoring in clinical settings. The system can simultaneously track multiple patients' respiratory patterns, heart rates, and movement with sub-millimeter precision from distances up to 3 meters. Philips has developed specialized algorithms that filter environmental interference and distinguish between multiple subjects in the same room. Their technology has been clinically validated to detect early signs of patient deterioration in hospital settings, potentially reducing code blue events by up to 35%. The system integrates with electronic health records and can trigger automated alerts when vital signs deviate from normal ranges.
Strengths: Extensive healthcare industry experience with established clinical validation protocols and regulatory expertise; comprehensive patient monitoring ecosystem. Weaknesses: Solutions primarily designed for clinical settings rather than consumer applications; higher implementation costs compared to consumer-grade alternatives.
Critical Patents and Research in Medical mmWave Sensing
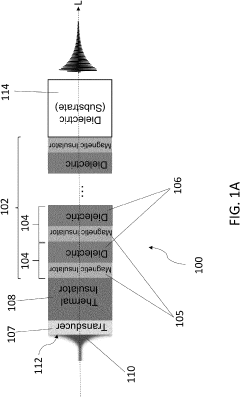
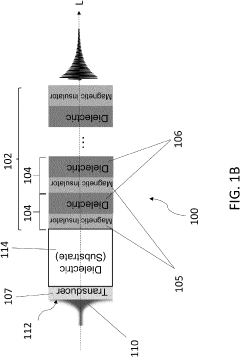
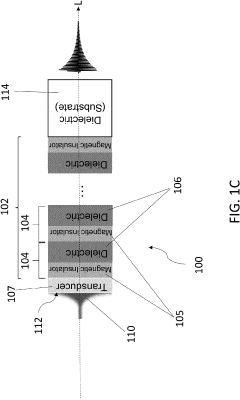
Magnonic electromagnetic radiation sources with high output power at high frequencies
PatentActiveUS20230154662A1
Innovation
- The development of acoustically mediated pulsed radiation sources based on superlattices with alternating magnetic insulator and dielectric layers, where acoustic pulses excite in-phase standing spin waves to generate electromagnetic radiation, enhancing output power across a wide frequency range including mmW frequencies.
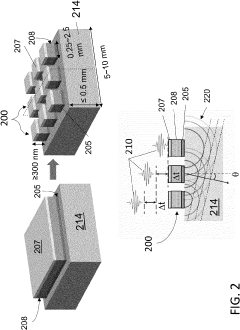
Millimeter- wave communication system and method for determining location of first device based on known location of second device
PatentWO2019181036A1
Innovation
- A method that utilizes multiple beam values sharing the same dominant path to resolve the ambiguity in beamforming models, determining the direction of the dominant path by evaluating a beamforming model that relates the deviation of beamforming angles from the dominant path with the energy received, and subsequently calculating the location of the first device relative to a second device with a known location.
Regulatory Framework for mmWave Medical Devices
The regulatory landscape for millimeter wave (mmWave) medical devices presents a complex framework that varies significantly across global jurisdictions. In the United States, the FDA has established specific guidelines for wireless medical devices operating in mmWave frequencies, with particular attention to radiation exposure limits and electromagnetic compatibility. These devices typically fall under Class II medical devices, requiring premarket notification (510(k)) or, in some cases, premarket approval (PMA) depending on their intended use and potential risks.
The European Union approaches mmWave medical technology regulation through the Medical Device Regulation (MDR) framework, which came into full effect in May 2021. The MDR imposes stricter requirements for clinical evaluation, post-market surveillance, and technical documentation compared to its predecessor. For mmWave devices specifically, compliance with harmonized standards related to radio equipment (RED Directive) and electromagnetic compatibility is mandatory.
In Asia, regulatory approaches differ substantially. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specific pathways for innovative medical technologies including mmWave applications, while China's National Medical Products Administration (NMPA) has recently updated its regulatory framework to accelerate approval for certain digital health technologies, though with stringent data localization requirements.
A critical regulatory consideration for mmWave medical devices is frequency allocation. The International Telecommunication Union (ITU) coordinates global spectrum management, but individual countries maintain sovereign control over their spectrum. Medical mmWave devices must operate within designated frequency bands to avoid interference with other services, with most jurisdictions allocating specific bands for medical applications.
Privacy and data security regulations present additional compliance challenges. The health data collected by mmWave devices falls under protected health information in many jurisdictions, requiring adherence to regulations such as HIPAA in the US or GDPR in Europe. These frameworks mandate robust data protection measures, informed consent protocols, and strict data breach notification procedures.
Emerging regulatory trends include the development of "regulatory sandboxes" in several countries, allowing controlled testing of innovative mmWave health applications with temporary regulatory flexibility. Additionally, international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to establish common principles for regulating software as a medical device (SaMD), which will impact many mmWave health monitoring applications.
The European Union approaches mmWave medical technology regulation through the Medical Device Regulation (MDR) framework, which came into full effect in May 2021. The MDR imposes stricter requirements for clinical evaluation, post-market surveillance, and technical documentation compared to its predecessor. For mmWave devices specifically, compliance with harmonized standards related to radio equipment (RED Directive) and electromagnetic compatibility is mandatory.
In Asia, regulatory approaches differ substantially. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specific pathways for innovative medical technologies including mmWave applications, while China's National Medical Products Administration (NMPA) has recently updated its regulatory framework to accelerate approval for certain digital health technologies, though with stringent data localization requirements.
A critical regulatory consideration for mmWave medical devices is frequency allocation. The International Telecommunication Union (ITU) coordinates global spectrum management, but individual countries maintain sovereign control over their spectrum. Medical mmWave devices must operate within designated frequency bands to avoid interference with other services, with most jurisdictions allocating specific bands for medical applications.
Privacy and data security regulations present additional compliance challenges. The health data collected by mmWave devices falls under protected health information in many jurisdictions, requiring adherence to regulations such as HIPAA in the US or GDPR in Europe. These frameworks mandate robust data protection measures, informed consent protocols, and strict data breach notification procedures.
Emerging regulatory trends include the development of "regulatory sandboxes" in several countries, allowing controlled testing of innovative mmWave health applications with temporary regulatory flexibility. Additionally, international harmonization efforts through the International Medical Device Regulators Forum (IMDRF) are working to establish common principles for regulating software as a medical device (SaMD), which will impact many mmWave health monitoring applications.
Data Privacy and Security in mmWave Health Monitoring
As millimeter wave (mmWave) technology increasingly integrates into digital health applications, data privacy and security concerns have emerged as critical challenges. The non-contact nature of mmWave sensing creates unique vulnerabilities that must be addressed to ensure patient confidentiality and regulatory compliance. Healthcare data collected through mmWave sensors is particularly sensitive, containing physiological parameters and behavioral patterns that could reveal intimate details about an individual's health status.
Current security frameworks for mmWave health monitoring systems implement multi-layered approaches, combining hardware-level encryption with secure software protocols. Advanced encryption standards (AES-256) are being deployed at the sensor level to protect data during transmission, while secure enclaves within processing units safeguard information during analysis. Despite these measures, the wireless nature of mmWave technology presents inherent risks for interception and unauthorized access.
Regulatory frameworks worldwide are evolving to address these emerging technologies. The Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe have begun incorporating guidelines specific to remote sensing technologies, though mmWave-specific regulations remain underdeveloped. This regulatory gap creates uncertainty for technology developers and healthcare providers implementing these solutions.
Authentication mechanisms represent another critical security component, with biometric verification and multi-factor authentication becoming standard features in mmWave health monitoring systems. These systems increasingly employ federated learning approaches that allow algorithm training across multiple devices without centralizing sensitive data, thereby reducing privacy risks while maintaining analytical capabilities.
The challenge of data minimization presents particular complexity in mmWave applications. Unlike traditional health monitoring, mmWave sensors often collect continuous streams of data that may capture more information than strictly necessary for the intended medical purpose. Implementing effective data minimization strategies without compromising diagnostic accuracy remains an active area of research and development.
Industry consortiums including the mmWave Security Alliance and Healthcare Information and Management Systems Society (HIMSS) have begun developing best practices specifically for securing mmWave health applications. These collaborative efforts focus on standardizing security protocols and establishing certification processes that can validate the security posture of mmWave health monitoring solutions before deployment in clinical settings.
Current security frameworks for mmWave health monitoring systems implement multi-layered approaches, combining hardware-level encryption with secure software protocols. Advanced encryption standards (AES-256) are being deployed at the sensor level to protect data during transmission, while secure enclaves within processing units safeguard information during analysis. Despite these measures, the wireless nature of mmWave technology presents inherent risks for interception and unauthorized access.
Regulatory frameworks worldwide are evolving to address these emerging technologies. The Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe have begun incorporating guidelines specific to remote sensing technologies, though mmWave-specific regulations remain underdeveloped. This regulatory gap creates uncertainty for technology developers and healthcare providers implementing these solutions.
Authentication mechanisms represent another critical security component, with biometric verification and multi-factor authentication becoming standard features in mmWave health monitoring systems. These systems increasingly employ federated learning approaches that allow algorithm training across multiple devices without centralizing sensitive data, thereby reducing privacy risks while maintaining analytical capabilities.
The challenge of data minimization presents particular complexity in mmWave applications. Unlike traditional health monitoring, mmWave sensors often collect continuous streams of data that may capture more information than strictly necessary for the intended medical purpose. Implementing effective data minimization strategies without compromising diagnostic accuracy remains an active area of research and development.
Industry consortiums including the mmWave Security Alliance and Healthcare Information and Management Systems Society (HIMSS) have begun developing best practices specifically for securing mmWave health applications. These collaborative efforts focus on standardizing security protocols and establishing certification processes that can validate the security posture of mmWave health monitoring solutions before deployment in clinical settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!