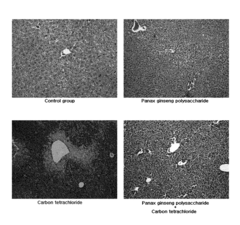
Carbon Tetrachloride Metabolites and Their Environmental Impact
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Metabolism Background and Research Objectives
Carbon tetrachloride (CCl4) has been a subject of significant environmental and toxicological concern for decades. Initially used as a solvent, cleaning agent, and refrigerant, its production and use have been largely phased out due to its ozone-depleting properties and potential health hazards. The metabolism of CCl4 in biological systems plays a crucial role in understanding its toxicity and environmental impact.
The primary objective of this research is to elucidate the metabolic pathways of CCl4 and identify its metabolites, as well as assess their environmental persistence and potential ecological effects. This investigation is critical for developing effective remediation strategies and improving risk assessment models for CCl4 contamination.
CCl4 metabolism primarily occurs in the liver, where it undergoes reductive dehalogenation by cytochrome P450 enzymes. This process generates highly reactive free radical intermediates, which are responsible for much of the compound's toxicity. The main metabolites include trichloromethane (chloroform), dichloromethane, and carbon monoxide. These metabolites can further react with cellular components, leading to lipid peroxidation, protein denaturation, and DNA damage.
Understanding the fate of these metabolites in the environment is essential for assessing the long-term impact of CCl4 contamination. Some metabolites, such as chloroform, can persist in soil and groundwater, potentially causing further environmental damage. Others may undergo further transformation, contributing to the formation of other chlorinated compounds or greenhouse gases.
Recent technological advancements in analytical chemistry, particularly in mass spectrometry and nuclear magnetic resonance spectroscopy, have enabled more precise identification and quantification of CCl4 metabolites. These tools allow researchers to track the transformation of CCl4 in various environmental matrices and biological systems with unprecedented accuracy.
The research aims to address several key questions: What are the primary and secondary metabolites of CCl4 in different environmental conditions? How do these metabolites interact with various ecosystems? What are the long-term effects of CCl4 metabolites on soil microbiota, aquatic organisms, and plant life? By answering these questions, we can develop more effective strategies for environmental remediation and improve our understanding of the global carbon and chlorine cycles.
Furthermore, this research seeks to explore potential biomarkers of CCl4 exposure and metabolism in various organisms. Such biomarkers could be invaluable for early detection of CCl4 contamination and for monitoring the effectiveness of remediation efforts. The study also aims to investigate potential natural attenuation processes that may mitigate the environmental impact of CCl4 and its metabolites over time.
The primary objective of this research is to elucidate the metabolic pathways of CCl4 and identify its metabolites, as well as assess their environmental persistence and potential ecological effects. This investigation is critical for developing effective remediation strategies and improving risk assessment models for CCl4 contamination.
CCl4 metabolism primarily occurs in the liver, where it undergoes reductive dehalogenation by cytochrome P450 enzymes. This process generates highly reactive free radical intermediates, which are responsible for much of the compound's toxicity. The main metabolites include trichloromethane (chloroform), dichloromethane, and carbon monoxide. These metabolites can further react with cellular components, leading to lipid peroxidation, protein denaturation, and DNA damage.
Understanding the fate of these metabolites in the environment is essential for assessing the long-term impact of CCl4 contamination. Some metabolites, such as chloroform, can persist in soil and groundwater, potentially causing further environmental damage. Others may undergo further transformation, contributing to the formation of other chlorinated compounds or greenhouse gases.
Recent technological advancements in analytical chemistry, particularly in mass spectrometry and nuclear magnetic resonance spectroscopy, have enabled more precise identification and quantification of CCl4 metabolites. These tools allow researchers to track the transformation of CCl4 in various environmental matrices and biological systems with unprecedented accuracy.
The research aims to address several key questions: What are the primary and secondary metabolites of CCl4 in different environmental conditions? How do these metabolites interact with various ecosystems? What are the long-term effects of CCl4 metabolites on soil microbiota, aquatic organisms, and plant life? By answering these questions, we can develop more effective strategies for environmental remediation and improve our understanding of the global carbon and chlorine cycles.
Furthermore, this research seeks to explore potential biomarkers of CCl4 exposure and metabolism in various organisms. Such biomarkers could be invaluable for early detection of CCl4 contamination and for monitoring the effectiveness of remediation efforts. The study also aims to investigate potential natural attenuation processes that may mitigate the environmental impact of CCl4 and its metabolites over time.
Environmental Concerns and Market Drivers
The growing concern over carbon tetrachloride (CCl4) and its metabolites has become a significant driver in environmental research and market dynamics. As a potent ozone-depleting substance and greenhouse gas, CCl4 has been phased out under the Montreal Protocol. However, its persistence in the environment and the potential impacts of its metabolites continue to raise alarm among scientists, policymakers, and environmentalists.
The environmental concerns surrounding CCl4 and its metabolites are multifaceted. Firstly, despite the global ban on its production and use, atmospheric concentrations of CCl4 have not declined as rapidly as expected. This discrepancy suggests ongoing emissions from unknown sources or previously unaccounted-for environmental processes. The slow degradation of CCl4 in the atmosphere, with a lifetime of about 26 years, means that its impact on ozone depletion and global warming persists long after its release.
Furthermore, the breakdown products of CCl4 in the environment, particularly in soil and groundwater, pose additional risks. These metabolites, including chloroform and carbon dioxide, can have their own set of environmental and health impacts. The potential for bioaccumulation of these compounds in the food chain and their effects on ecosystem health are areas of active research and growing concern.
The market drivers related to CCl4 research are closely tied to these environmental concerns. There is an increasing demand for advanced monitoring and detection technologies capable of identifying trace amounts of CCl4 and its metabolites in various environmental matrices. This has spurred innovation in analytical instrumentation and methodologies, creating new market opportunities for environmental monitoring equipment manufacturers.
Additionally, the remediation sector has seen growth in response to the need for effective cleanup strategies for CCl4-contaminated sites. Technologies for in-situ and ex-situ treatment of contaminated soil and groundwater are in high demand, driving research and development in this area. The market for environmental consulting services related to CCl4 assessment and remediation has also expanded.
The regulatory landscape plays a crucial role in shaping market dynamics. Stricter environmental regulations and enforcement of international agreements like the Montreal Protocol have created a compliance-driven market for CCl4 alternatives and mitigation technologies. Industries previously reliant on CCl4, such as dry cleaning and fire extinguisher manufacturing, have been forced to innovate and adopt alternative substances and processes.
Lastly, the growing emphasis on corporate environmental responsibility and sustainable practices has led to increased voluntary efforts by companies to address legacy CCl4 contamination and prevent future releases. This trend has created opportunities for environmental service providers and technology developers catering to proactive environmental management strategies.
The environmental concerns surrounding CCl4 and its metabolites are multifaceted. Firstly, despite the global ban on its production and use, atmospheric concentrations of CCl4 have not declined as rapidly as expected. This discrepancy suggests ongoing emissions from unknown sources or previously unaccounted-for environmental processes. The slow degradation of CCl4 in the atmosphere, with a lifetime of about 26 years, means that its impact on ozone depletion and global warming persists long after its release.
Furthermore, the breakdown products of CCl4 in the environment, particularly in soil and groundwater, pose additional risks. These metabolites, including chloroform and carbon dioxide, can have their own set of environmental and health impacts. The potential for bioaccumulation of these compounds in the food chain and their effects on ecosystem health are areas of active research and growing concern.
The market drivers related to CCl4 research are closely tied to these environmental concerns. There is an increasing demand for advanced monitoring and detection technologies capable of identifying trace amounts of CCl4 and its metabolites in various environmental matrices. This has spurred innovation in analytical instrumentation and methodologies, creating new market opportunities for environmental monitoring equipment manufacturers.
Additionally, the remediation sector has seen growth in response to the need for effective cleanup strategies for CCl4-contaminated sites. Technologies for in-situ and ex-situ treatment of contaminated soil and groundwater are in high demand, driving research and development in this area. The market for environmental consulting services related to CCl4 assessment and remediation has also expanded.
The regulatory landscape plays a crucial role in shaping market dynamics. Stricter environmental regulations and enforcement of international agreements like the Montreal Protocol have created a compliance-driven market for CCl4 alternatives and mitigation technologies. Industries previously reliant on CCl4, such as dry cleaning and fire extinguisher manufacturing, have been forced to innovate and adopt alternative substances and processes.
Lastly, the growing emphasis on corporate environmental responsibility and sustainable practices has led to increased voluntary efforts by companies to address legacy CCl4 contamination and prevent future releases. This trend has created opportunities for environmental service providers and technology developers catering to proactive environmental management strategies.
Current State of CCl4 Metabolite Research
Carbon tetrachloride (CCl4) metabolite research has made significant strides in recent years, shedding light on the environmental fate and impact of this persistent organic pollutant. Current studies focus on identifying and characterizing the metabolites formed during CCl4 degradation processes, both in environmental matrices and biological systems.
In environmental contexts, researchers have identified several key metabolites resulting from CCl4 transformation. These include chloroform (CHCl3), dichloromethane (CH2Cl2), and carbon monoxide (CO). The formation of these metabolites is primarily attributed to reductive dechlorination processes, which can occur under both aerobic and anaerobic conditions. Recent studies have also revealed the potential for CCl4 to form more complex organic compounds through various environmental reactions.
Biological systems, particularly microbial communities, play a crucial role in CCl4 metabolism. Current research has identified several bacterial strains capable of degrading CCl4, including members of the genera Pseudomonas, Desulfovibrio, and Methanosarcina. These microorganisms employ different enzymatic pathways to break down CCl4, often resulting in the formation of less chlorinated intermediates and ultimately, inorganic chloride.
Advanced analytical techniques have greatly enhanced our ability to detect and quantify CCl4 metabolites. High-resolution mass spectrometry, coupled with chromatographic separation methods, has enabled researchers to identify trace levels of metabolites in complex environmental matrices. Additionally, stable isotope probing techniques have provided valuable insights into the transformation pathways of CCl4 in various ecosystems.
The environmental impact of CCl4 metabolites remains a critical area of investigation. While some metabolites, such as chloroform, are known to pose significant environmental and health risks, the toxicity and persistence of other transformation products are still being evaluated. Current research efforts are focused on assessing the bioaccumulation potential and long-term ecological effects of these metabolites in aquatic and terrestrial ecosystems.
Remediation strategies for CCl4-contaminated sites are being developed based on our growing understanding of its metabolic pathways. Bioremediation approaches, leveraging the natural degradation capabilities of microorganisms, show promise for in situ treatment of CCl4 pollution. However, the potential formation of toxic intermediates during these processes necessitates careful monitoring and control.
Global efforts to phase out CCl4 production and use have led to a decline in atmospheric concentrations. However, legacy contamination in soil and groundwater continues to be a concern. Current research is focused on developing predictive models to assess the long-term behavior and impact of CCl4 and its metabolites in these environmental compartments.
In environmental contexts, researchers have identified several key metabolites resulting from CCl4 transformation. These include chloroform (CHCl3), dichloromethane (CH2Cl2), and carbon monoxide (CO). The formation of these metabolites is primarily attributed to reductive dechlorination processes, which can occur under both aerobic and anaerobic conditions. Recent studies have also revealed the potential for CCl4 to form more complex organic compounds through various environmental reactions.
Biological systems, particularly microbial communities, play a crucial role in CCl4 metabolism. Current research has identified several bacterial strains capable of degrading CCl4, including members of the genera Pseudomonas, Desulfovibrio, and Methanosarcina. These microorganisms employ different enzymatic pathways to break down CCl4, often resulting in the formation of less chlorinated intermediates and ultimately, inorganic chloride.
Advanced analytical techniques have greatly enhanced our ability to detect and quantify CCl4 metabolites. High-resolution mass spectrometry, coupled with chromatographic separation methods, has enabled researchers to identify trace levels of metabolites in complex environmental matrices. Additionally, stable isotope probing techniques have provided valuable insights into the transformation pathways of CCl4 in various ecosystems.
The environmental impact of CCl4 metabolites remains a critical area of investigation. While some metabolites, such as chloroform, are known to pose significant environmental and health risks, the toxicity and persistence of other transformation products are still being evaluated. Current research efforts are focused on assessing the bioaccumulation potential and long-term ecological effects of these metabolites in aquatic and terrestrial ecosystems.
Remediation strategies for CCl4-contaminated sites are being developed based on our growing understanding of its metabolic pathways. Bioremediation approaches, leveraging the natural degradation capabilities of microorganisms, show promise for in situ treatment of CCl4 pollution. However, the potential formation of toxic intermediates during these processes necessitates careful monitoring and control.
Global efforts to phase out CCl4 production and use have led to a decline in atmospheric concentrations. However, legacy contamination in soil and groundwater continues to be a concern. Current research is focused on developing predictive models to assess the long-term behavior and impact of CCl4 and its metabolites in these environmental compartments.
Analytical Methods for CCl4 Metabolites
01 Environmental fate and degradation of carbon tetrachloride
Carbon tetrachloride and its metabolites can persist in various environmental compartments, including soil, water, and air. The degradation process and environmental fate of these compounds are influenced by factors such as microbial activity, chemical reactions, and physical conditions. Understanding these processes is crucial for assessing the long-term environmental impact of carbon tetrachloride contamination.- Environmental fate and degradation of carbon tetrachloride: Carbon tetrachloride and its metabolites can persist in various environmental compartments, including soil, water, and air. The degradation process and environmental fate of these compounds are influenced by factors such as microbial activity, sunlight exposure, and chemical reactions. Understanding these processes is crucial for assessing the long-term environmental impact of carbon tetrachloride contamination.
- Bioaccumulation and toxicity in aquatic ecosystems: Carbon tetrachloride metabolites can accumulate in aquatic organisms, potentially leading to biomagnification through the food chain. This bioaccumulation can result in toxic effects on various aquatic species, including fish, invertebrates, and algae. The impact on aquatic ecosystems may include reduced biodiversity, altered food web dynamics, and impaired ecosystem functions.
- Soil contamination and impact on terrestrial ecosystems: Carbon tetrachloride and its metabolites can persist in soil, affecting soil microorganisms, plants, and terrestrial animals. The presence of these compounds in soil can lead to reduced soil fertility, altered microbial communities, and potential uptake by plants. This contamination may have cascading effects on terrestrial food webs and ecosystem services.
- Atmospheric effects and contribution to ozone depletion: Carbon tetrachloride and some of its metabolites can contribute to stratospheric ozone depletion when released into the atmosphere. These compounds can persist in the air for extended periods, potentially impacting global atmospheric chemistry and climate. The long-term consequences of these atmospheric effects on ecosystems and human health are of significant concern.
- Remediation and mitigation strategies: Various techniques and approaches have been developed to remediate environments contaminated with carbon tetrachloride and its metabolites. These may include bioremediation, chemical treatment, and physical removal methods. Implementing effective remediation strategies is essential for reducing the environmental impact of these compounds and restoring affected ecosystems.
02 Toxicity and bioaccumulation of carbon tetrachloride metabolites
Metabolites of carbon tetrachloride can exhibit varying degrees of toxicity to aquatic and terrestrial organisms. Some metabolites may bioaccumulate in the food chain, potentially causing long-term ecological effects. Research into the toxicological properties of these metabolites is essential for understanding their impact on ecosystems and human health.Expand Specific Solutions03 Remediation techniques for carbon tetrachloride contamination
Various remediation techniques have been developed to address carbon tetrachloride contamination in soil and groundwater. These methods may include bioremediation, chemical oxidation, and physical removal processes. The effectiveness of these techniques depends on the specific environmental conditions and the nature of the contamination.Expand Specific Solutions04 Monitoring and detection of carbon tetrachloride metabolites
Advanced analytical techniques are employed to monitor and detect carbon tetrachloride metabolites in environmental samples. These methods are crucial for assessing the extent of contamination, tracking the progress of remediation efforts, and evaluating the long-term environmental impact of carbon tetrachloride use.Expand Specific Solutions05 Regulatory and policy implications of carbon tetrachloride metabolites
The environmental impact of carbon tetrachloride metabolites has led to regulatory measures and policy changes aimed at controlling their release and mitigating their effects. These regulations may include restrictions on the use of carbon tetrachloride, guidelines for handling and disposal, and requirements for environmental monitoring and remediation.Expand Specific Solutions
Key Players in CCl4 Research
The research on Carbon Tetrachloride Metabolites and Their Environmental Impact is in a mature stage, with a well-established market and significant industry involvement. The field has progressed beyond initial exploration, with major players from academia and industry contributing to its development. Companies like Furukawa Electric, Chiyoda Corp, and Occidental Chemical Corp are actively engaged in this area, leveraging their expertise in chemical processing and environmental technologies. Universities such as the University of Tokyo, Central South University, and MIT are driving fundamental research, while government institutions like the Naval Research Laboratory provide additional support. The market size is substantial, reflecting the importance of understanding and mitigating the environmental impact of carbon tetrachloride metabolites in various industrial and ecological contexts.
University of Tokyo
Technical Solution: The University of Tokyo has made significant contributions to understanding the environmental fate of CCl4 metabolites. Their research has focused on the microbial degradation of CCl4 in anaerobic environments, identifying key bacterial species and enzymes involved in the process [7]. They have also studied the formation of chloroform and other halogenated byproducts during CCl4 degradation and their potential impact on ecosystems [8]. Additionally, the university has developed novel bioremediation techniques using genetically engineered microorganisms to enhance the breakdown of CCl4 and its metabolites in contaminated soil and groundwater [9].
Strengths: Strong focus on microbial processes and bioremediation strategies. Innovative approaches to enhancing natural degradation processes. Weaknesses: May have limited focus on abiotic degradation pathways and atmospheric chemistry of CCl4.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has focused on understanding the fate and transport of CCl4 and its metabolites in marine environments. Their research includes developing sensitive analytical methods for detecting trace levels of CCl4 and its breakdown products in seawater and marine sediments [13]. They have also studied the role of deep-sea hydrothermal vents in the natural production and degradation of CCl4, providing insights into global carbon cycling [14]. Additionally, the NRL has investigated the impact of CCl4 metabolites on marine ecosystems, including effects on phytoplankton and coral reef communities [15].
Strengths: Unique focus on marine environments and deep-sea processes. Advanced analytical capabilities for trace contaminant detection. Weaknesses: Research may be less applicable to terrestrial and freshwater environments.
Key Metabolites and Their Environmental Fate
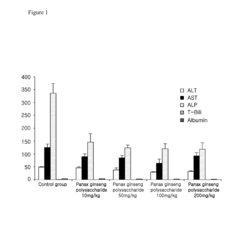
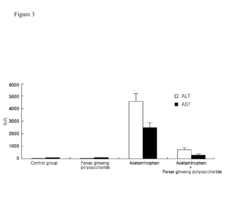
Composition Comprising Polysaccharide Extracted from Panax Ginseng Preventing and Treating Liver Diseases
PatentInactiveUS20110046086A1
Innovation
- A Panax ginseng polysaccharide extract comprising mannose, glucose, galactose, and arabinose, obtained through a method involving cold-infusion or heating of Panax ginseng in water, followed by concentration and precipitation, is used to create a composition for liver protection and treatment, including prevention and treatment of fatty liver, hepatic fibrosis, and liver cancer.
Composition comprising polysaccharide extracted from panax ginseng preventing and treating liver diseases
PatentInactiveEP2273999A2
Innovation
- A Panax ginseng polysaccharide extract is obtained through a method involving water extraction, concentration, and ethanol precipitation, comprising mannose, glucose, galactose, and arabinose, which demonstrates hepatoprotective and therapeutic effects in carbon tetrachloride-induced liver injury models.
Regulatory Framework for CCl4 and Its Metabolites
The regulatory framework for carbon tetrachloride (CCl4) and its metabolites has evolved significantly over the past few decades, reflecting growing concerns about their environmental and health impacts. At the international level, the Montreal Protocol, established in 1987, has been instrumental in phasing out the production and consumption of CCl4 due to its ozone-depleting properties. This agreement has led to a dramatic reduction in global CCl4 emissions, although unintentional releases and legacy contamination remain ongoing issues.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes, including the Clean Air Act, the Clean Water Act, and the Safe Drinking Water Act. The EPA has set a maximum contaminant level goal of zero for CCl4 in drinking water, with an enforceable standard of 0.005 mg/L. Additionally, CCl4 is listed as a hazardous air pollutant, subject to stringent emission controls in industrial settings.
The European Union has implemented similar regulations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its persistent, bioaccumulative, and toxic properties. This classification imposes strict controls on its use and requires manufacturers to seek authorization for specific applications.
Regarding CCl4 metabolites, regulatory approaches are less uniform and comprehensive. The primary metabolites, including phosgene and chloroform, are regulated individually based on their specific toxicological profiles. However, there is growing recognition of the need for a more integrated approach that considers the entire metabolic pathway of CCl4 and its environmental fate.
Recent scientific evidence highlighting the long-term persistence and potential toxicity of CCl4 metabolites in aquatic and soil ecosystems has prompted regulatory bodies to reassess their frameworks. Some jurisdictions are now exploring the implementation of biomonitoring programs to track CCl4 metabolites in environmental matrices and biota. These efforts aim to provide a more comprehensive understanding of the environmental impact of CCl4 and inform future regulatory decisions.
The challenge for regulators lies in balancing the need for stringent controls with the practical considerations of enforcement and economic impact. As analytical techniques improve, enabling the detection of CCl4 and its metabolites at increasingly lower concentrations, there is ongoing debate about appropriate threshold levels and the feasibility of achieving them in various environmental compartments.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes, including the Clean Air Act, the Clean Water Act, and the Safe Drinking Water Act. The EPA has set a maximum contaminant level goal of zero for CCl4 in drinking water, with an enforceable standard of 0.005 mg/L. Additionally, CCl4 is listed as a hazardous air pollutant, subject to stringent emission controls in industrial settings.
The European Union has implemented similar regulations through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) framework. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its persistent, bioaccumulative, and toxic properties. This classification imposes strict controls on its use and requires manufacturers to seek authorization for specific applications.
Regarding CCl4 metabolites, regulatory approaches are less uniform and comprehensive. The primary metabolites, including phosgene and chloroform, are regulated individually based on their specific toxicological profiles. However, there is growing recognition of the need for a more integrated approach that considers the entire metabolic pathway of CCl4 and its environmental fate.
Recent scientific evidence highlighting the long-term persistence and potential toxicity of CCl4 metabolites in aquatic and soil ecosystems has prompted regulatory bodies to reassess their frameworks. Some jurisdictions are now exploring the implementation of biomonitoring programs to track CCl4 metabolites in environmental matrices and biota. These efforts aim to provide a more comprehensive understanding of the environmental impact of CCl4 and inform future regulatory decisions.
The challenge for regulators lies in balancing the need for stringent controls with the practical considerations of enforcement and economic impact. As analytical techniques improve, enabling the detection of CCl4 and its metabolites at increasingly lower concentrations, there is ongoing debate about appropriate threshold levels and the feasibility of achieving them in various environmental compartments.
Ecological Risk Assessment of CCl4 Metabolites
The ecological risk assessment of carbon tetrachloride (CCl4) metabolites is a critical component in understanding the environmental impact of this widely used industrial solvent. CCl4 undergoes various transformation processes in the environment, resulting in the formation of several metabolites that can pose significant risks to ecosystems.
One of the primary metabolites of CCl4 is chloroform (CHCl3), which is formed through reductive dechlorination processes in anaerobic environments. Chloroform itself is a known environmental contaminant with potential carcinogenic properties. Its presence in soil and groundwater can lead to long-term ecological consequences, affecting microbial communities and potentially entering the food chain through plant uptake.
Another important metabolite is phosgene (COCl2), which can form under certain conditions when CCl4 is exposed to high temperatures or UV radiation. Phosgene is highly toxic and can cause severe damage to aquatic organisms, even at low concentrations. Its rapid hydrolysis in water produces hydrochloric acid and carbon dioxide, potentially altering the pH of aquatic environments and impacting sensitive species.
Carbon dioxide (CO2) and chloride ions (Cl-) are the ultimate products of CCl4 degradation. While CO2 contributes to global warming, the accumulation of chloride ions in soil and water bodies can lead to increased salinity, affecting plant growth and aquatic ecosystems. This is particularly concerning in freshwater environments where many organisms are sensitive to changes in salinity.
The ecological risk assessment of CCl4 metabolites must consider their persistence, bioaccumulation potential, and toxicity. Studies have shown that some metabolites, such as chloroform, can persist in the environment for extended periods, increasing the likelihood of long-term ecological impacts. The potential for bioaccumulation in aquatic organisms and subsequent biomagnification up the food chain is another critical factor in assessing ecological risk.
Furthermore, the synergistic effects of CCl4 metabolites with other environmental contaminants must be evaluated. The presence of multiple pollutants can lead to complex interactions, potentially amplifying the toxic effects on ecosystems. This complexity necessitates comprehensive risk assessment strategies that consider multiple stressors and their combined impacts on different trophic levels.
To accurately assess the ecological risks associated with CCl4 metabolites, a multifaceted approach is required. This includes conducting toxicity tests on various species representing different trophic levels, analyzing bioaccumulation patterns, and modeling the fate and transport of these compounds in different environmental compartments. Long-term monitoring programs are essential to track the persistence and distribution of CCl4 metabolites in affected ecosystems and to detect any delayed or chronic effects on biodiversity and ecosystem functions.
One of the primary metabolites of CCl4 is chloroform (CHCl3), which is formed through reductive dechlorination processes in anaerobic environments. Chloroform itself is a known environmental contaminant with potential carcinogenic properties. Its presence in soil and groundwater can lead to long-term ecological consequences, affecting microbial communities and potentially entering the food chain through plant uptake.
Another important metabolite is phosgene (COCl2), which can form under certain conditions when CCl4 is exposed to high temperatures or UV radiation. Phosgene is highly toxic and can cause severe damage to aquatic organisms, even at low concentrations. Its rapid hydrolysis in water produces hydrochloric acid and carbon dioxide, potentially altering the pH of aquatic environments and impacting sensitive species.
Carbon dioxide (CO2) and chloride ions (Cl-) are the ultimate products of CCl4 degradation. While CO2 contributes to global warming, the accumulation of chloride ions in soil and water bodies can lead to increased salinity, affecting plant growth and aquatic ecosystems. This is particularly concerning in freshwater environments where many organisms are sensitive to changes in salinity.
The ecological risk assessment of CCl4 metabolites must consider their persistence, bioaccumulation potential, and toxicity. Studies have shown that some metabolites, such as chloroform, can persist in the environment for extended periods, increasing the likelihood of long-term ecological impacts. The potential for bioaccumulation in aquatic organisms and subsequent biomagnification up the food chain is another critical factor in assessing ecological risk.
Furthermore, the synergistic effects of CCl4 metabolites with other environmental contaminants must be evaluated. The presence of multiple pollutants can lead to complex interactions, potentially amplifying the toxic effects on ecosystems. This complexity necessitates comprehensive risk assessment strategies that consider multiple stressors and their combined impacts on different trophic levels.
To accurately assess the ecological risks associated with CCl4 metabolites, a multifaceted approach is required. This includes conducting toxicity tests on various species representing different trophic levels, analyzing bioaccumulation patterns, and modeling the fate and transport of these compounds in different environmental compartments. Long-term monitoring programs are essential to track the persistence and distribution of CCl4 metabolites in affected ecosystems and to detect any delayed or chronic effects on biodiversity and ecosystem functions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!