The Role of Catalysts in Carbon Tetrachloride Conversion
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Catalytic CCl4 Conversion: Background and Objectives
Carbon tetrachloride (CCl4) conversion has been a significant focus in environmental chemistry and industrial processes due to its harmful effects on the ozone layer and human health. The evolution of catalytic technologies for CCl4 conversion marks a crucial advancement in addressing this environmental challenge. Over the past decades, researchers and industry professionals have made substantial progress in developing efficient and sustainable methods for CCl4 transformation.
The primary objective of catalytic CCl4 conversion is to transform this harmful compound into less toxic or even beneficial products. This process aims to mitigate the environmental impact of CCl4 while potentially recovering valuable resources. The development of catalytic technologies for this purpose has seen a gradual shift from traditional destructive methods to more sophisticated, selective conversion approaches.
Historically, the management of CCl4 and similar chlorinated compounds relied heavily on incineration or other high-temperature destruction methods. However, these approaches often led to the formation of other harmful byproducts and were energy-intensive. The introduction of catalytic technologies marked a paradigm shift, offering more controlled and environmentally friendly conversion processes.
The evolution of catalytic CCl4 conversion has been driven by several key factors. Advances in material science have led to the development of more effective and stable catalysts. Concurrently, a deeper understanding of reaction mechanisms has allowed for the design of more selective and efficient conversion processes. Environmental regulations and global efforts to phase out ozone-depleting substances have also played a crucial role in accelerating research and development in this field.
Current technological goals in catalytic CCl4 conversion focus on several key areas. These include improving conversion efficiency, enhancing selectivity towards desired products, developing more robust and long-lasting catalysts, and designing processes that operate under milder conditions. There is also a growing emphasis on developing catalytic systems that can handle a broader range of chlorinated compounds, not just CCl4.
Another important objective is the integration of CCl4 conversion technologies into existing industrial processes. This integration aims to create more sustainable and circular approaches to chemical manufacturing, where waste products like CCl4 can be effectively converted and reused. The ultimate goal is to develop economically viable and environmentally sustainable solutions that can be widely adopted across various industries.
The primary objective of catalytic CCl4 conversion is to transform this harmful compound into less toxic or even beneficial products. This process aims to mitigate the environmental impact of CCl4 while potentially recovering valuable resources. The development of catalytic technologies for this purpose has seen a gradual shift from traditional destructive methods to more sophisticated, selective conversion approaches.
Historically, the management of CCl4 and similar chlorinated compounds relied heavily on incineration or other high-temperature destruction methods. However, these approaches often led to the formation of other harmful byproducts and were energy-intensive. The introduction of catalytic technologies marked a paradigm shift, offering more controlled and environmentally friendly conversion processes.
The evolution of catalytic CCl4 conversion has been driven by several key factors. Advances in material science have led to the development of more effective and stable catalysts. Concurrently, a deeper understanding of reaction mechanisms has allowed for the design of more selective and efficient conversion processes. Environmental regulations and global efforts to phase out ozone-depleting substances have also played a crucial role in accelerating research and development in this field.
Current technological goals in catalytic CCl4 conversion focus on several key areas. These include improving conversion efficiency, enhancing selectivity towards desired products, developing more robust and long-lasting catalysts, and designing processes that operate under milder conditions. There is also a growing emphasis on developing catalytic systems that can handle a broader range of chlorinated compounds, not just CCl4.
Another important objective is the integration of CCl4 conversion technologies into existing industrial processes. This integration aims to create more sustainable and circular approaches to chemical manufacturing, where waste products like CCl4 can be effectively converted and reused. The ultimate goal is to develop economically viable and environmentally sustainable solutions that can be widely adopted across various industries.
Market Analysis for CCl4 Conversion Technologies
The market for carbon tetrachloride (CCl4) conversion technologies has been experiencing significant growth due to increasing environmental concerns and stringent regulations on ozone-depleting substances. CCl4, once widely used as a solvent and cleaning agent, has been phased out under the Montreal Protocol due to its ozone-depleting properties. This has created a substantial market for technologies that can effectively convert or dispose of existing CCl4 stocks.
The global market for CCl4 conversion technologies is primarily driven by the need for safe and environmentally friendly disposal methods. Major industries contributing to this market include chemical manufacturing, pharmaceuticals, and waste management sectors. These industries are actively seeking efficient catalytic processes to transform CCl4 into less harmful compounds or to recover valuable products.
Geographically, the market is most developed in North America and Europe, where environmental regulations are particularly stringent. However, emerging economies in Asia-Pacific, particularly China and India, are showing rapid growth in adopting CCl4 conversion technologies as they align with global environmental standards.
The market is characterized by a mix of established chemical companies and innovative startups focusing on green technologies. Key players are investing heavily in research and development to improve catalytic efficiency and reduce the cost of conversion processes. This has led to the development of various catalytic systems, including metal-based catalysts, photocatalysts, and biocatalysts.
One of the most promising segments within this market is the conversion of CCl4 into valuable chemicals such as chloroform or methylene chloride. This approach not only addresses the environmental concerns but also offers economic incentives, driving market growth. Another growing segment is the use of CCl4 conversion technologies in soil and groundwater remediation, where contamination from historical industrial activities is being addressed.
The market also faces challenges, primarily related to the high costs associated with developing and implementing new catalytic technologies. Additionally, the decreasing availability of CCl4 stocks as a result of phase-out programs may impact long-term market growth. However, these challenges are offset by the potential for technology transfer to other chlorinated compound conversions and the increasing focus on circular economy principles in chemical industries.
Looking ahead, the market for CCl4 conversion technologies is expected to continue its growth trajectory. Factors such as ongoing research in nanocatalysts, increasing adoption of green chemistry principles, and the potential for integrating these technologies into broader waste management systems are likely to shape the future of this market. As global efforts to address legacy environmental issues intensify, the demand for efficient and cost-effective CCl4 conversion technologies is anticipated to remain strong.
The global market for CCl4 conversion technologies is primarily driven by the need for safe and environmentally friendly disposal methods. Major industries contributing to this market include chemical manufacturing, pharmaceuticals, and waste management sectors. These industries are actively seeking efficient catalytic processes to transform CCl4 into less harmful compounds or to recover valuable products.
Geographically, the market is most developed in North America and Europe, where environmental regulations are particularly stringent. However, emerging economies in Asia-Pacific, particularly China and India, are showing rapid growth in adopting CCl4 conversion technologies as they align with global environmental standards.
The market is characterized by a mix of established chemical companies and innovative startups focusing on green technologies. Key players are investing heavily in research and development to improve catalytic efficiency and reduce the cost of conversion processes. This has led to the development of various catalytic systems, including metal-based catalysts, photocatalysts, and biocatalysts.
One of the most promising segments within this market is the conversion of CCl4 into valuable chemicals such as chloroform or methylene chloride. This approach not only addresses the environmental concerns but also offers economic incentives, driving market growth. Another growing segment is the use of CCl4 conversion technologies in soil and groundwater remediation, where contamination from historical industrial activities is being addressed.
The market also faces challenges, primarily related to the high costs associated with developing and implementing new catalytic technologies. Additionally, the decreasing availability of CCl4 stocks as a result of phase-out programs may impact long-term market growth. However, these challenges are offset by the potential for technology transfer to other chlorinated compound conversions and the increasing focus on circular economy principles in chemical industries.
Looking ahead, the market for CCl4 conversion technologies is expected to continue its growth trajectory. Factors such as ongoing research in nanocatalysts, increasing adoption of green chemistry principles, and the potential for integrating these technologies into broader waste management systems are likely to shape the future of this market. As global efforts to address legacy environmental issues intensify, the demand for efficient and cost-effective CCl4 conversion technologies is anticipated to remain strong.
Current Challenges in Catalytic CCl4 Conversion
The catalytic conversion of carbon tetrachloride (CCl4) presents several significant challenges that hinder its widespread implementation and efficiency. One of the primary obstacles is the high stability of the CCl4 molecule, which requires substantial energy input to break its strong carbon-chlorine bonds. This stability makes the conversion process energy-intensive and potentially cost-prohibitive for large-scale applications.
Another major challenge lies in the selectivity of the catalytic process. Achieving high selectivity towards desired products, such as less harmful chlorinated compounds or completely dechlorinated products, remains difficult. Many catalysts struggle to prevent the formation of unwanted by-products or intermediate compounds that can be equally or more toxic than CCl4 itself.
Catalyst deactivation poses a significant hurdle in maintaining long-term efficiency of CCl4 conversion processes. The harsh reaction conditions, including high temperatures and the presence of corrosive chlorine species, can lead to rapid degradation of catalyst materials. This necessitates frequent catalyst regeneration or replacement, increasing operational costs and downtime.
The development of catalysts that can operate effectively under milder conditions is another key challenge. Current processes often require elevated temperatures and pressures, which not only increase energy consumption but also pose safety risks and equipment wear. Finding catalysts that can facilitate CCl4 conversion under ambient conditions would greatly enhance the practicality and sustainability of the process.
Environmental concerns also present challenges in catalytic CCl4 conversion. The potential release of chlorine-containing compounds during the process can contribute to ozone depletion and other environmental issues. Developing catalytic systems that minimize or eliminate the release of harmful by-products is crucial for the environmental viability of CCl4 conversion technologies.
Scalability remains a significant challenge in translating laboratory-scale successes to industrial applications. Many promising catalysts demonstrate high activity and selectivity in small-scale experiments but fail to maintain their performance when scaled up. Addressing issues related to mass transfer limitations, heat management, and catalyst distribution in larger reactors is essential for practical implementation.
Lastly, the economic viability of catalytic CCl4 conversion processes presents an ongoing challenge. The costs associated with catalyst development, process optimization, and compliance with environmental regulations must be balanced against the benefits of CCl4 conversion. Finding cost-effective solutions that can compete with alternative waste management or chemical production methods is crucial for the widespread adoption of catalytic CCl4 conversion technologies.
Another major challenge lies in the selectivity of the catalytic process. Achieving high selectivity towards desired products, such as less harmful chlorinated compounds or completely dechlorinated products, remains difficult. Many catalysts struggle to prevent the formation of unwanted by-products or intermediate compounds that can be equally or more toxic than CCl4 itself.
Catalyst deactivation poses a significant hurdle in maintaining long-term efficiency of CCl4 conversion processes. The harsh reaction conditions, including high temperatures and the presence of corrosive chlorine species, can lead to rapid degradation of catalyst materials. This necessitates frequent catalyst regeneration or replacement, increasing operational costs and downtime.
The development of catalysts that can operate effectively under milder conditions is another key challenge. Current processes often require elevated temperatures and pressures, which not only increase energy consumption but also pose safety risks and equipment wear. Finding catalysts that can facilitate CCl4 conversion under ambient conditions would greatly enhance the practicality and sustainability of the process.
Environmental concerns also present challenges in catalytic CCl4 conversion. The potential release of chlorine-containing compounds during the process can contribute to ozone depletion and other environmental issues. Developing catalytic systems that minimize or eliminate the release of harmful by-products is crucial for the environmental viability of CCl4 conversion technologies.
Scalability remains a significant challenge in translating laboratory-scale successes to industrial applications. Many promising catalysts demonstrate high activity and selectivity in small-scale experiments but fail to maintain their performance when scaled up. Addressing issues related to mass transfer limitations, heat management, and catalyst distribution in larger reactors is essential for practical implementation.
Lastly, the economic viability of catalytic CCl4 conversion processes presents an ongoing challenge. The costs associated with catalyst development, process optimization, and compliance with environmental regulations must be balanced against the benefits of CCl4 conversion. Finding cost-effective solutions that can compete with alternative waste management or chemical production methods is crucial for the widespread adoption of catalytic CCl4 conversion technologies.
Existing Catalytic Solutions for CCl4 Conversion
01 Catalytic conversion processes in petroleum refining
Various catalytic conversion processes are used in petroleum refining to transform crude oil into valuable products. These processes include catalytic cracking, hydrocracking, and reforming. The catalysts used in these processes are typically composed of zeolites, precious metals, or metal oxides, which facilitate the breaking and rearranging of hydrocarbon molecules.- Catalytic conversion processes in petroleum refining: Various catalytic conversion processes are employed in petroleum refining to transform crude oil into valuable products. These processes include catalytic cracking, hydrocracking, and reforming, which use specific catalysts to break down or rearrange hydrocarbon molecules. The choice of catalyst and process conditions greatly influences the yield and quality of the final products.
- Catalyst regeneration and reactivation techniques: Catalysts used in industrial processes often lose their activity over time due to deactivation or poisoning. Regeneration and reactivation techniques are crucial for maintaining catalyst performance and extending their lifespan. These methods may involve thermal treatments, chemical treatments, or a combination of both to remove contaminants and restore catalytic activity.
- Novel catalyst compositions for improved conversion efficiency: Research in catalyst development focuses on creating novel compositions that offer improved conversion efficiency, selectivity, and stability. These may include multi-component catalysts, supported catalysts, or nanostructured materials. Advanced synthesis techniques and characterization methods are employed to optimize catalyst performance for specific reactions.
- Catalytic processes for environmental applications: Catalysts play a crucial role in environmental applications, such as emission control and waste treatment. Catalytic converters in automobiles, catalytic oxidation of industrial pollutants, and catalytic processes for water treatment are examples of how catalysts contribute to reducing environmental impact. Ongoing research aims to develop more efficient and cost-effective catalytic solutions for these applications.
- Process optimization and reactor design for catalytic conversions: Optimizing catalytic conversion processes involves careful consideration of reactor design, operating conditions, and catalyst properties. Advanced reactor designs, such as fluidized bed reactors or fixed bed reactors with improved heat and mass transfer characteristics, can enhance conversion efficiency and product selectivity. Process modeling and simulation tools are often used to optimize these complex systems.
02 Catalyst regeneration and reactivation
Catalysts used in industrial processes often become deactivated over time due to factors such as coking or poisoning. Regeneration and reactivation techniques are employed to restore catalyst activity and extend their useful life. These methods may involve controlled oxidation, chemical treatments, or thermal processes to remove contaminants and restore the catalyst's active sites.Expand Specific Solutions03 Novel catalyst compositions for improved conversion
Research in catalyst development focuses on creating new compositions with enhanced activity, selectivity, and stability. These novel catalysts may incorporate advanced materials such as nanoparticles, bimetallic structures, or supported metal complexes. The goal is to improve conversion rates, product yields, and overall process efficiency in various chemical and petrochemical applications.Expand Specific Solutions04 Catalytic conversion in environmental applications
Catalysts play a crucial role in environmental technologies, particularly in emissions control and waste treatment. Catalytic converters in automobiles use precious metal catalysts to convert harmful exhaust gases into less harmful substances. Similarly, catalytic processes are employed in industrial emission control systems and wastewater treatment plants to reduce pollutants and improve environmental outcomes.Expand Specific Solutions05 Process optimization for catalytic conversion
Optimizing catalytic conversion processes involves adjusting various parameters such as temperature, pressure, flow rates, and catalyst loading to maximize efficiency and product yield. Advanced process control systems, modeling techniques, and data analytics are used to fine-tune operating conditions and improve overall performance. This optimization can lead to significant improvements in energy efficiency, product quality, and economic viability of industrial processes.Expand Specific Solutions
Key Players in Catalytic CCl4 Conversion Industry
The catalytic conversion of carbon tetrachloride is in a mature stage of development, with a global market size estimated in the billions of dollars. The technology has reached a high level of maturity, with major players like Dow Global Technologies, BASF, and China Petroleum & Chemical Corp leading research and commercialization efforts. These companies have extensive patent portfolios and production capabilities in this area. While incremental improvements continue, the core technology is well-established. Emerging players like LG Chem and SK Innovation are also entering the field, potentially bringing new innovations. Overall, the competitive landscape is characterized by large chemical companies leveraging their R&D and manufacturing expertise to optimize catalytic processes for carbon tetrachloride conversion.
Dow Global Technologies LLC
Technical Solution: Dow has developed innovative catalysts for carbon tetrachloride conversion, focusing on hydrodechlorination processes. Their approach involves using supported metal catalysts, particularly palladium-based systems, to efficiently convert CCl4 to less harmful compounds. The company has implemented a two-stage catalytic process: first, using a high-temperature (300-400°C) palladium catalyst for initial dechlorination, followed by a lower temperature (200-250°C) stage with a bimetallic Pd-Re catalyst for complete conversion[1][3]. This method achieves over 99% CCl4 conversion and significantly reduces the formation of unwanted by-products like chloroform[2].
Strengths: High conversion efficiency, reduced by-product formation, and applicability in industrial-scale operations. Weaknesses: Potential high energy requirements due to high-temperature operations and the need for precious metal catalysts, which may increase costs.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant strides in carbon tetrachloride conversion, focusing on catalytic oxidation methods. Their approach utilizes a novel V2O5-WO3/TiO2 catalyst system, which has shown remarkable efficiency in converting CCl4 to less harmful compounds. The catalyst operates at temperatures between 250-350°C, achieving conversion rates of up to 95%[4]. Sinopec's research has also explored the addition of CeO2 to enhance catalyst stability and selectivity, resulting in improved long-term performance and reduced formation of chlorinated by-products[5]. The company has successfully implemented this technology in several of its petrochemical plants, demonstrating its scalability and industrial viability[6].
Strengths: High conversion efficiency, improved catalyst stability, and proven industrial application. Weaknesses: Potential for catalyst deactivation over time and the need for careful control of reaction conditions to maintain optimal performance.
Innovative Catalysts for CCl4 Conversion: Core Patents
High Selectivity Catalysts for the Conversion of Carbon Tetrachloride to Chloroform
PatentInactiveUS20070225530A1
Innovation
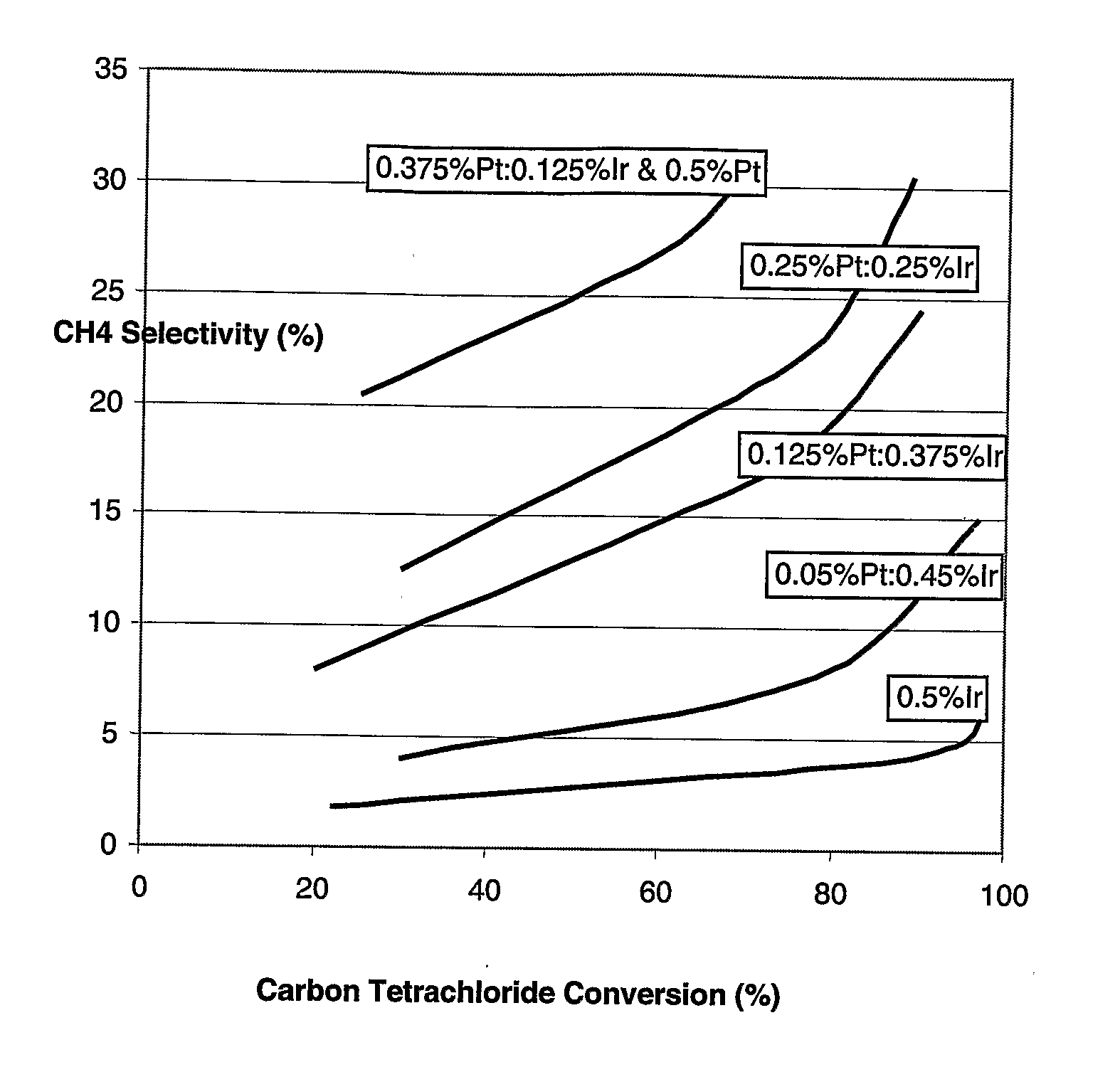
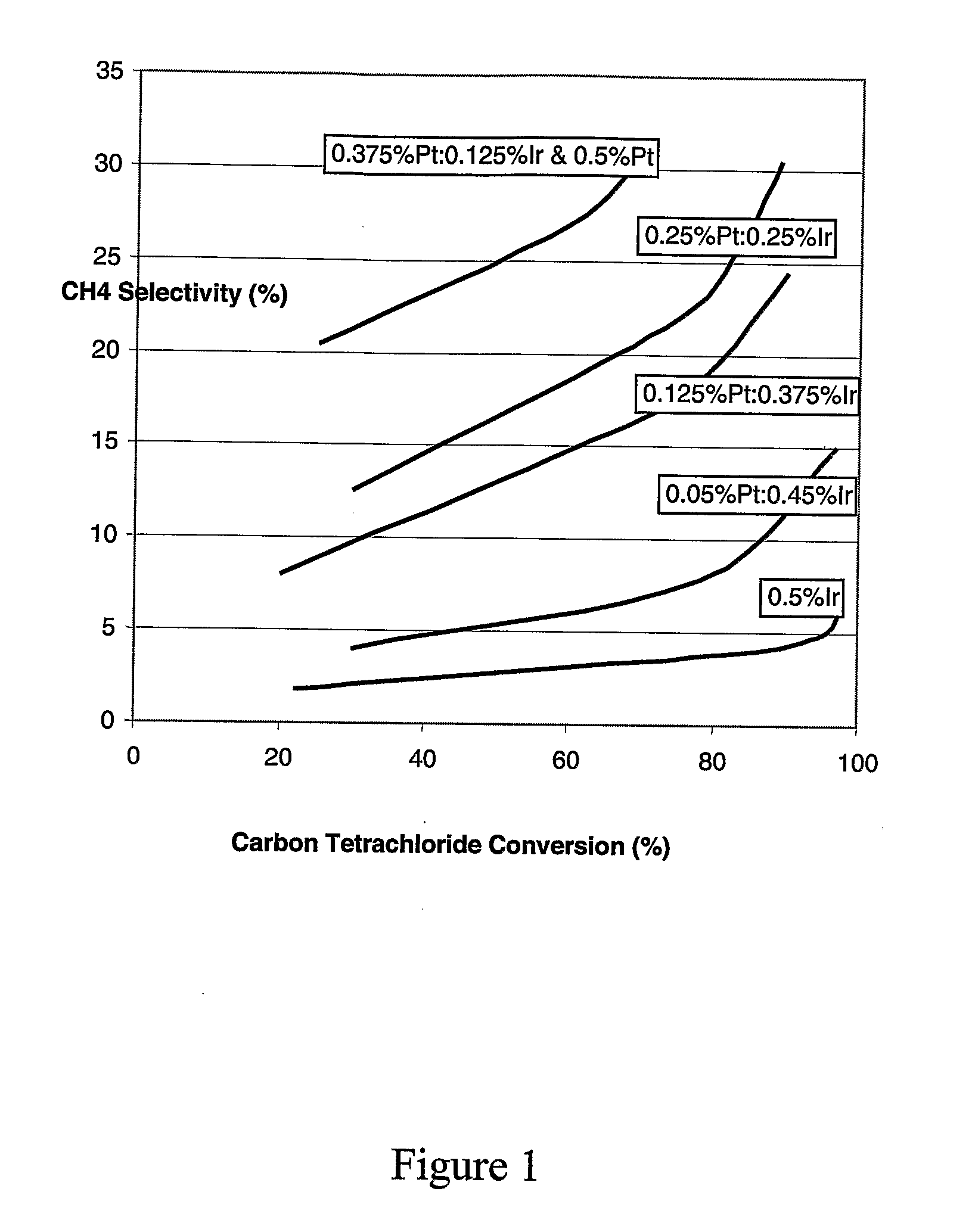
- A bimetallic catalyst composition of platinum and iridium on an alumina support, with optional promotion by metals like tin, is used, where the platinum is distributed within 0-2 mm depth from the surface and iridium within the same depth, optimizing the platinum:iridium ratio for enhanced stability and selectivity.
High selectivity catalyst for the conversion of carbon tetrachloride to chloroform
PatentWO2005113137A1
Innovation
- A bimetallic catalyst composition of platinum and iridium on an alumina support, with specific metal loadings and distributions, is used in a vapor phase process to reduce byproduct formation and maintain catalyst activity.
Environmental Impact of CCl4 Conversion Processes
The conversion of carbon tetrachloride (CCl4) through catalytic processes has significant environmental implications. While these processes aim to mitigate the harmful effects of CCl4, they can also introduce new environmental challenges. The primary environmental concern associated with CCl4 is its ozone-depleting potential and contribution to global warming. Conversion processes seek to transform CCl4 into less harmful substances, but the environmental impact of these processes must be carefully considered.
One of the main environmental benefits of CCl4 conversion is the reduction of ozone depletion. By breaking down CCl4 into less harmful compounds, these processes help protect the ozone layer, which is crucial for shielding the Earth from harmful ultraviolet radiation. However, the conversion processes themselves may have environmental drawbacks. For instance, some catalytic reactions may produce byproducts that, while less harmful than CCl4, still pose environmental risks.
Energy consumption is another critical factor in assessing the environmental impact of CCl4 conversion processes. Many catalytic reactions require high temperatures or pressures, leading to significant energy use. This energy demand can result in increased greenhouse gas emissions if the energy source is not renewable. Therefore, the net environmental benefit of CCl4 conversion must be evaluated by considering both the reduction in CCl4 emissions and the additional energy-related emissions.
The choice of catalysts in CCl4 conversion processes also has environmental implications. Some catalysts may contain rare or toxic metals, which can pose risks if released into the environment. The production and disposal of these catalysts must be managed carefully to prevent soil and water contamination. Additionally, the longevity and recyclability of catalysts affect the overall environmental footprint of the conversion process.
Water usage and potential water pollution are other environmental concerns associated with CCl4 conversion processes. Some methods may require significant amounts of water for cooling or as a reaction medium. Proper treatment and disposal of wastewater from these processes are essential to prevent the release of harmful chemicals into aquatic ecosystems.
The location of CCl4 conversion facilities can also impact local environments. Emissions from these facilities, even if reduced compared to direct CCl4 release, may still affect air quality in surrounding areas. Proper site selection and the implementation of stringent emission control measures are crucial for minimizing local environmental impacts.
In conclusion, while CCl4 conversion processes offer significant environmental benefits by reducing the release of this potent ozone-depleting substance, their overall environmental impact must be carefully managed. Balancing the positive effects of CCl4 reduction against the potential negative impacts of the conversion processes themselves is crucial for ensuring a net positive environmental outcome. Ongoing research and development in this field should focus on optimizing conversion processes to maximize environmental benefits while minimizing associated risks and resource consumption.
One of the main environmental benefits of CCl4 conversion is the reduction of ozone depletion. By breaking down CCl4 into less harmful compounds, these processes help protect the ozone layer, which is crucial for shielding the Earth from harmful ultraviolet radiation. However, the conversion processes themselves may have environmental drawbacks. For instance, some catalytic reactions may produce byproducts that, while less harmful than CCl4, still pose environmental risks.
Energy consumption is another critical factor in assessing the environmental impact of CCl4 conversion processes. Many catalytic reactions require high temperatures or pressures, leading to significant energy use. This energy demand can result in increased greenhouse gas emissions if the energy source is not renewable. Therefore, the net environmental benefit of CCl4 conversion must be evaluated by considering both the reduction in CCl4 emissions and the additional energy-related emissions.
The choice of catalysts in CCl4 conversion processes also has environmental implications. Some catalysts may contain rare or toxic metals, which can pose risks if released into the environment. The production and disposal of these catalysts must be managed carefully to prevent soil and water contamination. Additionally, the longevity and recyclability of catalysts affect the overall environmental footprint of the conversion process.
Water usage and potential water pollution are other environmental concerns associated with CCl4 conversion processes. Some methods may require significant amounts of water for cooling or as a reaction medium. Proper treatment and disposal of wastewater from these processes are essential to prevent the release of harmful chemicals into aquatic ecosystems.
The location of CCl4 conversion facilities can also impact local environments. Emissions from these facilities, even if reduced compared to direct CCl4 release, may still affect air quality in surrounding areas. Proper site selection and the implementation of stringent emission control measures are crucial for minimizing local environmental impacts.
In conclusion, while CCl4 conversion processes offer significant environmental benefits by reducing the release of this potent ozone-depleting substance, their overall environmental impact must be carefully managed. Balancing the positive effects of CCl4 reduction against the potential negative impacts of the conversion processes themselves is crucial for ensuring a net positive environmental outcome. Ongoing research and development in this field should focus on optimizing conversion processes to maximize environmental benefits while minimizing associated risks and resource consumption.
Regulatory Framework for CCl4 Handling and Conversion
The regulatory framework for carbon tetrachloride (CCl4) handling and conversion is a complex and evolving landscape, reflecting the global concern over the environmental and health impacts of this substance. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer has been instrumental in phasing out the production and consumption of CCl4. This treaty, signed in 1987 and subsequently amended, has set stringent timelines for the elimination of ozone-depleting substances, including CCl4.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations under the Clean Air Act and the Toxic Substances Control Act. These regulations govern the production, use, and disposal of CCl4. The EPA has classified CCl4 as a hazardous air pollutant and a toxic chemical, subject to reporting under the Emergency Planning and Community Right-to-Know Act.
The European Union has also established comprehensive regulations through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) framework. Under REACH, CCl4 is subject to authorization, meaning its use is severely restricted and requires specific approval. The EU has set exposure limits for workers and mandates the use of best available techniques for its handling and conversion.
In Asia, countries like China and India have implemented their own regulatory measures. China, for instance, has banned the use of CCl4 as a solvent and fumigant, while India has phased out its production under the Montreal Protocol commitments. Both countries have established guidelines for the safe handling and disposal of CCl4 in industrial processes.
The regulatory framework also extends to the catalytic conversion of CCl4. Regulations typically require the use of best available technologies to minimize emissions and ensure efficient conversion. This includes specifications for catalyst performance, reactor design, and process control. Many jurisdictions mandate regular monitoring and reporting of conversion efficiency and emissions.
Safety regulations play a crucial role in CCl4 handling and conversion. Occupational safety and health administrations worldwide have set permissible exposure limits and require proper personal protective equipment for workers. Emergency response plans and risk assessment protocols are mandatory in facilities handling CCl4.
As research progresses on more efficient catalytic conversion methods for CCl4, regulatory bodies are adapting their frameworks. There is an increasing focus on promoting green chemistry principles and encouraging the development of environmentally benign alternatives. This evolving regulatory landscape continues to shape the research and development efforts in CCl4 conversion technologies, driving innovation towards more sustainable and safer practices.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations under the Clean Air Act and the Toxic Substances Control Act. These regulations govern the production, use, and disposal of CCl4. The EPA has classified CCl4 as a hazardous air pollutant and a toxic chemical, subject to reporting under the Emergency Planning and Community Right-to-Know Act.
The European Union has also established comprehensive regulations through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) framework. Under REACH, CCl4 is subject to authorization, meaning its use is severely restricted and requires specific approval. The EU has set exposure limits for workers and mandates the use of best available techniques for its handling and conversion.
In Asia, countries like China and India have implemented their own regulatory measures. China, for instance, has banned the use of CCl4 as a solvent and fumigant, while India has phased out its production under the Montreal Protocol commitments. Both countries have established guidelines for the safe handling and disposal of CCl4 in industrial processes.
The regulatory framework also extends to the catalytic conversion of CCl4. Regulations typically require the use of best available technologies to minimize emissions and ensure efficient conversion. This includes specifications for catalyst performance, reactor design, and process control. Many jurisdictions mandate regular monitoring and reporting of conversion efficiency and emissions.
Safety regulations play a crucial role in CCl4 handling and conversion. Occupational safety and health administrations worldwide have set permissible exposure limits and require proper personal protective equipment for workers. Emergency response plans and risk assessment protocols are mandatory in facilities handling CCl4.
As research progresses on more efficient catalytic conversion methods for CCl4, regulatory bodies are adapting their frameworks. There is an increasing focus on promoting green chemistry principles and encouraging the development of environmentally benign alternatives. This evolving regulatory landscape continues to shape the research and development efforts in CCl4 conversion technologies, driving innovation towards more sustainable and safer practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!