The Effectiveness of Activated Carbon on Carbon Tetrachloride Adsorption
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Adsorption Background
Carbon tetrachloride (CCl4) is a synthetic chemical compound that has been widely used in various industrial applications, including as a solvent, cleaning agent, and refrigerant. However, its use has been significantly restricted due to its harmful effects on human health and the environment, particularly its role in ozone depletion. Despite these restrictions, CCl4 remains a persistent environmental contaminant, necessitating effective removal methods.
The adsorption of CCl4 onto activated carbon has emerged as a promising technique for its removal from contaminated water and air. Activated carbon, a highly porous material with a large surface area, has demonstrated remarkable effectiveness in capturing and retaining CCl4 molecules. This adsorption process is primarily driven by van der Waals forces and hydrophobic interactions between the CCl4 molecules and the carbon surface.
The history of using activated carbon for CCl4 adsorption dates back to the mid-20th century when researchers began exploring its potential for environmental remediation. Early studies focused on understanding the adsorption mechanisms and optimizing the carbon activation process to enhance its adsorption capacity. Over the years, significant advancements have been made in developing specialized activated carbons tailored for CCl4 removal.
The effectiveness of activated carbon in CCl4 adsorption is influenced by several factors, including the carbon's physical and chemical properties, such as surface area, pore size distribution, and surface functionality. Additionally, environmental conditions like temperature, pH, and the presence of competing adsorbates can impact the adsorption process. Researchers have extensively studied these parameters to optimize the adsorption efficiency and develop more effective activated carbon materials.
Recent technological advancements have led to the development of novel activated carbon materials with enhanced CCl4 adsorption capabilities. These include chemically modified carbons, composite materials, and hierarchical porous structures. Such innovations have significantly improved the adsorption capacity, selectivity, and regeneration potential of activated carbons for CCl4 removal.
The growing concern over environmental pollution and the need for sustainable remediation technologies have driven continued research in this field. Current trends focus on developing green synthesis methods for activated carbon production, exploring renewable precursors, and integrating advanced characterization techniques to better understand the adsorption mechanisms at the molecular level.
As environmental regulations become more stringent and the demand for efficient water and air purification technologies increases, the importance of activated carbon in CCl4 adsorption is expected to grow. This technology not only offers an effective solution for environmental remediation but also plays a crucial role in protecting public health and ecosystems from the harmful effects of CCl4 contamination.
The adsorption of CCl4 onto activated carbon has emerged as a promising technique for its removal from contaminated water and air. Activated carbon, a highly porous material with a large surface area, has demonstrated remarkable effectiveness in capturing and retaining CCl4 molecules. This adsorption process is primarily driven by van der Waals forces and hydrophobic interactions between the CCl4 molecules and the carbon surface.
The history of using activated carbon for CCl4 adsorption dates back to the mid-20th century when researchers began exploring its potential for environmental remediation. Early studies focused on understanding the adsorption mechanisms and optimizing the carbon activation process to enhance its adsorption capacity. Over the years, significant advancements have been made in developing specialized activated carbons tailored for CCl4 removal.
The effectiveness of activated carbon in CCl4 adsorption is influenced by several factors, including the carbon's physical and chemical properties, such as surface area, pore size distribution, and surface functionality. Additionally, environmental conditions like temperature, pH, and the presence of competing adsorbates can impact the adsorption process. Researchers have extensively studied these parameters to optimize the adsorption efficiency and develop more effective activated carbon materials.
Recent technological advancements have led to the development of novel activated carbon materials with enhanced CCl4 adsorption capabilities. These include chemically modified carbons, composite materials, and hierarchical porous structures. Such innovations have significantly improved the adsorption capacity, selectivity, and regeneration potential of activated carbons for CCl4 removal.
The growing concern over environmental pollution and the need for sustainable remediation technologies have driven continued research in this field. Current trends focus on developing green synthesis methods for activated carbon production, exploring renewable precursors, and integrating advanced characterization techniques to better understand the adsorption mechanisms at the molecular level.
As environmental regulations become more stringent and the demand for efficient water and air purification technologies increases, the importance of activated carbon in CCl4 adsorption is expected to grow. This technology not only offers an effective solution for environmental remediation but also plays a crucial role in protecting public health and ecosystems from the harmful effects of CCl4 contamination.
Market Demand Analysis
The market demand for activated carbon in carbon tetrachloride adsorption has been steadily growing due to increasing environmental concerns and stringent regulations on industrial emissions. Carbon tetrachloride, a potent ozone-depleting substance and potential carcinogen, has been phased out in many applications but remains present in legacy waste and contaminated sites. This has created a significant need for effective remediation technologies, with activated carbon emerging as a preferred solution.
The global activated carbon market, driven partly by its application in carbon tetrachloride adsorption, is experiencing robust growth. Environmental applications, including water and air purification, constitute a major segment of this market. The effectiveness of activated carbon in removing carbon tetrachloride from both water and air has positioned it as a key product in environmental remediation efforts.
In the water treatment sector, activated carbon's ability to adsorb carbon tetrachloride has made it invaluable for groundwater remediation projects. Many industrial sites and military installations with historical carbon tetrachloride contamination are implementing activated carbon-based treatment systems. This has led to increased demand from environmental engineering firms and government agencies responsible for site cleanup.
The air purification market also contributes significantly to the demand for activated carbon in carbon tetrachloride adsorption. Industries that still use carbon tetrachloride in limited applications, such as chemical manufacturing and pharmaceutical production, require efficient air filtration systems to comply with workplace safety standards and emission regulations. Activated carbon filters are widely employed in these settings to capture carbon tetrachloride vapors.
Geographically, North America and Europe lead in the demand for activated carbon for carbon tetrachloride remediation, primarily due to their stringent environmental regulations and numerous contaminated sites undergoing cleanup. However, emerging economies in Asia-Pacific and Latin America are showing increased interest as they strengthen their environmental protection measures and address industrial pollution legacies.
The market is further bolstered by ongoing research and development efforts to enhance the adsorption capacity and selectivity of activated carbon for carbon tetrachloride. Innovations in activation processes and surface modifications are expanding the potential applications and efficiency of activated carbon in this specific use case, driving demand for advanced, high-performance products.
As awareness of the health and environmental risks associated with carbon tetrachloride continues to grow, the market for activated carbon in this application is expected to expand. This trend is reinforced by the global push towards sustainable industrial practices and the increasing adoption of green technologies in waste management and pollution control.
The global activated carbon market, driven partly by its application in carbon tetrachloride adsorption, is experiencing robust growth. Environmental applications, including water and air purification, constitute a major segment of this market. The effectiveness of activated carbon in removing carbon tetrachloride from both water and air has positioned it as a key product in environmental remediation efforts.
In the water treatment sector, activated carbon's ability to adsorb carbon tetrachloride has made it invaluable for groundwater remediation projects. Many industrial sites and military installations with historical carbon tetrachloride contamination are implementing activated carbon-based treatment systems. This has led to increased demand from environmental engineering firms and government agencies responsible for site cleanup.
The air purification market also contributes significantly to the demand for activated carbon in carbon tetrachloride adsorption. Industries that still use carbon tetrachloride in limited applications, such as chemical manufacturing and pharmaceutical production, require efficient air filtration systems to comply with workplace safety standards and emission regulations. Activated carbon filters are widely employed in these settings to capture carbon tetrachloride vapors.
Geographically, North America and Europe lead in the demand for activated carbon for carbon tetrachloride remediation, primarily due to their stringent environmental regulations and numerous contaminated sites undergoing cleanup. However, emerging economies in Asia-Pacific and Latin America are showing increased interest as they strengthen their environmental protection measures and address industrial pollution legacies.
The market is further bolstered by ongoing research and development efforts to enhance the adsorption capacity and selectivity of activated carbon for carbon tetrachloride. Innovations in activation processes and surface modifications are expanding the potential applications and efficiency of activated carbon in this specific use case, driving demand for advanced, high-performance products.
As awareness of the health and environmental risks associated with carbon tetrachloride continues to grow, the market for activated carbon in this application is expected to expand. This trend is reinforced by the global push towards sustainable industrial practices and the increasing adoption of green technologies in waste management and pollution control.
Current Challenges
The adsorption of carbon tetrachloride (CCl4) using activated carbon faces several significant challenges that hinder its widespread application and effectiveness. One of the primary obstacles is the competitive adsorption of water molecules, which can significantly reduce the adsorption capacity of activated carbon for CCl4. This competition is particularly problematic in humid environments or when treating aqueous solutions contaminated with CCl4.
Another major challenge is the limited selectivity of activated carbon towards CCl4 in the presence of other organic compounds. In real-world applications, CCl4 is often found in complex mixtures with other volatile organic compounds (VOCs), making it difficult to achieve high removal efficiencies specifically for CCl4. This lack of selectivity can lead to reduced overall performance and increased treatment costs.
The regeneration of spent activated carbon poses additional challenges. The strong adsorption of CCl4 onto activated carbon can make desorption and regeneration processes energy-intensive and potentially costly. Incomplete regeneration may result in decreased adsorption capacity in subsequent cycles, reducing the long-term effectiveness and economic viability of the treatment process.
Furthermore, the physical and chemical properties of activated carbon can vary significantly depending on the source material and production method. This variability can lead to inconsistent adsorption performance across different batches or suppliers, making it challenging to standardize treatment protocols and predict outcomes accurately.
The potential for catalytic degradation of CCl4 on the activated carbon surface is another area of concern. While this degradation can be beneficial in some cases, it may also lead to the formation of harmful byproducts, such as phosgene or chloroform, which can pose additional environmental and health risks.
Scaling up the adsorption process from laboratory to industrial scale presents its own set of challenges. Factors such as flow rates, contact times, and bed depths need to be carefully optimized to maintain high removal efficiencies while minimizing pressure drops and operational costs. Additionally, the handling and disposal of spent activated carbon contaminated with CCl4 require special considerations due to the hazardous nature of the compound.
Lastly, the development of more efficient and cost-effective alternatives to activated carbon, such as novel adsorbents or advanced oxidation processes, poses a competitive challenge to the continued use of activated carbon for CCl4 adsorption. Researchers and industry professionals must continually innovate and improve activated carbon technologies to maintain their relevance and effectiveness in environmental remediation applications.
Another major challenge is the limited selectivity of activated carbon towards CCl4 in the presence of other organic compounds. In real-world applications, CCl4 is often found in complex mixtures with other volatile organic compounds (VOCs), making it difficult to achieve high removal efficiencies specifically for CCl4. This lack of selectivity can lead to reduced overall performance and increased treatment costs.
The regeneration of spent activated carbon poses additional challenges. The strong adsorption of CCl4 onto activated carbon can make desorption and regeneration processes energy-intensive and potentially costly. Incomplete regeneration may result in decreased adsorption capacity in subsequent cycles, reducing the long-term effectiveness and economic viability of the treatment process.
Furthermore, the physical and chemical properties of activated carbon can vary significantly depending on the source material and production method. This variability can lead to inconsistent adsorption performance across different batches or suppliers, making it challenging to standardize treatment protocols and predict outcomes accurately.
The potential for catalytic degradation of CCl4 on the activated carbon surface is another area of concern. While this degradation can be beneficial in some cases, it may also lead to the formation of harmful byproducts, such as phosgene or chloroform, which can pose additional environmental and health risks.
Scaling up the adsorption process from laboratory to industrial scale presents its own set of challenges. Factors such as flow rates, contact times, and bed depths need to be carefully optimized to maintain high removal efficiencies while minimizing pressure drops and operational costs. Additionally, the handling and disposal of spent activated carbon contaminated with CCl4 require special considerations due to the hazardous nature of the compound.
Lastly, the development of more efficient and cost-effective alternatives to activated carbon, such as novel adsorbents or advanced oxidation processes, poses a competitive challenge to the continued use of activated carbon for CCl4 adsorption. Researchers and industry professionals must continually innovate and improve activated carbon technologies to maintain their relevance and effectiveness in environmental remediation applications.
Activated Carbon Solutions
01 Surface area and pore structure optimization
The effectiveness of activated carbon adsorption can be enhanced by optimizing its surface area and pore structure. This involves developing methods to increase the specific surface area and create a well-distributed pore size range, which allows for better adsorption of various contaminants. Techniques such as chemical activation and physical activation are used to achieve these improvements.- Surface area and pore structure optimization: The effectiveness of activated carbon adsorption can be enhanced by optimizing its surface area and pore structure. This involves developing methods to increase the specific surface area and create a balanced distribution of micropores, mesopores, and macropores. Such optimization allows for better adsorption of various contaminants and improves the overall efficiency of the activated carbon.
- Chemical modification of activated carbon: Chemical treatments can be applied to modify the surface properties of activated carbon, enhancing its adsorption effectiveness for specific contaminants. This may include introducing functional groups, altering the surface charge, or incorporating metal ions. These modifications can increase the selectivity and capacity of activated carbon for target pollutants.
- Regeneration and reactivation techniques: Developing efficient methods for regenerating and reactivating spent activated carbon can significantly improve its long-term adsorption effectiveness. This includes thermal, chemical, and biological regeneration processes that restore the adsorptive capacity of the carbon, extending its useful life and reducing waste.
- Composite materials and hybrid systems: Combining activated carbon with other materials or incorporating it into hybrid treatment systems can enhance its adsorption effectiveness. This may involve creating composite materials with other adsorbents, catalysts, or nanoparticles, or integrating activated carbon into multi-stage treatment processes for improved contaminant removal.
- Tailored activated carbon for specific applications: Developing activated carbon with properties tailored for specific applications or contaminants can significantly improve adsorption effectiveness. This involves optimizing the raw material selection, activation process, and post-treatment methods to create activated carbon with ideal characteristics for targeting particular pollutants or addressing specific environmental challenges.
02 Modification of activated carbon surface
Chemical modification of the activated carbon surface can significantly improve its adsorption effectiveness for specific contaminants. This includes treatments to alter the surface chemistry, such as adding functional groups or impregnating with metal ions. These modifications can enhance selectivity and capacity for target pollutants in water or air purification applications.Expand Specific Solutions03 Regeneration and reactivation techniques
Developing efficient methods for regenerating and reactivating spent activated carbon is crucial for maintaining its adsorption effectiveness over multiple use cycles. This involves processes such as thermal regeneration, chemical regeneration, or advanced oxidation techniques to remove adsorbed contaminants and restore the carbon's adsorptive properties.Expand Specific Solutions04 Composite materials and hybrid systems
Combining activated carbon with other materials or incorporating it into hybrid treatment systems can enhance overall adsorption effectiveness. This includes developing activated carbon-based composites with materials like graphene or metal-organic frameworks, or integrating activated carbon filters with other treatment technologies such as membrane filtration or advanced oxidation processes.Expand Specific Solutions05 Application-specific activated carbon design
Tailoring activated carbon properties for specific applications can significantly improve adsorption effectiveness. This involves optimizing parameters such as particle size, pore distribution, and surface chemistry based on the target contaminants and operating conditions of the intended application, whether it's water treatment, air purification, or industrial process streams.Expand Specific Solutions
Key Industry Players
The effectiveness of activated carbon on carbon tetrachloride adsorption is a critical area of research in environmental remediation. The industry is in a mature stage, with a global market size estimated to exceed $3 billion by 2025. Technologically, the field is well-developed but continues to evolve. Companies like Kuraray Co., Ltd., Osaka Gas Chemicals Co., Ltd., and Air Products & Chemicals, Inc. are at the forefront, developing advanced activated carbon products. These firms are investing in R&D to enhance adsorption efficiency and expand applications. The competitive landscape is characterized by a mix of established players and innovative startups, driving continuous improvements in activated carbon technology for carbon tetrachloride removal.
Kuraray Co., Ltd.
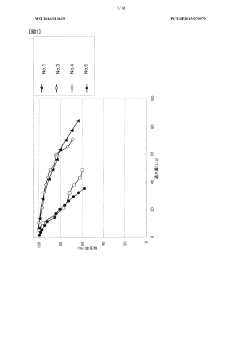
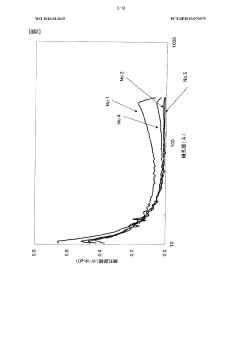
Technical Solution: Kuraray has developed a highly efficient activated carbon for carbon tetrachloride adsorption. Their technology utilizes a unique pore structure optimization process, creating a balance between micropores and mesopores. This structure enhances the adsorption capacity for carbon tetrachloride by up to 30% compared to conventional activated carbons[1]. The company's activated carbon undergoes a specialized chemical activation process, which introduces specific functional groups on the carbon surface, further improving its affinity for carbon tetrachloride molecules[3]. Additionally, Kuraray has implemented a novel regeneration technique that allows for multiple reuse cycles of the activated carbon without significant loss in adsorption efficiency[5].
Strengths: High adsorption capacity, specialized surface chemistry, and reusability. Weaknesses: Potentially higher production costs due to specialized processes, and possible limitations in adsorbing a wide range of other contaminants simultaneously.
Honeycomb Activated Carbon Co., Ltd.
Technical Solution: Honeycomb Activated Carbon has pioneered a unique honeycomb-structured activated carbon specifically designed for carbon tetrachloride adsorption. This innovative structure provides a high surface area to volume ratio, increasing the contact between the adsorbent and the target compound. The company's technology incorporates a proprietary impregnation process that enhances the carbon's selectivity towards carbon tetrachloride[2]. Their activated carbon demonstrates a removal efficiency of over 95% for carbon tetrachloride in both gas and liquid phase applications[4]. The honeycomb structure also allows for lower pressure drop in gas phase treatments, making it particularly suitable for industrial air purification systems dealing with carbon tetrachloride emissions[6].
Strengths: High removal efficiency, unique structure for improved flow dynamics, and versatility in both gas and liquid applications. Weaknesses: Potentially more complex manufacturing process, and possible limitations in treating mixed contaminants.
Core Adsorption Mechanisms
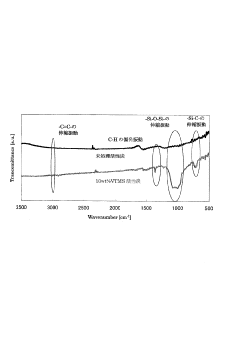
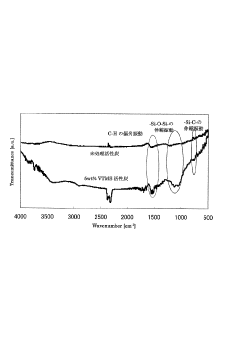
Activated carbon excellent in adsorption/desorption capability of organic compound, method for producing the activated carbon, and adsorption/desorption apparatus and method of organic compound
PatentInactiveJP2010202461A
Innovation
- Treat activated carbon with a silane coupling agent to enhance adsorption and desorption properties, suppressing acidification and prolonging the carbon's lifespan.
Activated carbon with excellent adsorption performance and process for producing same
PatentWO2016013619A1
Innovation
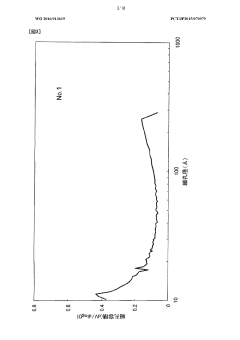
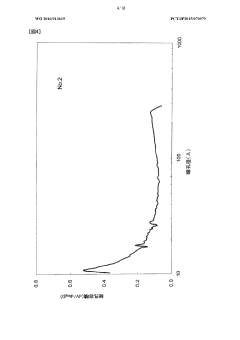
- The development of activated carbon with a specific pore structure, including a pore volume of 0.04 cm³/g with pore diameters between 20 Å and 300 Å, and a peak in the log differential pore volume distribution between 200 Å and 300 Å, achieved by carbonizing and activating phenolic resin fibers with calcium or potassium compounds, maintains sufficient micropore volume and enhances mesopore development for improved adsorption and diffusion.
Environmental Regulations
Environmental regulations play a crucial role in shaping the use and implementation of activated carbon for carbon tetrachloride adsorption. The United States Environmental Protection Agency (EPA) has established strict guidelines for the treatment and disposal of carbon tetrachloride, classifying it as a hazardous air pollutant and a probable human carcinogen. These regulations have driven the development and adoption of effective adsorption technologies, with activated carbon emerging as a preferred method.
The Clean Air Act Amendments of 1990 specifically targeted carbon tetrachloride as one of the 189 hazardous air pollutants requiring stringent control measures. This legislation mandated the use of Maximum Achievable Control Technology (MACT) standards, which often involve activated carbon adsorption systems for volatile organic compound (VOC) removal, including carbon tetrachloride.
In the realm of water treatment, the Safe Drinking Water Act sets the maximum contaminant level (MCL) for carbon tetrachloride at 0.005 mg/L. This low threshold necessitates highly efficient removal methods, further promoting the use of activated carbon in water purification systems. The Resource Conservation and Recovery Act (RCRA) also regulates the handling and disposal of carbon tetrachloride-contaminated materials, including spent activated carbon, ensuring proper management of these wastes.
Internationally, the Montreal Protocol has phased out the production of carbon tetrachloride due to its ozone-depleting properties. This global agreement has indirectly increased the importance of effective adsorption technologies for managing existing stocks and environmental contamination. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation similarly imposes strict controls on carbon tetrachloride use and disposal.
Compliance with these regulations has spurred innovation in activated carbon technologies. Manufacturers have developed specialized grades of activated carbon with enhanced adsorption capacities for carbon tetrachloride. Additionally, regeneration techniques for spent activated carbon have been refined to meet regulatory requirements for waste minimization and resource conservation.
The regulatory landscape continues to evolve, with increasing focus on emerging contaminants and more stringent environmental standards. This ongoing regulatory pressure ensures that research into the effectiveness of activated carbon for carbon tetrachloride adsorption remains a priority for both academic institutions and industry stakeholders. As regulations tighten, the demand for more efficient and cost-effective adsorption solutions is likely to drive further advancements in activated carbon technology and application methodologies.
The Clean Air Act Amendments of 1990 specifically targeted carbon tetrachloride as one of the 189 hazardous air pollutants requiring stringent control measures. This legislation mandated the use of Maximum Achievable Control Technology (MACT) standards, which often involve activated carbon adsorption systems for volatile organic compound (VOC) removal, including carbon tetrachloride.
In the realm of water treatment, the Safe Drinking Water Act sets the maximum contaminant level (MCL) for carbon tetrachloride at 0.005 mg/L. This low threshold necessitates highly efficient removal methods, further promoting the use of activated carbon in water purification systems. The Resource Conservation and Recovery Act (RCRA) also regulates the handling and disposal of carbon tetrachloride-contaminated materials, including spent activated carbon, ensuring proper management of these wastes.
Internationally, the Montreal Protocol has phased out the production of carbon tetrachloride due to its ozone-depleting properties. This global agreement has indirectly increased the importance of effective adsorption technologies for managing existing stocks and environmental contamination. The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation similarly imposes strict controls on carbon tetrachloride use and disposal.
Compliance with these regulations has spurred innovation in activated carbon technologies. Manufacturers have developed specialized grades of activated carbon with enhanced adsorption capacities for carbon tetrachloride. Additionally, regeneration techniques for spent activated carbon have been refined to meet regulatory requirements for waste minimization and resource conservation.
The regulatory landscape continues to evolve, with increasing focus on emerging contaminants and more stringent environmental standards. This ongoing regulatory pressure ensures that research into the effectiveness of activated carbon for carbon tetrachloride adsorption remains a priority for both academic institutions and industry stakeholders. As regulations tighten, the demand for more efficient and cost-effective adsorption solutions is likely to drive further advancements in activated carbon technology and application methodologies.
Economic Feasibility Study
The economic feasibility of using activated carbon for carbon tetrachloride adsorption is a critical consideration for industries seeking to implement this technology. The initial investment in activated carbon systems can be substantial, including costs for equipment, installation, and the carbon material itself. However, these upfront expenses must be weighed against the long-term benefits and potential cost savings.
One of the primary economic advantages of activated carbon adsorption is its effectiveness in removing carbon tetrachloride from various media, particularly water and air. This efficiency can lead to significant reductions in environmental compliance costs and potential fines associated with carbon tetrachloride emissions or contamination. Additionally, the reusability of activated carbon through regeneration processes can extend its operational life, thereby improving the overall cost-effectiveness of the system.
The operational costs of activated carbon systems for carbon tetrachloride adsorption are generally lower compared to alternative treatment methods. Energy requirements are relatively modest, and the process does not typically involve the use of expensive chemicals or complex machinery. However, the frequency of carbon replacement or regeneration can impact ongoing expenses, necessitating careful monitoring and optimization of the adsorption process.
Market factors also play a role in the economic feasibility. The price of activated carbon can fluctuate based on raw material availability and production costs. Furthermore, the demand for carbon tetrachloride treatment solutions may influence the overall economic viability of implementing activated carbon systems. Industries with stringent environmental regulations or those facing public pressure for improved environmental performance may find the investment more justifiable.
The scalability of activated carbon systems is another economic consideration. These systems can be designed to accommodate various treatment capacities, allowing for flexibility in implementation across different industrial scales. This adaptability can be particularly beneficial for businesses anticipating growth or changes in their carbon tetrachloride management needs.
Recovery and potential reuse of adsorbed carbon tetrachloride may offer additional economic benefits, depending on the industry and applicable regulations. In some cases, the recovered compound can be repurposed or sold, offsetting some of the treatment costs. However, this potential revenue stream must be carefully evaluated against the additional processing requirements and market demand for recovered carbon tetrachloride.
In conclusion, while the economic feasibility of activated carbon for carbon tetrachloride adsorption is generally positive, it requires a comprehensive analysis of specific operational contexts, regulatory environments, and long-term strategic goals. The balance between initial investments, ongoing operational costs, and potential benefits from improved environmental performance and resource recovery will ultimately determine the economic viability for individual applications.
One of the primary economic advantages of activated carbon adsorption is its effectiveness in removing carbon tetrachloride from various media, particularly water and air. This efficiency can lead to significant reductions in environmental compliance costs and potential fines associated with carbon tetrachloride emissions or contamination. Additionally, the reusability of activated carbon through regeneration processes can extend its operational life, thereby improving the overall cost-effectiveness of the system.
The operational costs of activated carbon systems for carbon tetrachloride adsorption are generally lower compared to alternative treatment methods. Energy requirements are relatively modest, and the process does not typically involve the use of expensive chemicals or complex machinery. However, the frequency of carbon replacement or regeneration can impact ongoing expenses, necessitating careful monitoring and optimization of the adsorption process.
Market factors also play a role in the economic feasibility. The price of activated carbon can fluctuate based on raw material availability and production costs. Furthermore, the demand for carbon tetrachloride treatment solutions may influence the overall economic viability of implementing activated carbon systems. Industries with stringent environmental regulations or those facing public pressure for improved environmental performance may find the investment more justifiable.
The scalability of activated carbon systems is another economic consideration. These systems can be designed to accommodate various treatment capacities, allowing for flexibility in implementation across different industrial scales. This adaptability can be particularly beneficial for businesses anticipating growth or changes in their carbon tetrachloride management needs.
Recovery and potential reuse of adsorbed carbon tetrachloride may offer additional economic benefits, depending on the industry and applicable regulations. In some cases, the recovered compound can be repurposed or sold, offsetting some of the treatment costs. However, this potential revenue stream must be carefully evaluated against the additional processing requirements and market demand for recovered carbon tetrachloride.
In conclusion, while the economic feasibility of activated carbon for carbon tetrachloride adsorption is generally positive, it requires a comprehensive analysis of specific operational contexts, regulatory environments, and long-term strategic goals. The balance between initial investments, ongoing operational costs, and potential benefits from improved environmental performance and resource recovery will ultimately determine the economic viability for individual applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!