Implementing mmWave for Interconnected Medical Devices
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
mmWave Technology Background and Objectives
Millimeter wave (mmWave) technology operates in the frequency spectrum between 30 GHz and 300 GHz, with wavelengths ranging from 1 to 10 millimeters. This technology has evolved significantly since its initial development in the mid-20th century for military and radar applications. The miniaturization of electronic components and advancements in semiconductor technology have enabled mmWave to transition from specialized applications to more mainstream use cases, particularly in telecommunications with the advent of 5G networks.
In the medical device sector, mmWave technology represents a promising frontier for interconnected healthcare systems. The evolution of medical devices has progressed from standalone equipment to networked systems that require reliable, high-speed, and secure communication channels. Traditional wireless technologies like Bluetooth and Wi-Fi face limitations in bandwidth, latency, and interference in complex clinical environments, creating a technological gap that mmWave can potentially address.
The primary objective of implementing mmWave for interconnected medical devices is to establish ultra-high-speed, low-latency communication networks within healthcare settings. These networks would support real-time data transmission between diagnostic equipment, monitoring systems, and centralized healthcare platforms. The technology aims to facilitate seamless integration of various medical devices, enabling comprehensive patient monitoring and more efficient clinical workflows.
Another critical goal is to leverage mmWave's high bandwidth capacity to support the increasing data demands of modern medical imaging and diagnostic tools. As medical imaging resolution continues to improve and diagnostic systems generate larger datasets, the need for robust data transmission capabilities becomes paramount. mmWave technology, with its potential for multi-gigabit data rates, presents a viable solution for handling these expanding data requirements.
The implementation of mmWave also aims to address the growing need for wireless medical device networks that can operate reliably in densely populated healthcare environments without interference. The directional nature of mmWave signals and their limited penetration through physical barriers offer inherent security advantages and reduced interference compared to lower-frequency alternatives.
Looking forward, the technological trajectory for mmWave in medical applications is focused on overcoming current limitations related to power consumption, signal attenuation, and implementation costs. Research efforts are directed toward developing more energy-efficient mmWave transceivers, advanced beamforming techniques to improve signal reliability, and cost-effective manufacturing processes to make the technology more accessible for widespread medical device integration.
In the medical device sector, mmWave technology represents a promising frontier for interconnected healthcare systems. The evolution of medical devices has progressed from standalone equipment to networked systems that require reliable, high-speed, and secure communication channels. Traditional wireless technologies like Bluetooth and Wi-Fi face limitations in bandwidth, latency, and interference in complex clinical environments, creating a technological gap that mmWave can potentially address.
The primary objective of implementing mmWave for interconnected medical devices is to establish ultra-high-speed, low-latency communication networks within healthcare settings. These networks would support real-time data transmission between diagnostic equipment, monitoring systems, and centralized healthcare platforms. The technology aims to facilitate seamless integration of various medical devices, enabling comprehensive patient monitoring and more efficient clinical workflows.
Another critical goal is to leverage mmWave's high bandwidth capacity to support the increasing data demands of modern medical imaging and diagnostic tools. As medical imaging resolution continues to improve and diagnostic systems generate larger datasets, the need for robust data transmission capabilities becomes paramount. mmWave technology, with its potential for multi-gigabit data rates, presents a viable solution for handling these expanding data requirements.
The implementation of mmWave also aims to address the growing need for wireless medical device networks that can operate reliably in densely populated healthcare environments without interference. The directional nature of mmWave signals and their limited penetration through physical barriers offer inherent security advantages and reduced interference compared to lower-frequency alternatives.
Looking forward, the technological trajectory for mmWave in medical applications is focused on overcoming current limitations related to power consumption, signal attenuation, and implementation costs. Research efforts are directed toward developing more energy-efficient mmWave transceivers, advanced beamforming techniques to improve signal reliability, and cost-effective manufacturing processes to make the technology more accessible for widespread medical device integration.
Medical Device Connectivity Market Analysis
The medical device connectivity market is experiencing robust growth, driven by the increasing adoption of interconnected healthcare solutions. Current market valuations place this sector at approximately 2.5 billion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 25.3% through 2030. This exceptional growth trajectory is primarily fueled by healthcare facilities' digital transformation initiatives and the rising demand for real-time patient monitoring systems.
Demand analysis reveals several key market segments where mmWave technology for interconnected medical devices shows particular promise. Hospital settings represent the largest market share at 42%, followed by ambulatory care centers at 28%, and home healthcare environments at 18%. The remaining 12% encompasses specialized care facilities and research institutions. Within these segments, critical care monitoring, surgical suite integration, and remote patient monitoring emerge as the highest-value application areas.
Regional market assessment indicates North America currently dominates with 38% market share, followed closely by Europe at 32%. However, the Asia-Pacific region demonstrates the fastest growth rate at 29.7% annually, primarily driven by rapid healthcare infrastructure development in China and India. Latin America and Middle East/Africa regions, while smaller in market size, show increasing adoption rates as healthcare modernization efforts accelerate.
Key demand drivers include the growing prevalence of chronic diseases requiring continuous monitoring, increasing healthcare expenditure on digital infrastructure, and regulatory policies favoring interoperability standards. The COVID-19 pandemic has significantly accelerated market growth by highlighting the necessity for remote monitoring capabilities and reducing physical contact requirements in healthcare settings.
Customer pain points identified through market research include concerns about data security (cited by 78% of healthcare providers), system interoperability challenges (65%), implementation costs (59%), and technical complexity (52%). These factors represent both market barriers and opportunities for mmWave technology implementation.
Future market trends indicate a shift toward edge computing capabilities in medical devices, increased demand for AI-integrated monitoring solutions, and growing preference for wireless technologies that minimize physical infrastructure requirements. The mmWave technology segment specifically is projected to grow at 31.2% annually, outpacing the overall market due to its superior bandwidth capabilities and potential for enabling advanced applications like high-resolution real-time imaging and ultra-reliable low-latency communications essential for critical care scenarios.
Demand analysis reveals several key market segments where mmWave technology for interconnected medical devices shows particular promise. Hospital settings represent the largest market share at 42%, followed by ambulatory care centers at 28%, and home healthcare environments at 18%. The remaining 12% encompasses specialized care facilities and research institutions. Within these segments, critical care monitoring, surgical suite integration, and remote patient monitoring emerge as the highest-value application areas.
Regional market assessment indicates North America currently dominates with 38% market share, followed closely by Europe at 32%. However, the Asia-Pacific region demonstrates the fastest growth rate at 29.7% annually, primarily driven by rapid healthcare infrastructure development in China and India. Latin America and Middle East/Africa regions, while smaller in market size, show increasing adoption rates as healthcare modernization efforts accelerate.
Key demand drivers include the growing prevalence of chronic diseases requiring continuous monitoring, increasing healthcare expenditure on digital infrastructure, and regulatory policies favoring interoperability standards. The COVID-19 pandemic has significantly accelerated market growth by highlighting the necessity for remote monitoring capabilities and reducing physical contact requirements in healthcare settings.
Customer pain points identified through market research include concerns about data security (cited by 78% of healthcare providers), system interoperability challenges (65%), implementation costs (59%), and technical complexity (52%). These factors represent both market barriers and opportunities for mmWave technology implementation.
Future market trends indicate a shift toward edge computing capabilities in medical devices, increased demand for AI-integrated monitoring solutions, and growing preference for wireless technologies that minimize physical infrastructure requirements. The mmWave technology segment specifically is projected to grow at 31.2% annually, outpacing the overall market due to its superior bandwidth capabilities and potential for enabling advanced applications like high-resolution real-time imaging and ultra-reliable low-latency communications essential for critical care scenarios.
mmWave Implementation Challenges in Healthcare
The implementation of millimeter wave (mmWave) technology in healthcare environments presents several significant challenges that must be addressed for successful deployment. The high-frequency nature of mmWave signals (typically 30-300 GHz) creates unique obstacles in medical settings that differ substantially from consumer or industrial applications.
Signal propagation represents a primary concern, as mmWave signals experience severe attenuation when encountering physical barriers. In healthcare facilities with numerous walls, medical equipment, and constantly moving personnel, maintaining reliable connectivity becomes problematic. The short-range characteristics of mmWave signals necessitate careful planning of access point placement to ensure comprehensive coverage throughout medical facilities.
Power consumption poses another critical challenge, particularly for battery-operated medical devices. The high-frequency operation of mmWave technology inherently demands greater power, which conflicts with the need for extended battery life in portable medical devices. Engineers must develop innovative power management solutions to balance the high-bandwidth benefits of mmWave with energy efficiency requirements.
Interference management presents complex challenges in healthcare settings where numerous electronic devices operate simultaneously. Medical equipment sensitivity to electromagnetic interference requires robust protocols to prevent signal conflicts that could potentially compromise critical care systems. The dense deployment of interconnected devices in hospital environments exacerbates this challenge.
Regulatory compliance adds another layer of complexity. Medical device implementations must adhere to stringent healthcare regulations beyond standard telecommunications requirements. This includes compliance with patient data security standards, electromagnetic compatibility with life-supporting equipment, and adherence to specific power emission limits in medical environments.
Miniaturization of mmWave components represents a significant engineering challenge. Medical devices often have strict size constraints, yet mmWave technology typically requires specialized antennas and signal processing components. Integrating these elements into compact medical devices while maintaining performance standards demands advanced design approaches.
Cost considerations cannot be overlooked, as healthcare systems operate under strict budget constraints. The specialized nature of mmWave components and the engineering expertise required for implementation contribute to higher development and deployment costs compared to conventional wireless technologies.
Reliability and redundancy requirements in healthcare are exceptionally high, as device failures could have life-threatening consequences. Implementing fail-safe mechanisms and redundant systems while maintaining the performance benefits of mmWave technology presents significant design challenges that exceed those in consumer applications.
Signal propagation represents a primary concern, as mmWave signals experience severe attenuation when encountering physical barriers. In healthcare facilities with numerous walls, medical equipment, and constantly moving personnel, maintaining reliable connectivity becomes problematic. The short-range characteristics of mmWave signals necessitate careful planning of access point placement to ensure comprehensive coverage throughout medical facilities.
Power consumption poses another critical challenge, particularly for battery-operated medical devices. The high-frequency operation of mmWave technology inherently demands greater power, which conflicts with the need for extended battery life in portable medical devices. Engineers must develop innovative power management solutions to balance the high-bandwidth benefits of mmWave with energy efficiency requirements.
Interference management presents complex challenges in healthcare settings where numerous electronic devices operate simultaneously. Medical equipment sensitivity to electromagnetic interference requires robust protocols to prevent signal conflicts that could potentially compromise critical care systems. The dense deployment of interconnected devices in hospital environments exacerbates this challenge.
Regulatory compliance adds another layer of complexity. Medical device implementations must adhere to stringent healthcare regulations beyond standard telecommunications requirements. This includes compliance with patient data security standards, electromagnetic compatibility with life-supporting equipment, and adherence to specific power emission limits in medical environments.
Miniaturization of mmWave components represents a significant engineering challenge. Medical devices often have strict size constraints, yet mmWave technology typically requires specialized antennas and signal processing components. Integrating these elements into compact medical devices while maintaining performance standards demands advanced design approaches.
Cost considerations cannot be overlooked, as healthcare systems operate under strict budget constraints. The specialized nature of mmWave components and the engineering expertise required for implementation contribute to higher development and deployment costs compared to conventional wireless technologies.
Reliability and redundancy requirements in healthcare are exceptionally high, as device failures could have life-threatening consequences. Implementing fail-safe mechanisms and redundant systems while maintaining the performance benefits of mmWave technology presents significant design challenges that exceed those in consumer applications.
Current mmWave Implementation Architectures
01 mmWave communication systems and protocols
mmWave technology enables high-speed wireless communication through the use of millimeter wave frequency bands (typically 30-300 GHz). These systems implement specialized protocols for beam management, channel access, and signal processing to overcome the propagation challenges inherent to high-frequency transmission. Advanced modulation schemes and multiple access techniques are employed to maximize data throughput while maintaining reliable connections in various environments.- mmWave communication systems and protocols: mmWave technology enables high-speed wireless communication by utilizing frequency bands between 30-300 GHz. These systems implement specialized protocols for efficient data transmission, beamforming techniques to overcome signal attenuation, and advanced modulation schemes to maximize bandwidth utilization. The technology supports multi-gigabit data rates essential for 5G networks and beyond, while addressing challenges related to signal propagation and interference management.
- mmWave antenna design and beam management: Advanced antenna designs are crucial for mmWave technology implementation, featuring phased array configurations that enable precise beam steering and focusing. These systems incorporate multiple antenna elements to form highly directional beams, compensating for the high path loss characteristic of mmWave frequencies. Beam management techniques include tracking algorithms, adaptive beam switching, and spatial multiplexing to maintain reliable connections as devices move or encounter obstacles.
- mmWave semiconductor and integrated circuit technologies: Specialized semiconductor technologies and integrated circuits are developed specifically for mmWave applications, featuring high-frequency transistors, low-noise amplifiers, and power-efficient designs. These components are fabricated using advanced processes to minimize signal loss and maximize performance at extremely high frequencies. Integration techniques combine RF components, digital processing, and power management on compact chipsets suitable for mobile and IoT devices operating in mmWave bands.
- mmWave applications in sensing and imaging: Beyond communications, mmWave technology enables high-resolution sensing and imaging applications due to its short wavelength characteristics. These systems can detect objects, measure distances with high precision, and create detailed images through various materials. Applications include automotive radar, security scanning, medical imaging, industrial quality control, and gesture recognition interfaces. The technology offers advantages in terms of resolution, penetration capabilities, and operation in challenging environmental conditions.
- mmWave network infrastructure and deployment: Implementing mmWave technology in network infrastructure requires specialized equipment and deployment strategies to overcome coverage limitations. Small cell architectures, distributed antenna systems, and intelligent network planning are employed to ensure adequate coverage despite the limited propagation range of mmWave signals. These networks incorporate advanced backhaul solutions, edge computing capabilities, and dynamic resource allocation to support high-bandwidth applications while maintaining reliability and quality of service.
02 Antenna array designs for mmWave applications
Specialized antenna arrays are critical for mmWave technology implementation, featuring beamforming capabilities to overcome the high path loss at these frequencies. These designs include phased arrays, MIMO configurations, and adaptive beam steering mechanisms that enable directional transmission and reception. Compact antenna structures with high gain characteristics help maximize signal strength while minimizing interference in dense deployment scenarios.Expand Specific Solutions03 mmWave semiconductor and integrated circuit technologies
Advanced semiconductor technologies are developed specifically for mmWave applications, including specialized RF front-end modules, power amplifiers, and low-noise amplifiers capable of operating at extremely high frequencies. These integrated circuits employ novel materials and fabrication techniques to achieve the necessary performance characteristics while managing thermal issues and power consumption. System-on-chip solutions integrate multiple mmWave components to reduce size and improve overall system efficiency.Expand Specific Solutions04 mmWave applications in sensing and imaging
mmWave technology enables high-resolution sensing and imaging applications due to the short wavelength characteristics. These systems can penetrate certain materials while providing detailed object detection and classification. Applications include automotive radar, security scanning, medical imaging, and industrial quality control. Advanced signal processing algorithms extract meaningful information from mmWave reflections to create detailed environmental maps or identify specific materials and objects.Expand Specific Solutions05 mmWave network infrastructure and deployment
The deployment of mmWave technology in network infrastructure requires specialized planning and equipment to address coverage challenges. Small cell architectures, distributed antenna systems, and intelligent repeaters are employed to extend coverage in urban environments. Network planning tools incorporate detailed propagation models specific to mmWave frequencies, accounting for atmospheric attenuation, building penetration losses, and reflection characteristics. Integration with existing sub-6 GHz networks enables seamless connectivity across different coverage zones.Expand Specific Solutions
Key Industry Players in Medical mmWave Solutions
The mmWave technology for interconnected medical devices market is in its early growth phase, characterized by significant R&D investment and emerging commercial applications. The global market is projected to expand rapidly, driven by increasing demand for high-bandwidth, low-latency medical connectivity solutions. Leading technology companies like Qualcomm, Samsung, and Huawei are leveraging their 5G expertise to develop medical-specific mmWave applications, while specialized medical technology firms such as Beijing Zhongchengkangfu and Cardiac Pacemakers are focusing on clinical implementations. Academic institutions including Xidian University and University of California are contributing fundamental research. The technology is approaching commercial maturity in certain applications, with companies like Welch Allyn and Wisonic integrating mmWave capabilities into next-generation medical devices, though regulatory approval processes remain a significant hurdle for widespread adoption.
QUALCOMM, Inc.
Technical Solution: Qualcomm has developed a comprehensive mmWave solution for interconnected medical devices that leverages their expertise in wireless communications. Their approach integrates 5G mmWave technology with specialized medical device connectivity protocols to enable high-bandwidth, low-latency communication between medical devices. The system operates in the 60GHz frequency band, providing data rates up to 7Gbps while maintaining medical-grade reliability. Qualcomm's implementation includes custom antenna arrays with beamforming capabilities specifically designed for medical environments, minimizing interference with existing hospital equipment. Their solution incorporates advanced security features including hardware-level encryption and authentication mechanisms that comply with HIPAA and other healthcare data protection standards. The architecture supports mesh networking capabilities allowing for seamless device-to-device communication without requiring constant connection to central infrastructure, critical for maintaining connectivity in complex hospital environments.
Strengths: Qualcomm's extensive experience in wireless chip development provides superior integration capabilities and power efficiency. Their solution offers industry-leading data throughput with minimal latency, critical for real-time medical applications. Weaknesses: The implementation requires specialized hardware components that may increase device costs and complexity compared to lower-frequency alternatives. Signal penetration limitations of mmWave technology may necessitate more access points in medical facilities.
Huawei Technologies Co., Ltd.
Technical Solution: Huawei has developed a comprehensive mmWave solution for medical device interconnectivity that leverages their extensive expertise in 5G communications. Their implementation utilizes the 60GHz frequency band with specialized protocols optimized for medical applications, delivering data rates up to 10Gbps with sub-millisecond latency. The system features Huawei's proprietary Massive MIMO antenna arrays with 256 elements, providing exceptional coverage and penetration capabilities in complex hospital environments. Their solution incorporates AI-driven network optimization that continuously adjusts transmission parameters based on environmental conditions and device priorities, ensuring critical medical devices receive bandwidth priority. Huawei's implementation includes a hierarchical security architecture with multiple encryption layers and authentication mechanisms specifically designed to meet healthcare data protection requirements across different regulatory frameworks. The platform supports seamless integration with hospital information systems through standardized interfaces, enabling comprehensive device management and data aggregation capabilities.
Strengths: Huawei's extensive telecommunications infrastructure experience provides superior scalability and reliability for large-scale hospital deployments. Their AI-driven network optimization delivers exceptional performance in challenging RF environments compared to static configurations. Weaknesses: Regulatory concerns in some markets may limit adoption despite technical merits. The comprehensive nature of their solution may present implementation challenges for smaller healthcare facilities with limited IT resources.
Critical Patents and Technical Literature Review
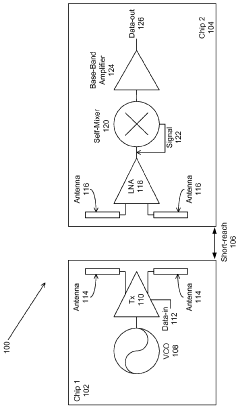
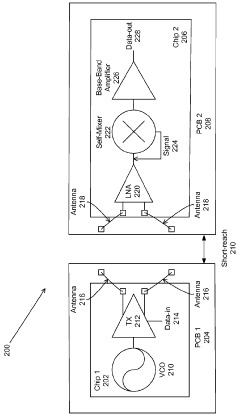
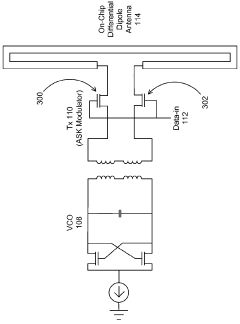
Milli-meter-wave-wireless-interconnect (m2w2 - interconnect) method for short-range communications with ultra-high data rate capability
PatentActiveEP2441313A2
Innovation
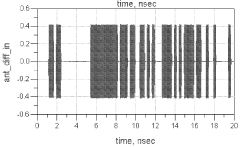
- A millimeter wave wireless interconnect using asynchronous modulation and differential signaling, with a transmitter modulating a millimeter-wave carrier signal that is amplified and radiated by a transmitter antenna, and received by a receiver antenna for demodulation to a full swing digital signal, eliminating the need for power-hungry equalization circuits and phase lock loops.
Milli-meter-wave-wireless-interconnect (m2w2 - interconnect) method for short-range communications with ultra-high data rate capability
PatentWO2010144617A2
Innovation
- A millimeter wave wireless interconnect using asynchronous modulation and differential signaling, with a transmitter modulating a millimeter-wave carrier signal that is amplified and radiated by a transmitter antenna, and received by a receiver antenna for demodulation to a full swing digital signal, eliminating the need for power-hungry equalization circuits and phase lock loops.
Regulatory Compliance for Medical mmWave Devices
The regulatory landscape for mmWave medical devices presents significant complexity due to the intersection of telecommunications standards and medical device regulations. In the United States, the FDA's Center for Devices and Radiological Health (CDRH) oversees medical devices utilizing mmWave technology, requiring manufacturers to demonstrate both safety and efficacy through clinical validation. These devices typically fall under Class II medical devices, necessitating 510(k) clearance with particular emphasis on electromagnetic compatibility and radiation safety assessments.
The European Union applies the Medical Device Regulation (MDR) framework, which imposes stringent requirements for clinical evidence, post-market surveillance, and risk management. For mmWave devices, manufacturers must additionally comply with the Radio Equipment Directive (RED) 2014/53/EU, addressing specific radio frequency requirements. The CE marking process requires verification of compliance with both directives before market access is permitted.
International Electrotechnical Commission (IEC) standards, particularly IEC 60601-1-2 for electromagnetic compatibility in medical environments, establish critical safety parameters for mmWave device operation. These standards ensure devices function reliably without interference in clinical settings where multiple electronic systems operate simultaneously. The IEEE 802.15.6 standard specifically addresses wireless body area networks, providing technical guidelines for short-range, low-power communication systems used in medical applications.
Exposure limits represent another critical regulatory consideration. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) guidelines establish safety thresholds for human exposure to electromagnetic fields, including the mmWave spectrum. Medical device manufacturers must demonstrate compliance with these exposure limits through comprehensive testing and documentation.
Privacy and cybersecurity regulations add another layer of complexity. In the EU, the General Data Protection Regulation (GDPR) imposes strict requirements on the handling of patient data transmitted via mmWave devices. In the US, HIPAA compliance is mandatory for any medical device that processes protected health information. The FDA has also published guidance on cybersecurity for networked medical devices, requiring manufacturers to implement robust security measures throughout the product lifecycle.
Regulatory harmonization efforts are underway through the International Medical Device Regulators Forum (IMDRF), which aims to streamline approval processes across different jurisdictions. However, significant regional variations persist, creating challenges for global market access. Manufacturers must develop comprehensive regulatory strategies that address these variations while maintaining compliance with evolving standards as mmWave technology continues to advance in medical applications.
The European Union applies the Medical Device Regulation (MDR) framework, which imposes stringent requirements for clinical evidence, post-market surveillance, and risk management. For mmWave devices, manufacturers must additionally comply with the Radio Equipment Directive (RED) 2014/53/EU, addressing specific radio frequency requirements. The CE marking process requires verification of compliance with both directives before market access is permitted.
International Electrotechnical Commission (IEC) standards, particularly IEC 60601-1-2 for electromagnetic compatibility in medical environments, establish critical safety parameters for mmWave device operation. These standards ensure devices function reliably without interference in clinical settings where multiple electronic systems operate simultaneously. The IEEE 802.15.6 standard specifically addresses wireless body area networks, providing technical guidelines for short-range, low-power communication systems used in medical applications.
Exposure limits represent another critical regulatory consideration. The International Commission on Non-Ionizing Radiation Protection (ICNIRP) guidelines establish safety thresholds for human exposure to electromagnetic fields, including the mmWave spectrum. Medical device manufacturers must demonstrate compliance with these exposure limits through comprehensive testing and documentation.
Privacy and cybersecurity regulations add another layer of complexity. In the EU, the General Data Protection Regulation (GDPR) imposes strict requirements on the handling of patient data transmitted via mmWave devices. In the US, HIPAA compliance is mandatory for any medical device that processes protected health information. The FDA has also published guidance on cybersecurity for networked medical devices, requiring manufacturers to implement robust security measures throughout the product lifecycle.
Regulatory harmonization efforts are underway through the International Medical Device Regulators Forum (IMDRF), which aims to streamline approval processes across different jurisdictions. However, significant regional variations persist, creating challenges for global market access. Manufacturers must develop comprehensive regulatory strategies that address these variations while maintaining compliance with evolving standards as mmWave technology continues to advance in medical applications.
Security and Privacy Considerations in mmWave Healthcare
The implementation of millimeter wave (mmWave) technology in interconnected medical devices introduces significant security and privacy challenges that must be addressed comprehensively. Patient data transmitted via mmWave signals contains highly sensitive information protected by regulations such as HIPAA in the United States and GDPR in Europe, requiring robust security frameworks specifically designed for high-frequency wireless communications.
mmWave's physical characteristics present unique security considerations. The directional nature and limited penetration capabilities of these signals provide inherent protection against certain types of eavesdropping attacks, as interception requires line-of-sight positioning. However, this physical layer security is insufficient when considering sophisticated attack vectors targeting modern healthcare environments.
Authentication mechanisms for mmWave medical devices must be strengthened beyond traditional approaches. Multi-factor authentication protocols specifically optimized for low-latency medical applications are essential, as conventional authentication methods may introduce unacceptable delays in critical care scenarios. Biometric authentication integrated with mmWave sensing capabilities offers promising solutions for continuous user verification without compromising operational efficiency.
Encryption standards for mmWave medical communications require adaptation to address the technology's high bandwidth capabilities. While AES-256 encryption remains viable, the implementation must be optimized to handle the multi-gigabit data rates characteristic of mmWave transmissions without introducing latency that could compromise real-time medical applications. Hardware-accelerated encryption solutions specifically designed for mmWave bandwidths are emerging as necessary components.
Privacy concerns extend beyond data transmission to the mmWave sensors themselves. The high-resolution imaging capabilities of mmWave technology can potentially capture detailed physiological data beyond what patients have explicitly consented to share. Privacy-by-design approaches must include data minimization techniques that process only clinically relevant information while discarding extraneous personal data at the sensor level.
Regulatory frameworks are still evolving to address mmWave-specific security requirements in healthcare. The FDA's premarket cybersecurity guidance and the NIST Cybersecurity Framework provide foundational principles, but industry-specific standards for mmWave medical device security remain underdeveloped. Healthcare institutions implementing these technologies must participate in developing best practices while regulatory standards mature.
Interference detection and mitigation strategies represent another critical security consideration. Malicious signal jamming could disrupt critical care operations, necessitating robust detection algorithms and automated channel-switching capabilities to maintain connectivity during attempted security breaches or unintentional interference events.
mmWave's physical characteristics present unique security considerations. The directional nature and limited penetration capabilities of these signals provide inherent protection against certain types of eavesdropping attacks, as interception requires line-of-sight positioning. However, this physical layer security is insufficient when considering sophisticated attack vectors targeting modern healthcare environments.
Authentication mechanisms for mmWave medical devices must be strengthened beyond traditional approaches. Multi-factor authentication protocols specifically optimized for low-latency medical applications are essential, as conventional authentication methods may introduce unacceptable delays in critical care scenarios. Biometric authentication integrated with mmWave sensing capabilities offers promising solutions for continuous user verification without compromising operational efficiency.
Encryption standards for mmWave medical communications require adaptation to address the technology's high bandwidth capabilities. While AES-256 encryption remains viable, the implementation must be optimized to handle the multi-gigabit data rates characteristic of mmWave transmissions without introducing latency that could compromise real-time medical applications. Hardware-accelerated encryption solutions specifically designed for mmWave bandwidths are emerging as necessary components.
Privacy concerns extend beyond data transmission to the mmWave sensors themselves. The high-resolution imaging capabilities of mmWave technology can potentially capture detailed physiological data beyond what patients have explicitly consented to share. Privacy-by-design approaches must include data minimization techniques that process only clinically relevant information while discarding extraneous personal data at the sensor level.
Regulatory frameworks are still evolving to address mmWave-specific security requirements in healthcare. The FDA's premarket cybersecurity guidance and the NIST Cybersecurity Framework provide foundational principles, but industry-specific standards for mmWave medical device security remain underdeveloped. Healthcare institutions implementing these technologies must participate in developing best practices while regulatory standards mature.
Interference detection and mitigation strategies represent another critical security consideration. Malicious signal jamming could disrupt critical care operations, necessitating robust detection algorithms and automated channel-switching capabilities to maintain connectivity during attempted security breaches or unintentional interference events.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!