The Influence of pH on Carbon Tetrachloride Degradation Pathways
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Degradation Background and Objectives
Carbon tetrachloride (CCl4) degradation has been a significant environmental concern for decades due to its widespread use as a solvent and its persistence in soil and groundwater. The background of CCl4 degradation research dates back to the 1970s when its harmful effects on the ozone layer and human health were first recognized. Since then, extensive studies have been conducted to understand its behavior in various environmental conditions and to develop effective remediation strategies.
The evolution of CCl4 degradation research has seen a shift from physical and chemical treatment methods to more sustainable biological approaches. Early efforts focused on pump-and-treat systems and chemical oxidation techniques. However, these methods often proved costly and inefficient for long-term remediation. The discovery of microbial degradation pathways in the 1990s opened new avenues for bioremediation strategies, which have since become a primary focus of research and development.
The influence of pH on CCl4 degradation pathways has emerged as a critical area of study in recent years. pH plays a crucial role in determining the dominant degradation mechanisms, reaction rates, and end products. Understanding these pH-dependent pathways is essential for optimizing remediation strategies and predicting the environmental fate of CCl4 in diverse ecosystems.
The primary objective of current research in this field is to elucidate the specific degradation pathways of CCl4 under various pH conditions. This includes identifying the key chemical and biological processes involved, characterizing intermediate and end products, and quantifying reaction kinetics. Researchers aim to develop comprehensive models that can accurately predict CCl4 degradation behavior across a wide range of pH values typically encountered in natural and engineered systems.
Another important goal is to leverage this knowledge to enhance existing remediation technologies and develop novel approaches. By understanding how pH influences degradation pathways, scientists and engineers can design more effective treatment systems tailored to specific site conditions. This may involve optimizing pH levels in bioremediation processes, developing pH-responsive catalysts for chemical degradation, or creating innovative hybrid technologies that combine multiple degradation mechanisms.
Furthermore, the research aims to assess the environmental implications of different CCl4 degradation pathways. This includes evaluating the toxicity and persistence of degradation products formed under various pH conditions, as well as their potential impact on ecosystem health and human exposure risks. Such information is crucial for conducting accurate risk assessments and making informed decisions about remediation strategies.
The evolution of CCl4 degradation research has seen a shift from physical and chemical treatment methods to more sustainable biological approaches. Early efforts focused on pump-and-treat systems and chemical oxidation techniques. However, these methods often proved costly and inefficient for long-term remediation. The discovery of microbial degradation pathways in the 1990s opened new avenues for bioremediation strategies, which have since become a primary focus of research and development.
The influence of pH on CCl4 degradation pathways has emerged as a critical area of study in recent years. pH plays a crucial role in determining the dominant degradation mechanisms, reaction rates, and end products. Understanding these pH-dependent pathways is essential for optimizing remediation strategies and predicting the environmental fate of CCl4 in diverse ecosystems.
The primary objective of current research in this field is to elucidate the specific degradation pathways of CCl4 under various pH conditions. This includes identifying the key chemical and biological processes involved, characterizing intermediate and end products, and quantifying reaction kinetics. Researchers aim to develop comprehensive models that can accurately predict CCl4 degradation behavior across a wide range of pH values typically encountered in natural and engineered systems.
Another important goal is to leverage this knowledge to enhance existing remediation technologies and develop novel approaches. By understanding how pH influences degradation pathways, scientists and engineers can design more effective treatment systems tailored to specific site conditions. This may involve optimizing pH levels in bioremediation processes, developing pH-responsive catalysts for chemical degradation, or creating innovative hybrid technologies that combine multiple degradation mechanisms.
Furthermore, the research aims to assess the environmental implications of different CCl4 degradation pathways. This includes evaluating the toxicity and persistence of degradation products formed under various pH conditions, as well as their potential impact on ecosystem health and human exposure risks. Such information is crucial for conducting accurate risk assessments and making informed decisions about remediation strategies.
Environmental Remediation Market Analysis
The environmental remediation market has experienced significant growth in recent years, driven by increasing awareness of environmental pollution and stricter regulations. The global market for environmental remediation was valued at approximately $85 billion in 2020 and is projected to reach $128 billion by 2025, growing at a CAGR of 8.5% during the forecast period.
Carbon tetrachloride (CCl4) contamination is a critical concern within this market, particularly in soil and groundwater remediation sectors. The demand for effective CCl4 degradation technologies has been steadily rising due to its persistence in the environment and potential health risks. The influence of pH on CCl4 degradation pathways has become a focal point for research and development in the remediation industry.
The market for pH-dependent CCl4 remediation technologies is expected to grow substantially, with North America and Europe leading in terms of market share. This growth is attributed to the presence of stringent environmental regulations and a high concentration of industrial sites with historical CCl4 contamination. The Asia-Pacific region is anticipated to witness the fastest growth rate in this sector, driven by rapid industrialization and increasing environmental awareness.
Key market players in this segment include environmental engineering firms, specialized remediation technology providers, and chemical companies developing advanced treatment solutions. These companies are investing heavily in research and development to optimize CCl4 degradation processes across various pH conditions, aiming to improve efficiency and reduce treatment costs.
The market is seeing a shift towards in-situ remediation techniques that leverage pH manipulation to enhance CCl4 degradation. This trend is driven by the cost-effectiveness and minimal site disruption associated with in-situ methods compared to traditional ex-situ treatments. Additionally, there is growing interest in combining pH-controlled degradation with other remediation technologies, such as chemical oxidation or bioremediation, to achieve more comprehensive and efficient site cleanup.
Regulatory frameworks play a crucial role in shaping the market dynamics for CCl4 remediation. Stringent cleanup standards and increasing enforcement of environmental regulations are driving the adoption of advanced remediation technologies that can effectively address CCl4 contamination across various pH ranges. This regulatory landscape is expected to continue propelling market growth and innovation in pH-dependent CCl4 degradation technologies.
Carbon tetrachloride (CCl4) contamination is a critical concern within this market, particularly in soil and groundwater remediation sectors. The demand for effective CCl4 degradation technologies has been steadily rising due to its persistence in the environment and potential health risks. The influence of pH on CCl4 degradation pathways has become a focal point for research and development in the remediation industry.
The market for pH-dependent CCl4 remediation technologies is expected to grow substantially, with North America and Europe leading in terms of market share. This growth is attributed to the presence of stringent environmental regulations and a high concentration of industrial sites with historical CCl4 contamination. The Asia-Pacific region is anticipated to witness the fastest growth rate in this sector, driven by rapid industrialization and increasing environmental awareness.
Key market players in this segment include environmental engineering firms, specialized remediation technology providers, and chemical companies developing advanced treatment solutions. These companies are investing heavily in research and development to optimize CCl4 degradation processes across various pH conditions, aiming to improve efficiency and reduce treatment costs.
The market is seeing a shift towards in-situ remediation techniques that leverage pH manipulation to enhance CCl4 degradation. This trend is driven by the cost-effectiveness and minimal site disruption associated with in-situ methods compared to traditional ex-situ treatments. Additionally, there is growing interest in combining pH-controlled degradation with other remediation technologies, such as chemical oxidation or bioremediation, to achieve more comprehensive and efficient site cleanup.
Regulatory frameworks play a crucial role in shaping the market dynamics for CCl4 remediation. Stringent cleanup standards and increasing enforcement of environmental regulations are driving the adoption of advanced remediation technologies that can effectively address CCl4 contamination across various pH ranges. This regulatory landscape is expected to continue propelling market growth and innovation in pH-dependent CCl4 degradation technologies.
pH Effects on CCl4 Degradation: Current Understanding
The influence of pH on carbon tetrachloride (CCl4) degradation pathways has been a subject of extensive research due to its significant impact on remediation strategies for contaminated sites. Current understanding suggests that pH plays a crucial role in determining the efficiency and mechanisms of CCl4 degradation.
In acidic conditions (pH < 7), CCl4 degradation tends to follow reductive pathways. The presence of hydrogen ions facilitates the reduction of CCl4 to less chlorinated compounds, such as chloroform (CHCl3) and dichloromethane (CH2Cl2). This process is often mediated by iron-based reductants, including zero-valent iron (ZVI) and ferrous iron (Fe2+). The efficiency of these reductive pathways generally increases as pH decreases, with optimal degradation rates observed in the pH range of 3-5.
Neutral to slightly alkaline conditions (pH 7-9) present a transition zone where both reductive and oxidative pathways may occur. In this pH range, the degradation of CCl4 can proceed through a combination of mechanisms, including reductive dechlorination and hydrolysis. The relative contribution of each pathway depends on the specific environmental conditions and the presence of various reactive species.
Under alkaline conditions (pH > 9), oxidative pathways become more prominent in CCl4 degradation. At higher pH levels, the formation of hydroxyl radicals (OH•) is favored, which can initiate the oxidative breakdown of CCl4. This process often leads to the formation of intermediate products such as phosgene (COCl2) and carbon dioxide (CO2). Additionally, alkaline hydrolysis of CCl4 becomes more significant, resulting in the formation of carbonate ions and chloride ions as end products.
The pH-dependent behavior of CCl4 degradation has important implications for in situ remediation techniques. For instance, the application of ZVI for CCl4 treatment is most effective in acidic to neutral conditions, while advanced oxidation processes utilizing hydroxyl radicals perform better in alkaline environments. Understanding these pH effects allows for the optimization of treatment strategies and the selection of appropriate remediation technologies based on site-specific conditions.
Recent studies have also highlighted the importance of pH in controlling the microbial degradation of CCl4. Many anaerobic microorganisms capable of degrading CCl4 exhibit optimal activity within specific pH ranges. For example, some methanogenic and sulfate-reducing bacteria show enhanced CCl4 degradation capabilities at near-neutral pH, while certain Dehalobacter species perform better under slightly acidic conditions.
The current understanding of pH effects on CCl4 degradation pathways provides valuable insights for designing effective remediation strategies. However, it is important to note that pH is just one of many factors influencing CCl4 degradation in complex environmental systems. Factors such as redox conditions, presence of co-contaminants, and soil characteristics also play significant roles in determining the overall degradation behavior of CCl4 in the environment.
In acidic conditions (pH < 7), CCl4 degradation tends to follow reductive pathways. The presence of hydrogen ions facilitates the reduction of CCl4 to less chlorinated compounds, such as chloroform (CHCl3) and dichloromethane (CH2Cl2). This process is often mediated by iron-based reductants, including zero-valent iron (ZVI) and ferrous iron (Fe2+). The efficiency of these reductive pathways generally increases as pH decreases, with optimal degradation rates observed in the pH range of 3-5.
Neutral to slightly alkaline conditions (pH 7-9) present a transition zone where both reductive and oxidative pathways may occur. In this pH range, the degradation of CCl4 can proceed through a combination of mechanisms, including reductive dechlorination and hydrolysis. The relative contribution of each pathway depends on the specific environmental conditions and the presence of various reactive species.
Under alkaline conditions (pH > 9), oxidative pathways become more prominent in CCl4 degradation. At higher pH levels, the formation of hydroxyl radicals (OH•) is favored, which can initiate the oxidative breakdown of CCl4. This process often leads to the formation of intermediate products such as phosgene (COCl2) and carbon dioxide (CO2). Additionally, alkaline hydrolysis of CCl4 becomes more significant, resulting in the formation of carbonate ions and chloride ions as end products.
The pH-dependent behavior of CCl4 degradation has important implications for in situ remediation techniques. For instance, the application of ZVI for CCl4 treatment is most effective in acidic to neutral conditions, while advanced oxidation processes utilizing hydroxyl radicals perform better in alkaline environments. Understanding these pH effects allows for the optimization of treatment strategies and the selection of appropriate remediation technologies based on site-specific conditions.
Recent studies have also highlighted the importance of pH in controlling the microbial degradation of CCl4. Many anaerobic microorganisms capable of degrading CCl4 exhibit optimal activity within specific pH ranges. For example, some methanogenic and sulfate-reducing bacteria show enhanced CCl4 degradation capabilities at near-neutral pH, while certain Dehalobacter species perform better under slightly acidic conditions.
The current understanding of pH effects on CCl4 degradation pathways provides valuable insights for designing effective remediation strategies. However, it is important to note that pH is just one of many factors influencing CCl4 degradation in complex environmental systems. Factors such as redox conditions, presence of co-contaminants, and soil characteristics also play significant roles in determining the overall degradation behavior of CCl4 in the environment.
Existing pH-Dependent CCl4 Degradation Methods
01 Microbial degradation of carbon tetrachloride
Utilizing specific microorganisms or microbial communities to break down carbon tetrachloride in contaminated environments. This biological approach can be more environmentally friendly and cost-effective compared to chemical methods.- Biological degradation methods: Utilizing microorganisms or enzymes to break down carbon tetrachloride into less harmful compounds. This approach involves the use of specific bacteria or fungi that can metabolize carbon tetrachloride, often under controlled environmental conditions such as temperature and pH.
- Chemical reduction techniques: Employing chemical reducing agents to convert carbon tetrachloride into less toxic substances. This method often involves the use of zero-valent metals or other strong reducing agents to facilitate the breakdown of the carbon-chlorine bonds in carbon tetrachloride.
- Advanced oxidation processes: Utilizing advanced oxidation techniques such as UV irradiation, ozonation, or Fenton's reagent to degrade carbon tetrachloride. These methods generate highly reactive species like hydroxyl radicals that can effectively break down the compound into simpler, less harmful molecules.
- Thermal decomposition methods: Applying high temperatures to break down carbon tetrachloride into its constituent elements or simpler compounds. This approach may involve incineration, pyrolysis, or other high-temperature treatment processes in controlled environments to ensure complete degradation and prevent the formation of harmful by-products.
- Catalytic degradation techniques: Using catalysts to enhance the degradation of carbon tetrachloride under milder conditions. This method often involves the use of metal-based catalysts or nanoparticles that can facilitate the breakdown of carbon tetrachloride through various reaction pathways, potentially combining elements of reduction, oxidation, or thermal decomposition.
02 Chemical degradation methods
Employing various chemical reactions and processes to decompose carbon tetrachloride. This may include oxidation, reduction, or other chemical transformations to convert the compound into less harmful substances.Expand Specific Solutions03 Catalytic degradation techniques
Using catalysts to enhance the breakdown of carbon tetrachloride. This approach can increase the efficiency of degradation processes and potentially reduce energy requirements.Expand Specific Solutions04 Physical treatment methods
Applying physical processes such as adsorption, filtration, or thermal treatment to remove or degrade carbon tetrachloride from contaminated media. These methods can be effective for treating water or soil contamination.Expand Specific Solutions05 Combined degradation approaches
Integrating multiple degradation methods, such as combining biological, chemical, and physical processes, to achieve more effective and comprehensive carbon tetrachloride degradation in complex environmental scenarios.Expand Specific Solutions
Key Organizations in CCl4 Degradation Research
The carbon tetrachloride degradation pathway research is in a mature stage, with significant contributions from academic institutions and industry players. The market for remediation technologies is substantial, driven by environmental regulations and sustainability initiatives. Lanzhou University, Arizona State University, and Zhejiang University are leading academic research in this field, while companies like Tepha, Inc. and Scientific Design Co., Inc. are developing innovative solutions. The technology's maturity is evident in the diverse range of players, from established universities to specialized biotech firms like BluCon Biotech GmbH, indicating a well-developed ecosystem of research and commercial applications in environmental remediation and chemical processing industries.
Lanzhou University
Technical Solution: Lanzhou University has conducted extensive research on the influence of pH on carbon tetrachloride (CCl4) degradation pathways. Their approach involves using advanced spectroscopic techniques to monitor the degradation process in real-time. They have developed a novel pH-controlled reactor system that allows for precise manipulation of solution acidity during CCl4 degradation[1]. Their studies have shown that alkaline conditions (pH > 8) promote hydrolysis pathways, while acidic conditions (pH < 6) favor reductive dechlorination[2]. The university has also explored the use of various catalysts, such as zero-valent iron nanoparticles, to enhance CCl4 degradation efficiency across different pH ranges[3].
Strengths: Comprehensive pH range analysis, real-time monitoring capabilities, and innovative reactor design. Weaknesses: Potential scalability issues for industrial applications and limited field testing.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed proprietary technologies for CCl4 degradation that are adaptable to various pH conditions. Their approach focuses on industrial-scale applications, incorporating pH-responsive polymer systems for controlled release of degradation catalysts. They have engineered a modular treatment system that can automatically adjust pH levels to optimize CCl4 removal efficiency in wastewater streams[10]. Additionally, Dow has explored the use of pH-sensitive nanomaterials for selective adsorption and degradation of CCl4 in complex chemical mixtures, addressing challenges in industrial effluent treatment[11].
Strengths: Industrial-scale solutions, automated pH control systems, and expertise in handling complex chemical mixtures. Weaknesses: Potential high implementation costs and proprietary nature limiting widespread adoption.
Critical Patents in pH-Controlled CCl4 Degradation
Anionic electrochemical compressor and refrigeration system employing same
PatentPendingUS20240240839A1
Innovation
- An anionic electrochemical compressor using a pH swing absorption-desorption system with an anionic membrane, where a hydroxide/hydronium gradient is imposed by an electric field to efficiently compress carbon dioxide, eliminating the need for mechanical parts and reducing energy consumption.
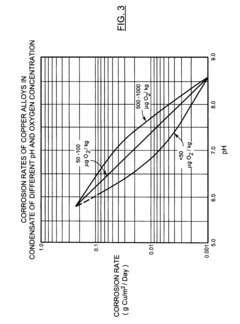
Corrosion reduction system for power generation plants during shutdown
PatentInactiveUS20110070123A1
Innovation
- The implementation of a water anion polisher and acidic gases filter system to remove acidic contaminants from water and air, maintaining optimal pH and reducing oxygen concentrations within the power generation cycle, using anion exchangers and active media filters that require periodic regeneration.
Regulatory Framework for CCl4 Remediation
The regulatory framework for Carbon Tetrachloride (CCl4) remediation is a complex and evolving landscape that reflects the growing understanding of the environmental and health impacts of this persistent organic pollutant. At the federal level in the United States, the Environmental Protection Agency (EPA) has established comprehensive guidelines under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), also known as Superfund. These regulations set stringent standards for the identification, assessment, and cleanup of CCl4-contaminated sites.
The Safe Drinking Water Act (SDWA) plays a crucial role in regulating CCl4 levels in drinking water sources. The EPA has set a maximum contaminant level (MCL) for CCl4 at 0.005 mg/L, reflecting the compound's potential carcinogenicity. This standard drives remediation efforts to ensure that groundwater resources meet these stringent quality requirements.
State-level regulations often complement federal standards, with some states implementing even more stringent cleanup goals. For instance, California's Environmental Protection Agency (CalEPA) has established more conservative risk-based screening levels for CCl4 in soil and groundwater, reflecting the state's proactive approach to environmental protection.
International agreements also influence CCl4 remediation efforts. The Montreal Protocol, while primarily focused on ozone-depleting substances, has indirectly impacted CCl4 management due to its historical use in the production of chlorofluorocarbons (CFCs). This global treaty has led to increased scrutiny and regulation of CCl4 production and disposal practices worldwide.
The regulatory framework also addresses the influence of pH on CCl4 degradation pathways. Recognizing that pH can significantly affect the efficiency and byproducts of CCl4 degradation, regulators have incorporated pH considerations into remediation guidelines. For example, the EPA's technical guidance for in situ chemical reduction (ISCR) treatments emphasizes the importance of pH control in optimizing CCl4 degradation processes.
Emerging regulations are beginning to focus on the long-term management of CCl4-contaminated sites. These include requirements for ongoing monitoring, the implementation of institutional controls, and the development of contingency plans for potential recontamination scenarios. Such comprehensive approaches reflect a growing recognition of the persistent nature of CCl4 contamination and the need for sustained remediation efforts.
As scientific understanding of CCl4 behavior in the environment continues to evolve, regulatory frameworks are expected to adapt. Future regulations may incorporate more nuanced approaches to pH-dependent degradation pathways, potentially leading to site-specific remediation strategies that optimize treatment effectiveness while minimizing undesirable byproduct formation.
The Safe Drinking Water Act (SDWA) plays a crucial role in regulating CCl4 levels in drinking water sources. The EPA has set a maximum contaminant level (MCL) for CCl4 at 0.005 mg/L, reflecting the compound's potential carcinogenicity. This standard drives remediation efforts to ensure that groundwater resources meet these stringent quality requirements.
State-level regulations often complement federal standards, with some states implementing even more stringent cleanup goals. For instance, California's Environmental Protection Agency (CalEPA) has established more conservative risk-based screening levels for CCl4 in soil and groundwater, reflecting the state's proactive approach to environmental protection.
International agreements also influence CCl4 remediation efforts. The Montreal Protocol, while primarily focused on ozone-depleting substances, has indirectly impacted CCl4 management due to its historical use in the production of chlorofluorocarbons (CFCs). This global treaty has led to increased scrutiny and regulation of CCl4 production and disposal practices worldwide.
The regulatory framework also addresses the influence of pH on CCl4 degradation pathways. Recognizing that pH can significantly affect the efficiency and byproducts of CCl4 degradation, regulators have incorporated pH considerations into remediation guidelines. For example, the EPA's technical guidance for in situ chemical reduction (ISCR) treatments emphasizes the importance of pH control in optimizing CCl4 degradation processes.
Emerging regulations are beginning to focus on the long-term management of CCl4-contaminated sites. These include requirements for ongoing monitoring, the implementation of institutional controls, and the development of contingency plans for potential recontamination scenarios. Such comprehensive approaches reflect a growing recognition of the persistent nature of CCl4 contamination and the need for sustained remediation efforts.
As scientific understanding of CCl4 behavior in the environment continues to evolve, regulatory frameworks are expected to adapt. Future regulations may incorporate more nuanced approaches to pH-dependent degradation pathways, potentially leading to site-specific remediation strategies that optimize treatment effectiveness while minimizing undesirable byproduct formation.
Ecological Impact of CCl4 Degradation Byproducts
The degradation of carbon tetrachloride (CCl4) in the environment can lead to the formation of various byproducts, which may have significant ecological impacts. These impacts can vary depending on the specific degradation pathways and environmental conditions, particularly pH levels.
One of the primary concerns is the formation of chloroform (CHCl3) as a byproduct of CCl4 degradation. Chloroform is known to be toxic to aquatic organisms and can persist in the environment for extended periods. In aquatic ecosystems, it can accumulate in sediments and bioaccumulate in the food chain, potentially affecting a wide range of organisms from microorganisms to fish and higher-level predators.
The pH of the environment plays a crucial role in determining the degradation pathways and, consequently, the types and quantities of byproducts formed. In acidic conditions, the formation of phosgene (COCl2) may be favored, which is highly toxic and can have severe impacts on both aquatic and terrestrial ecosystems. Phosgene can react with water to form hydrochloric acid and carbon dioxide, further altering the local pH and potentially causing acidification of water bodies.
Under alkaline conditions, the degradation of CCl4 may lead to the formation of carbonate ions and chloride ions. While these ions are generally less toxic than chloroform or phosgene, they can still impact the chemical balance of ecosystems, particularly in freshwater environments where changes in ion concentrations can affect the osmotic balance of aquatic organisms.
The presence of CCl4 degradation byproducts can also indirectly affect ecosystems by altering microbial communities. Some microorganisms may be more tolerant to these byproducts, leading to shifts in community composition and potentially disrupting important ecological processes such as nutrient cycling and organic matter decomposition.
Furthermore, the persistence of CCl4 and its degradation byproducts in soil can impact plant growth and soil fertility. This can lead to changes in vegetation patterns and, consequently, affect the entire ecosystem structure, including habitat availability for various animal species.
In marine environments, the ecological impact of CCl4 degradation byproducts can be particularly concerning due to the potential for long-range transport and the sensitivity of many marine organisms to chemical contaminants. Coral reefs, for instance, are known to be highly susceptible to chemical pollutants and may suffer from exposure to CCl4 byproducts, potentially leading to bleaching events and reduced biodiversity.
One of the primary concerns is the formation of chloroform (CHCl3) as a byproduct of CCl4 degradation. Chloroform is known to be toxic to aquatic organisms and can persist in the environment for extended periods. In aquatic ecosystems, it can accumulate in sediments and bioaccumulate in the food chain, potentially affecting a wide range of organisms from microorganisms to fish and higher-level predators.
The pH of the environment plays a crucial role in determining the degradation pathways and, consequently, the types and quantities of byproducts formed. In acidic conditions, the formation of phosgene (COCl2) may be favored, which is highly toxic and can have severe impacts on both aquatic and terrestrial ecosystems. Phosgene can react with water to form hydrochloric acid and carbon dioxide, further altering the local pH and potentially causing acidification of water bodies.
Under alkaline conditions, the degradation of CCl4 may lead to the formation of carbonate ions and chloride ions. While these ions are generally less toxic than chloroform or phosgene, they can still impact the chemical balance of ecosystems, particularly in freshwater environments where changes in ion concentrations can affect the osmotic balance of aquatic organisms.
The presence of CCl4 degradation byproducts can also indirectly affect ecosystems by altering microbial communities. Some microorganisms may be more tolerant to these byproducts, leading to shifts in community composition and potentially disrupting important ecological processes such as nutrient cycling and organic matter decomposition.
Furthermore, the persistence of CCl4 and its degradation byproducts in soil can impact plant growth and soil fertility. This can lead to changes in vegetation patterns and, consequently, affect the entire ecosystem structure, including habitat availability for various animal species.
In marine environments, the ecological impact of CCl4 degradation byproducts can be particularly concerning due to the potential for long-range transport and the sensitivity of many marine organisms to chemical contaminants. Coral reefs, for instance, are known to be highly susceptible to chemical pollutants and may suffer from exposure to CCl4 byproducts, potentially leading to bleaching events and reduced biodiversity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!