Carbon Tetrachloride's Role in the Decline of Ozone Layer Health
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 and Ozone Depletion: Historical Context
Carbon tetrachloride (CCl4) has played a significant role in the historical context of ozone layer depletion. The discovery of its impact on the ozone layer dates back to the 1970s when scientists first began to understand the complex chemistry of the stratosphere. Initially, CCl4 was widely used in various industrial applications, including as a solvent, cleaning agent, and fire extinguisher, due to its non-flammable properties and effectiveness.
The connection between CCl4 and ozone depletion was established through extensive atmospheric research and modeling. Scientists discovered that when CCl4 molecules reach the stratosphere, they are broken down by ultraviolet radiation, releasing chlorine atoms. These chlorine atoms then catalyze the destruction of ozone molecules, contributing to the thinning of the ozone layer.
The recognition of CCl4's ozone-depleting potential led to its inclusion in the Montreal Protocol on Substances that Deplete the Ozone Layer in 1987. This international treaty aimed to phase out the production and consumption of ozone-depleting substances, including CCl4. The protocol marked a turning point in global efforts to protect the ozone layer and address the environmental consequences of human activities.
Following the implementation of the Montreal Protocol, global production and use of CCl4 declined significantly. Many countries banned its use in consumer products and restricted its industrial applications. This shift in policy and practice resulted in a gradual decrease in atmospheric concentrations of CCl4, contributing to the slow recovery of the ozone layer.
However, despite these efforts, CCl4 continues to pose challenges to ozone layer health. Recent studies have shown that emissions of CCl4 have not decreased as rapidly as expected, suggesting ongoing production or release from unknown sources. This persistence highlights the complexity of addressing ozone depletion and the need for continued vigilance and research.
The historical context of CCl4 and ozone depletion serves as a crucial case study in environmental science and policy. It demonstrates the importance of scientific research in identifying environmental threats, the potential for international cooperation in addressing global challenges, and the ongoing need for monitoring and enforcement of environmental regulations.
As we continue to study the ozone layer and atmospheric chemistry, the lessons learned from the CCl4 experience inform current efforts to address other environmental issues, such as climate change and air pollution. The historical trajectory of CCl4 regulation and its impact on the ozone layer provides valuable insights for developing strategies to mitigate other anthropogenic environmental threats.
The connection between CCl4 and ozone depletion was established through extensive atmospheric research and modeling. Scientists discovered that when CCl4 molecules reach the stratosphere, they are broken down by ultraviolet radiation, releasing chlorine atoms. These chlorine atoms then catalyze the destruction of ozone molecules, contributing to the thinning of the ozone layer.
The recognition of CCl4's ozone-depleting potential led to its inclusion in the Montreal Protocol on Substances that Deplete the Ozone Layer in 1987. This international treaty aimed to phase out the production and consumption of ozone-depleting substances, including CCl4. The protocol marked a turning point in global efforts to protect the ozone layer and address the environmental consequences of human activities.
Following the implementation of the Montreal Protocol, global production and use of CCl4 declined significantly. Many countries banned its use in consumer products and restricted its industrial applications. This shift in policy and practice resulted in a gradual decrease in atmospheric concentrations of CCl4, contributing to the slow recovery of the ozone layer.
However, despite these efforts, CCl4 continues to pose challenges to ozone layer health. Recent studies have shown that emissions of CCl4 have not decreased as rapidly as expected, suggesting ongoing production or release from unknown sources. This persistence highlights the complexity of addressing ozone depletion and the need for continued vigilance and research.
The historical context of CCl4 and ozone depletion serves as a crucial case study in environmental science and policy. It demonstrates the importance of scientific research in identifying environmental threats, the potential for international cooperation in addressing global challenges, and the ongoing need for monitoring and enforcement of environmental regulations.
As we continue to study the ozone layer and atmospheric chemistry, the lessons learned from the CCl4 experience inform current efforts to address other environmental issues, such as climate change and air pollution. The historical trajectory of CCl4 regulation and its impact on the ozone layer provides valuable insights for developing strategies to mitigate other anthropogenic environmental threats.
Global Demand for Ozone-Safe Alternatives
The global demand for ozone-safe alternatives has experienced a significant surge in recent years, driven by increasing awareness of the detrimental effects of ozone-depleting substances on the Earth's protective ozone layer. This shift in market dynamics has been largely influenced by international agreements such as the Montreal Protocol, which has phased out the production and consumption of numerous ozone-depleting substances, including carbon tetrachloride.
The refrigeration and air conditioning sectors have been at the forefront of this transition, as they were major consumers of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). These industries have seen a rapid adoption of hydrofluorocarbons (HFCs) as a replacement, though concerns about their high global warming potential have led to further research into more environmentally friendly alternatives like hydrofluoroolefins (HFOs) and natural refrigerants.
The foam blowing industry, another significant user of ozone-depleting substances, has also witnessed a shift towards alternatives such as hydrocarbons, CO2, and water-based systems. This transition has been driven by both regulatory pressures and consumer demand for more sustainable products.
In the solvent sector, which previously relied heavily on carbon tetrachloride and other ozone-depleting substances, there has been a move towards aqueous and semi-aqueous cleaning systems, as well as the development of new, less harmful solvents. This has opened up opportunities for innovation in green chemistry and sustainable manufacturing processes.
The agricultural sector has also been impacted, with the phaseout of methyl bromide as a soil fumigant leading to increased demand for alternative pest control methods and fumigants. This has spurred research into integrated pest management strategies and the development of new, ozone-safe chemical alternatives.
The aerosol industry has successfully transitioned from CFCs to alternatives such as hydrocarbons, dimethyl ether, and compressed gases. This shift has not only benefited the ozone layer but has also led to improvements in product performance and safety.
As the global community continues to prioritize environmental protection, the demand for ozone-safe alternatives is expected to grow further. This trend is likely to drive innovation in various sectors, leading to the development of more efficient, cost-effective, and environmentally friendly technologies. The market for these alternatives is projected to expand, creating new opportunities for businesses that can provide sustainable solutions to meet the evolving needs of industries and consumers worldwide.
The refrigeration and air conditioning sectors have been at the forefront of this transition, as they were major consumers of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). These industries have seen a rapid adoption of hydrofluorocarbons (HFCs) as a replacement, though concerns about their high global warming potential have led to further research into more environmentally friendly alternatives like hydrofluoroolefins (HFOs) and natural refrigerants.
The foam blowing industry, another significant user of ozone-depleting substances, has also witnessed a shift towards alternatives such as hydrocarbons, CO2, and water-based systems. This transition has been driven by both regulatory pressures and consumer demand for more sustainable products.
In the solvent sector, which previously relied heavily on carbon tetrachloride and other ozone-depleting substances, there has been a move towards aqueous and semi-aqueous cleaning systems, as well as the development of new, less harmful solvents. This has opened up opportunities for innovation in green chemistry and sustainable manufacturing processes.
The agricultural sector has also been impacted, with the phaseout of methyl bromide as a soil fumigant leading to increased demand for alternative pest control methods and fumigants. This has spurred research into integrated pest management strategies and the development of new, ozone-safe chemical alternatives.
The aerosol industry has successfully transitioned from CFCs to alternatives such as hydrocarbons, dimethyl ether, and compressed gases. This shift has not only benefited the ozone layer but has also led to improvements in product performance and safety.
As the global community continues to prioritize environmental protection, the demand for ozone-safe alternatives is expected to grow further. This trend is likely to drive innovation in various sectors, leading to the development of more efficient, cost-effective, and environmentally friendly technologies. The market for these alternatives is projected to expand, creating new opportunities for businesses that can provide sustainable solutions to meet the evolving needs of industries and consumers worldwide.
Current CCl4 Emissions and Atmospheric Concentrations
Recent studies indicate that global emissions of carbon tetrachloride (CCl4) have significantly decreased since the 1980s due to the implementation of the Montreal Protocol. However, despite this reduction, atmospheric concentrations of CCl4 remain higher than expected, suggesting ongoing emissions from various sources.
Current estimates place global CCl4 emissions at approximately 35 ± 16 Gg yr−1. This figure is substantially lower than peak emissions in the 1980s but still represents a significant contribution to ozone depletion. The discrepancy between observed atmospheric concentrations and expected levels based on reported production and consumption has led researchers to investigate potential unreported sources.
Atmospheric measurements show that CCl4 concentrations have been declining at a rate of about 1% per year since the early 2000s. As of 2021, the global average concentration of CCl4 in the troposphere was approximately 80 parts per trillion (ppt). This level is about 10% higher than what models predict based on reported emissions, indicating the presence of unknown or underestimated sources.
Regional variations in CCl4 concentrations have been observed, with higher levels typically found in industrialized areas. East Asia, particularly China, has been identified as a significant source region, accounting for a substantial portion of global emissions. Recent studies using high-frequency atmospheric measurements and inverse modeling techniques have helped to pinpoint specific industrial areas contributing to these emissions.
The persistence of CCl4 in the atmosphere is partly due to its long lifetime, estimated at 32 years. This longevity means that even small ongoing emissions can maintain elevated concentrations for extended periods. Additionally, the discovery of natural sources of CCl4, such as emissions from soils and industrial processes, has further complicated efforts to reconcile observed concentrations with anthropogenic emission estimates.
Efforts to reduce CCl4 emissions have focused on improving industrial practices, enhancing monitoring and reporting mechanisms, and investigating potential feedstock and process agent uses that may be exempt from current regulations. The scientific community continues to refine measurement techniques and modeling approaches to better quantify and attribute CCl4 emissions, aiming to close the gap between observed concentrations and reported emissions.
Current estimates place global CCl4 emissions at approximately 35 ± 16 Gg yr−1. This figure is substantially lower than peak emissions in the 1980s but still represents a significant contribution to ozone depletion. The discrepancy between observed atmospheric concentrations and expected levels based on reported production and consumption has led researchers to investigate potential unreported sources.
Atmospheric measurements show that CCl4 concentrations have been declining at a rate of about 1% per year since the early 2000s. As of 2021, the global average concentration of CCl4 in the troposphere was approximately 80 parts per trillion (ppt). This level is about 10% higher than what models predict based on reported emissions, indicating the presence of unknown or underestimated sources.
Regional variations in CCl4 concentrations have been observed, with higher levels typically found in industrialized areas. East Asia, particularly China, has been identified as a significant source region, accounting for a substantial portion of global emissions. Recent studies using high-frequency atmospheric measurements and inverse modeling techniques have helped to pinpoint specific industrial areas contributing to these emissions.
The persistence of CCl4 in the atmosphere is partly due to its long lifetime, estimated at 32 years. This longevity means that even small ongoing emissions can maintain elevated concentrations for extended periods. Additionally, the discovery of natural sources of CCl4, such as emissions from soils and industrial processes, has further complicated efforts to reconcile observed concentrations with anthropogenic emission estimates.
Efforts to reduce CCl4 emissions have focused on improving industrial practices, enhancing monitoring and reporting mechanisms, and investigating potential feedstock and process agent uses that may be exempt from current regulations. The scientific community continues to refine measurement techniques and modeling approaches to better quantify and attribute CCl4 emissions, aiming to close the gap between observed concentrations and reported emissions.
Existing Mitigation Strategies for CCl4 Emissions
01 Environmental impact of carbon tetrachloride on ozone layer
Carbon tetrachloride is a known ozone-depleting substance that contributes to the destruction of the Earth's protective ozone layer. Its release into the atmosphere can lead to increased UV radiation reaching the Earth's surface, potentially causing harm to human health and ecosystems.- Impact of carbon tetrachloride on ozone layer depletion: Carbon tetrachloride is a significant ozone-depleting substance that contributes to the thinning of the Earth's ozone layer. When released into the atmosphere, it breaks down and releases chlorine atoms, which catalyze ozone destruction in the stratosphere. This process leads to increased UV radiation reaching the Earth's surface, posing various environmental and health risks.
- Health effects of carbon tetrachloride exposure: Exposure to carbon tetrachloride can have severe health consequences. It is known to be toxic to the liver, kidneys, and central nervous system. Inhalation or skin contact can cause symptoms such as dizziness, nausea, and in severe cases, liver and kidney damage. Long-term exposure may increase the risk of certain cancers and other chronic health issues.
- Alternatives and substitutes for carbon tetrachloride: Due to its harmful effects on the ozone layer and human health, efforts have been made to find safer alternatives to carbon tetrachloride. Various industries have developed and implemented substitute compounds and processes that have lower ozone depletion potential and reduced toxicity, while still maintaining the desired functional properties.
- Regulations and phase-out of carbon tetrachloride: International agreements and national regulations have been implemented to phase out the production and use of carbon tetrachloride. The Montreal Protocol and subsequent amendments have set timelines for the elimination of ozone-depleting substances, including carbon tetrachloride. Many countries have enacted laws to restrict its use and promote the adoption of safer alternatives.
- Monitoring and remediation of carbon tetrachloride in the environment: Environmental monitoring programs have been established to track the presence and concentration of carbon tetrachloride in the atmosphere, soil, and water. Remediation techniques have been developed to clean up contaminated sites and reduce the environmental impact of historical carbon tetrachloride use. These efforts aim to mitigate the long-term effects on the ozone layer and human health.
02 Health effects of carbon tetrachloride exposure
Exposure to carbon tetrachloride can have significant health impacts on humans. It can cause liver and kidney damage, central nervous system depression, and in severe cases, may lead to coma or death. Long-term exposure may increase the risk of certain cancers.Expand Specific Solutions03 Alternatives and substitutes for carbon tetrachloride
Due to its harmful effects on the ozone layer and human health, efforts have been made to develop and implement alternatives to carbon tetrachloride in various industrial applications. These substitutes aim to provide similar functionality while minimizing environmental and health risks.Expand Specific Solutions04 Monitoring and detection of carbon tetrachloride in the atmosphere
Advanced monitoring techniques and detection methods have been developed to measure carbon tetrachloride levels in the atmosphere. These technologies help track the effectiveness of regulations and mitigation efforts aimed at reducing its presence in the environment.Expand Specific Solutions05 Regulations and international efforts to phase out carbon tetrachloride
Various international agreements and regulations have been implemented to phase out the production and use of carbon tetrachloride. These efforts aim to protect the ozone layer and mitigate the associated health risks by reducing the global emissions of this harmful substance.Expand Specific Solutions
Key Stakeholders in Ozone Protection Efforts
The carbon tetrachloride market is in a mature phase, with declining demand due to environmental regulations aimed at protecting the ozone layer. The global market size has significantly decreased since the Montreal Protocol banned its production for emissive uses. Technologically, alternatives have largely replaced carbon tetrachloride in most applications. Companies like Carrier Corp., L'Oréal SA, and BASF SE have likely shifted away from its use, focusing on developing ozone-friendly substitutes. Research institutions such as Arizona State University and the Korea Institute of Radiological & Medical Sciences may be studying its environmental impacts and potential remediation strategies. Overall, the competitive landscape is characterized by a shrinking market and a focus on sustainable alternatives.
Carrier Corp.
Technical Solution: Carrier Corp., a leading HVAC manufacturer, has been actively working to address the environmental impact of refrigerants, including those that contribute to ozone depletion like carbon tetrachloride. The company has developed a range of products using alternative refrigerants with lower global warming potential and zero ozone depletion potential[13]. Carrier has invested in research and development to improve the energy efficiency of its cooling systems, which indirectly reduces the demand for refrigerants and their potential environmental impact[14]. The company has also implemented advanced leak detection and prevention technologies in its products to minimize the release of refrigerants into the atmosphere. Additionally, Carrier has established recycling and reclamation programs for refrigerants, helping to reduce the overall environmental footprint of its products throughout their lifecycle[15].
Strengths: Extensive industry experience, global market presence, and strong focus on sustainable technologies. Weaknesses: Potential challenges in rapidly transitioning existing product lines to new refrigerant technologies, market competition in the green HVAC sector.
Research Center For Eco-Environmental Sciences
Technical Solution: The Research Center for Eco-Environmental Sciences has conducted extensive studies on the impact of carbon tetrachloride on the ozone layer and developed strategies for mitigation. Their research has focused on atmospheric monitoring, modeling the transport and degradation of carbon tetrachloride in the stratosphere, and assessing the long-term effects on ozone layer recovery[7]. The center has developed advanced analytical techniques for detecting trace amounts of carbon tetrachloride in the atmosphere, allowing for more accurate global measurements and trend analysis[8]. Additionally, they have investigated natural sinks for carbon tetrachloride, such as microbial degradation in soil and oceans, which could potentially be enhanced to accelerate the removal of this compound from the environment[9]. The center has also collaborated with industry partners to develop cleaner production processes that minimize the generation of carbon tetrachloride as a byproduct.
Strengths: Cutting-edge research capabilities, strong international collaborations, and comprehensive approach to environmental issues. Weaknesses: Limited direct influence on industrial practices, dependence on external funding for large-scale projects.
Scientific Breakthroughs in CCl4-Ozone Interaction
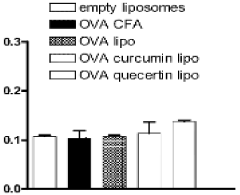
Improving the ability of quercetin loaded niosomes to reverse CCL4 intoxication and to carry out an antioxidant effect
PatentPendingIN202221057113A
Innovation
- Quercetin-loaded niosomes are developed, comprising specific lipid compositions and formulations to enhance the delivery and antioxidant efficacy, including glucose, lauric acid, cholesterol, and NF-kB inhibitors, to target and mitigate CCL4-induced oxidative stress and inflammation.
The antipollution system on the road
PatentInactiveIN201821029033A
Innovation
- An antipollution system that uses water to absorb and filter exhaust smoke and dust from vehicles, employing a fountain wall mechanism where pollutants come into contact with water, separating from the air, and are collected in an underground tank for filtration, with filtered water reused.
International Policies on Ozone-Depleting Substances
The international community has recognized the critical importance of addressing ozone depletion, leading to the development of comprehensive policies and agreements to regulate ozone-depleting substances (ODS), including carbon tetrachloride. The cornerstone of these efforts is the Montreal Protocol on Substances that Deplete the Ozone Layer, adopted in 1987 and subsequently amended to strengthen its provisions.
The Montreal Protocol established a framework for the phase-out of ODS production and consumption, with differentiated schedules for developed and developing countries. For carbon tetrachloride, developed countries were required to phase out production and consumption by 1996, while developing countries had until 2010. The protocol also introduced a licensing system for the import and export of ODS, ensuring better control and monitoring of these substances.
In addition to the Montreal Protocol, various regional and national policies have been implemented to support the global effort. The European Union, for instance, adopted Regulation (EC) No 1005/2009 on substances that deplete the ozone layer, which goes beyond the requirements of the Montreal Protocol in some aspects. This regulation prohibits the production, placing on the market, and use of ODS, including carbon tetrachloride, with limited exemptions for essential uses.
The United States Environmental Protection Agency (EPA) has implemented regulations under the Clean Air Act to control ODS. These include the phase-out of production and import of carbon tetrachloride, as well as restrictions on its use in various applications. The EPA also manages a system of allowances and permits for any remaining essential uses of ODS.
Developing countries have also taken significant steps in implementing ODS regulations. China, for example, has established a national management plan for the phase-out of ODS, including carbon tetrachloride, supported by the Multilateral Fund for the Implementation of the Montreal Protocol.
International cooperation and financial mechanisms have been crucial in supporting the implementation of these policies. The Multilateral Fund provides financial and technical assistance to developing countries to help them meet their obligations under the Montreal Protocol. This support has been instrumental in facilitating the transition to ozone-friendly alternatives and technologies.
Enforcement and compliance mechanisms are integral to the success of these international policies. The Montreal Protocol includes provisions for non-compliance procedures, which have been effective in addressing instances of non-compliance and ensuring that parties meet their obligations. Regular reporting and review processes also help track progress and identify areas requiring further attention.
As the global community continues to address the challenges of ozone layer protection, these international policies serve as a model for effective environmental governance and demonstrate the potential for concerted global action in addressing complex environmental issues.
The Montreal Protocol established a framework for the phase-out of ODS production and consumption, with differentiated schedules for developed and developing countries. For carbon tetrachloride, developed countries were required to phase out production and consumption by 1996, while developing countries had until 2010. The protocol also introduced a licensing system for the import and export of ODS, ensuring better control and monitoring of these substances.
In addition to the Montreal Protocol, various regional and national policies have been implemented to support the global effort. The European Union, for instance, adopted Regulation (EC) No 1005/2009 on substances that deplete the ozone layer, which goes beyond the requirements of the Montreal Protocol in some aspects. This regulation prohibits the production, placing on the market, and use of ODS, including carbon tetrachloride, with limited exemptions for essential uses.
The United States Environmental Protection Agency (EPA) has implemented regulations under the Clean Air Act to control ODS. These include the phase-out of production and import of carbon tetrachloride, as well as restrictions on its use in various applications. The EPA also manages a system of allowances and permits for any remaining essential uses of ODS.
Developing countries have also taken significant steps in implementing ODS regulations. China, for example, has established a national management plan for the phase-out of ODS, including carbon tetrachloride, supported by the Multilateral Fund for the Implementation of the Montreal Protocol.
International cooperation and financial mechanisms have been crucial in supporting the implementation of these policies. The Multilateral Fund provides financial and technical assistance to developing countries to help them meet their obligations under the Montreal Protocol. This support has been instrumental in facilitating the transition to ozone-friendly alternatives and technologies.
Enforcement and compliance mechanisms are integral to the success of these international policies. The Montreal Protocol includes provisions for non-compliance procedures, which have been effective in addressing instances of non-compliance and ensuring that parties meet their obligations. Regular reporting and review processes also help track progress and identify areas requiring further attention.
As the global community continues to address the challenges of ozone layer protection, these international policies serve as a model for effective environmental governance and demonstrate the potential for concerted global action in addressing complex environmental issues.
Environmental Impact Assessment of CCl4
Carbon tetrachloride (CCl4) has been identified as a significant contributor to the depletion of the ozone layer, leading to severe environmental consequences. This assessment aims to evaluate the environmental impact of CCl4 on the Earth's atmosphere and ecosystems.
The primary concern regarding CCl4 is its role in ozone depletion. When released into the atmosphere, CCl4 molecules rise to the stratosphere, where they are broken down by ultraviolet radiation. This process releases chlorine atoms, which catalyze the destruction of ozone molecules. A single chlorine atom can destroy thousands of ozone molecules before being removed from the stratosphere, making CCl4 a potent ozone-depleting substance.
The thinning of the ozone layer results in increased ultraviolet (UV) radiation reaching the Earth's surface. This heightened UV exposure has far-reaching effects on both terrestrial and aquatic ecosystems. In terrestrial environments, increased UV radiation can damage plant DNA, reduce crop yields, and alter plant species composition. It also affects animal populations by causing skin damage, eye problems, and weakened immune systems in various species.
Aquatic ecosystems are particularly vulnerable to increased UV radiation. Phytoplankton, the foundation of many aquatic food chains, can experience reduced growth rates and altered species composition. This disruption at the base of the food web can have cascading effects throughout entire marine ecosystems, potentially impacting fisheries and global ocean productivity.
The impact of CCl4 on climate change is also noteworthy. While not as potent as some other greenhouse gases, CCl4 does contribute to global warming. Its long atmospheric lifetime allows it to accumulate in the atmosphere, trapping heat and exacerbating climate change effects. This can lead to changes in temperature patterns, precipitation, and extreme weather events, further stressing ecosystems already affected by ozone depletion.
Human health is another critical concern related to CCl4 emissions. The breakdown of the ozone layer increases human exposure to UV radiation, leading to higher rates of skin cancer, cataracts, and immune system suppression. Additionally, direct exposure to CCl4 through contaminated air, water, or soil can cause liver and kidney damage, and in high concentrations, it can be fatal.
The global nature of CCl4's impact necessitates international cooperation for effective mitigation. The Montreal Protocol, an international treaty designed to protect the ozone layer, has been instrumental in phasing out the production and consumption of ozone-depleting substances, including CCl4. However, ongoing monitoring and enforcement are crucial to ensure compliance and address any illegal production or use.
In conclusion, the environmental impact of CCl4 is extensive and multifaceted, affecting atmospheric chemistry, ecosystem health, climate patterns, and human well-being. Continued research, monitoring, and global efforts to eliminate CCl4 emissions are essential to mitigate these impacts and protect the Earth's ozone layer and broader environmental health.
The primary concern regarding CCl4 is its role in ozone depletion. When released into the atmosphere, CCl4 molecules rise to the stratosphere, where they are broken down by ultraviolet radiation. This process releases chlorine atoms, which catalyze the destruction of ozone molecules. A single chlorine atom can destroy thousands of ozone molecules before being removed from the stratosphere, making CCl4 a potent ozone-depleting substance.
The thinning of the ozone layer results in increased ultraviolet (UV) radiation reaching the Earth's surface. This heightened UV exposure has far-reaching effects on both terrestrial and aquatic ecosystems. In terrestrial environments, increased UV radiation can damage plant DNA, reduce crop yields, and alter plant species composition. It also affects animal populations by causing skin damage, eye problems, and weakened immune systems in various species.
Aquatic ecosystems are particularly vulnerable to increased UV radiation. Phytoplankton, the foundation of many aquatic food chains, can experience reduced growth rates and altered species composition. This disruption at the base of the food web can have cascading effects throughout entire marine ecosystems, potentially impacting fisheries and global ocean productivity.
The impact of CCl4 on climate change is also noteworthy. While not as potent as some other greenhouse gases, CCl4 does contribute to global warming. Its long atmospheric lifetime allows it to accumulate in the atmosphere, trapping heat and exacerbating climate change effects. This can lead to changes in temperature patterns, precipitation, and extreme weather events, further stressing ecosystems already affected by ozone depletion.
Human health is another critical concern related to CCl4 emissions. The breakdown of the ozone layer increases human exposure to UV radiation, leading to higher rates of skin cancer, cataracts, and immune system suppression. Additionally, direct exposure to CCl4 through contaminated air, water, or soil can cause liver and kidney damage, and in high concentrations, it can be fatal.
The global nature of CCl4's impact necessitates international cooperation for effective mitigation. The Montreal Protocol, an international treaty designed to protect the ozone layer, has been instrumental in phasing out the production and consumption of ozone-depleting substances, including CCl4. However, ongoing monitoring and enforcement are crucial to ensure compliance and address any illegal production or use.
In conclusion, the environmental impact of CCl4 is extensive and multifaceted, affecting atmospheric chemistry, ecosystem health, climate patterns, and human well-being. Continued research, monitoring, and global efforts to eliminate CCl4 emissions are essential to mitigate these impacts and protect the Earth's ozone layer and broader environmental health.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!