How Reactive Oxygen Species Are Involved in Carbon Tetrachloride Toxicity
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ROS in CCl4 Toxicity: Background and Objectives
Carbon tetrachloride (CCl4) has long been recognized as a potent hepatotoxin, with its toxicity primarily attributed to the generation of reactive oxygen species (ROS). This research aims to elucidate the intricate mechanisms by which ROS contribute to CCl4-induced liver damage, a critical area of study in toxicology and hepatology.
The historical context of CCl4 toxicity research dates back to the early 20th century when it was widely used as a solvent and fire extinguishing agent. However, its severe hepatotoxic effects led to its ban in consumer products. Understanding the role of ROS in CCl4 toxicity has evolved significantly over the past few decades, with advancements in molecular biology and biochemistry providing deeper insights into the underlying mechanisms.
ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radicals, are highly reactive molecules that can cause oxidative stress and cellular damage. In the context of CCl4 toxicity, these species are primarily generated through the metabolic activation of CCl4 by cytochrome P450 enzymes in the liver. This process leads to the formation of trichloromethyl radicals, which rapidly react with oxygen to produce more reactive species.
The objectives of this technical research report are multifaceted. Firstly, it aims to provide a comprehensive review of the current understanding of how ROS are involved in CCl4 toxicity. This includes examining the specific pathways of ROS generation, their interactions with cellular components, and the resulting cascade of events leading to liver injury.
Secondly, the report seeks to explore the latest advancements in detecting and quantifying ROS in biological systems, particularly in the context of CCl4 exposure. This involves evaluating cutting-edge techniques such as fluorescence-based assays, electron spin resonance spectroscopy, and redox-sensitive protein markers.
Furthermore, this research aims to investigate the potential protective mechanisms against CCl4-induced oxidative stress. This includes examining endogenous antioxidant systems and their regulation in response to CCl4 exposure, as well as exploring novel antioxidant therapies that could mitigate CCl4 toxicity.
Lastly, the report will address the broader implications of understanding ROS involvement in CCl4 toxicity. This includes its relevance to other hepatotoxic compounds, potential applications in drug development and toxicity screening, and implications for liver disease prevention and treatment strategies.
By comprehensively examining these aspects, this technical research report aims to provide valuable insights into the role of ROS in CCl4 toxicity, contributing to the advancement of toxicology research and potentially informing future therapeutic approaches for liver diseases.
The historical context of CCl4 toxicity research dates back to the early 20th century when it was widely used as a solvent and fire extinguishing agent. However, its severe hepatotoxic effects led to its ban in consumer products. Understanding the role of ROS in CCl4 toxicity has evolved significantly over the past few decades, with advancements in molecular biology and biochemistry providing deeper insights into the underlying mechanisms.
ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radicals, are highly reactive molecules that can cause oxidative stress and cellular damage. In the context of CCl4 toxicity, these species are primarily generated through the metabolic activation of CCl4 by cytochrome P450 enzymes in the liver. This process leads to the formation of trichloromethyl radicals, which rapidly react with oxygen to produce more reactive species.
The objectives of this technical research report are multifaceted. Firstly, it aims to provide a comprehensive review of the current understanding of how ROS are involved in CCl4 toxicity. This includes examining the specific pathways of ROS generation, their interactions with cellular components, and the resulting cascade of events leading to liver injury.
Secondly, the report seeks to explore the latest advancements in detecting and quantifying ROS in biological systems, particularly in the context of CCl4 exposure. This involves evaluating cutting-edge techniques such as fluorescence-based assays, electron spin resonance spectroscopy, and redox-sensitive protein markers.
Furthermore, this research aims to investigate the potential protective mechanisms against CCl4-induced oxidative stress. This includes examining endogenous antioxidant systems and their regulation in response to CCl4 exposure, as well as exploring novel antioxidant therapies that could mitigate CCl4 toxicity.
Lastly, the report will address the broader implications of understanding ROS involvement in CCl4 toxicity. This includes its relevance to other hepatotoxic compounds, potential applications in drug development and toxicity screening, and implications for liver disease prevention and treatment strategies.
By comprehensively examining these aspects, this technical research report aims to provide valuable insights into the role of ROS in CCl4 toxicity, contributing to the advancement of toxicology research and potentially informing future therapeutic approaches for liver diseases.
Market Demand for CCl4 Toxicity Research
The market demand for research into carbon tetrachloride (CCl4) toxicity, particularly focusing on the role of reactive oxygen species (ROS), has been steadily increasing in recent years. This growing interest is driven by several factors, including heightened environmental awareness, stricter regulatory requirements, and the ongoing need for safer industrial practices.
Environmental concerns have become a significant driver for CCl4 toxicity research. As global efforts to reduce pollution and protect ecosystems intensify, there is an increased focus on understanding the environmental impact of industrial chemicals. CCl4, once widely used in various applications, has been identified as a potent environmental pollutant. The demand for research into its toxicity mechanisms, especially the involvement of ROS, has grown as industries and regulators seek to mitigate its environmental effects and develop safer alternatives.
The healthcare sector represents another substantial market for CCl4 toxicity research. With liver damage being a primary concern in CCl4 exposure, medical professionals and pharmaceutical companies are increasingly interested in understanding the role of ROS in this process. This knowledge is crucial for developing more effective treatments for liver diseases and improving diagnostic methods for chemical-induced liver injuries.
Regulatory bodies worldwide have been tightening controls on the use and disposal of CCl4, creating a robust market for toxicity research. Companies involved in the production, use, or disposal of CCl4 are now required to provide more comprehensive safety data, including detailed information on toxicity mechanisms. This regulatory pressure has led to increased investment in research, particularly in understanding how ROS contribute to CCl4 toxicity.
The occupational health and safety sector also contributes significantly to the market demand for CCl4 toxicity research. As workplace safety standards continue to evolve, there is a growing need for detailed understanding of chemical hazards. Research into the ROS-mediated toxicity of CCl4 is crucial for developing better protective measures and exposure limits for workers in industries where CCl4 is still used or produced as a byproduct.
Academic institutions and research organizations form another key market segment. The complex nature of CCl4 toxicity and the involvement of ROS present numerous opportunities for scientific exploration and publication. This academic interest not only drives fundamental research but also supports the development of new analytical techniques and methodologies for studying chemical toxicity.
In conclusion, the market demand for research into CCl4 toxicity, particularly regarding the role of ROS, is multifaceted and growing. It spans environmental protection, healthcare, regulatory compliance, occupational safety, and academic research. This diverse demand is likely to continue driving investment and innovation in this field for the foreseeable future.
Environmental concerns have become a significant driver for CCl4 toxicity research. As global efforts to reduce pollution and protect ecosystems intensify, there is an increased focus on understanding the environmental impact of industrial chemicals. CCl4, once widely used in various applications, has been identified as a potent environmental pollutant. The demand for research into its toxicity mechanisms, especially the involvement of ROS, has grown as industries and regulators seek to mitigate its environmental effects and develop safer alternatives.
The healthcare sector represents another substantial market for CCl4 toxicity research. With liver damage being a primary concern in CCl4 exposure, medical professionals and pharmaceutical companies are increasingly interested in understanding the role of ROS in this process. This knowledge is crucial for developing more effective treatments for liver diseases and improving diagnostic methods for chemical-induced liver injuries.
Regulatory bodies worldwide have been tightening controls on the use and disposal of CCl4, creating a robust market for toxicity research. Companies involved in the production, use, or disposal of CCl4 are now required to provide more comprehensive safety data, including detailed information on toxicity mechanisms. This regulatory pressure has led to increased investment in research, particularly in understanding how ROS contribute to CCl4 toxicity.
The occupational health and safety sector also contributes significantly to the market demand for CCl4 toxicity research. As workplace safety standards continue to evolve, there is a growing need for detailed understanding of chemical hazards. Research into the ROS-mediated toxicity of CCl4 is crucial for developing better protective measures and exposure limits for workers in industries where CCl4 is still used or produced as a byproduct.
Academic institutions and research organizations form another key market segment. The complex nature of CCl4 toxicity and the involvement of ROS present numerous opportunities for scientific exploration and publication. This academic interest not only drives fundamental research but also supports the development of new analytical techniques and methodologies for studying chemical toxicity.
In conclusion, the market demand for research into CCl4 toxicity, particularly regarding the role of ROS, is multifaceted and growing. It spans environmental protection, healthcare, regulatory compliance, occupational safety, and academic research. This diverse demand is likely to continue driving investment and innovation in this field for the foreseeable future.
Current Understanding of ROS in CCl4-Induced Damage
The current understanding of reactive oxygen species (ROS) in carbon tetrachloride (CCl4)-induced damage has significantly advanced in recent years. CCl4 is a well-known hepatotoxin that has been extensively studied as a model compound for liver injury. Its toxicity is primarily mediated through the generation of ROS, which play a crucial role in the pathogenesis of CCl4-induced liver damage.
Upon entering the body, CCl4 is metabolized by cytochrome P450 enzymes, particularly CYP2E1, in the endoplasmic reticulum of hepatocytes. This biotransformation process results in the formation of the trichloromethyl radical (CCl3•), which rapidly reacts with oxygen to form the highly reactive trichloromethyl peroxy radical (CCl3OO•). These free radicals initiate a cascade of events leading to oxidative stress and cellular damage.
The generated ROS, including superoxide anion (O2•-), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), cause lipid peroxidation of cellular membranes. This process disrupts membrane integrity and leads to the release of pro-inflammatory mediators. Additionally, ROS directly damage cellular proteins and DNA, further compromising cellular function and viability.
Research has shown that CCl4-induced ROS production triggers the activation of various stress-responsive pathways, including nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs). These signaling cascades contribute to the inflammatory response and the progression of liver injury. Moreover, ROS-mediated oxidative stress has been linked to the induction of apoptosis in hepatocytes, a key feature of CCl4-induced liver damage.
Recent studies have also highlighted the role of ROS in modulating the balance between pro-oxidant and antioxidant systems in the liver. CCl4 exposure has been shown to deplete cellular antioxidants such as glutathione (GSH) and impair the activity of antioxidant enzymes like superoxide dismutase (SOD) and catalase. This disruption of the cellular redox balance further exacerbates oxidative stress and tissue damage.
Furthermore, the involvement of ROS in CCl4-induced fibrogenesis has gained attention. Oxidative stress activates hepatic stellate cells, promoting their transformation into myofibroblasts and increasing collagen production. This process contributes to the development of liver fibrosis, a hallmark of chronic CCl4 exposure.
Recent research has also explored the potential of various antioxidant interventions in mitigating CCl4-induced liver injury. Natural compounds with antioxidant properties, such as flavonoids and polyphenols, have shown promise in attenuating ROS-mediated damage and improving liver function in experimental models of CCl4 toxicity.
Upon entering the body, CCl4 is metabolized by cytochrome P450 enzymes, particularly CYP2E1, in the endoplasmic reticulum of hepatocytes. This biotransformation process results in the formation of the trichloromethyl radical (CCl3•), which rapidly reacts with oxygen to form the highly reactive trichloromethyl peroxy radical (CCl3OO•). These free radicals initiate a cascade of events leading to oxidative stress and cellular damage.
The generated ROS, including superoxide anion (O2•-), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), cause lipid peroxidation of cellular membranes. This process disrupts membrane integrity and leads to the release of pro-inflammatory mediators. Additionally, ROS directly damage cellular proteins and DNA, further compromising cellular function and viability.
Research has shown that CCl4-induced ROS production triggers the activation of various stress-responsive pathways, including nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs). These signaling cascades contribute to the inflammatory response and the progression of liver injury. Moreover, ROS-mediated oxidative stress has been linked to the induction of apoptosis in hepatocytes, a key feature of CCl4-induced liver damage.
Recent studies have also highlighted the role of ROS in modulating the balance between pro-oxidant and antioxidant systems in the liver. CCl4 exposure has been shown to deplete cellular antioxidants such as glutathione (GSH) and impair the activity of antioxidant enzymes like superoxide dismutase (SOD) and catalase. This disruption of the cellular redox balance further exacerbates oxidative stress and tissue damage.
Furthermore, the involvement of ROS in CCl4-induced fibrogenesis has gained attention. Oxidative stress activates hepatic stellate cells, promoting their transformation into myofibroblasts and increasing collagen production. This process contributes to the development of liver fibrosis, a hallmark of chronic CCl4 exposure.
Recent research has also explored the potential of various antioxidant interventions in mitigating CCl4-induced liver injury. Natural compounds with antioxidant properties, such as flavonoids and polyphenols, have shown promise in attenuating ROS-mediated damage and improving liver function in experimental models of CCl4 toxicity.
Mechanisms of ROS Generation by CCl4
01 Antioxidant compounds for reducing ROS toxicity
Various antioxidant compounds can be used to mitigate the toxic effects of reactive oxygen species (ROS). These compounds work by neutralizing free radicals and preventing oxidative damage to cellular components. Antioxidants can be derived from natural sources or synthesized chemically, and they play a crucial role in protecting cells from ROS-induced stress and damage.- Antioxidant compounds for reducing ROS toxicity: Various antioxidant compounds can be used to mitigate the toxic effects of reactive oxygen species (ROS). These compounds work by neutralizing free radicals and preventing oxidative stress in cells. Natural and synthetic antioxidants can be incorporated into formulations to protect against ROS-induced damage in biological systems.
- Nanoparticle-based ROS scavenging systems: Nanoparticle-based systems have been developed to effectively scavenge reactive oxygen species. These nanoparticles can be engineered to target specific cellular compartments or tissues, providing localized protection against ROS toxicity. The high surface area and customizable properties of nanoparticles make them promising candidates for ROS detoxification.
- Peptide and protein-based ROS modulators: Specific peptides and proteins have been identified or engineered to modulate ROS levels in biological systems. These biomolecules can either directly neutralize ROS or enhance the body's natural antioxidant defense mechanisms. Some peptides may also help in repairing ROS-induced cellular damage.
- Gene therapy approaches for ROS toxicity prevention: Gene therapy techniques are being explored to enhance cellular resistance to ROS toxicity. This involves introducing genes that code for antioxidant enzymes or proteins that can regulate ROS levels. By modifying the genetic makeup of cells, it may be possible to create long-lasting protection against oxidative stress.
- Plant-derived compounds for ROS detoxification: Natural compounds derived from plants have shown promise in combating ROS toxicity. These phytochemicals, including flavonoids, polyphenols, and terpenes, possess strong antioxidant properties. Extracts or purified compounds from various plant sources can be formulated into products aimed at reducing oxidative stress and ROS-induced cellular damage.
02 Nanoparticle-based ROS scavenging systems
Nanoparticles can be engineered to act as efficient ROS scavengers, reducing oxidative stress and toxicity in biological systems. These nanoparticles can be designed with specific surface properties and functionalities to target and neutralize various types of ROS. The use of nanoparticle-based systems offers advantages such as improved cellular uptake and prolonged antioxidant activity.Expand Specific Solutions03 Peptide-based approaches for ROS detoxification
Specific peptides can be designed and synthesized to combat ROS toxicity. These peptides may possess antioxidant properties or act as mimetics of natural antioxidant enzymes. Peptide-based approaches offer the advantage of high specificity and potential for targeted delivery to cellular compartments prone to ROS damage.Expand Specific Solutions04 Gene therapy strategies for enhancing cellular ROS defense
Gene therapy approaches can be employed to enhance the cellular defense mechanisms against ROS toxicity. This may involve the overexpression of antioxidant enzymes or the introduction of genes that regulate cellular redox balance. By modifying the genetic makeup of cells, their resilience to oxidative stress can be improved, potentially mitigating ROS-induced damage.Expand Specific Solutions05 Plant-derived compounds for ROS protection
Natural compounds derived from plants have shown promise in protecting against ROS toxicity. These phytochemicals, including flavonoids, polyphenols, and terpenes, possess antioxidant properties and can help neutralize harmful free radicals. Extracts or purified compounds from various plant sources can be utilized to develop novel therapeutic strategies for combating oxidative stress.Expand Specific Solutions
Key Players in CCl4 and ROS Research
The competitive landscape for research on reactive oxygen species (ROS) involvement in carbon tetrachloride toxicity is in a mature stage, with established market players and ongoing research efforts. The market size is significant, given the importance of understanding toxicity mechanisms for drug development and environmental safety. Technologically, the field is well-developed, with companies like Quark Pharmaceuticals and Silence Therapeutics leading in RNAi-based approaches. Academic institutions such as Maastricht University and the University of Tokyo contribute substantially to basic research. Pharmaceutical giants like Vertex Pharmaceuticals and MedImmune bring considerable resources to translational research, while specialized firms like Clean Chemistry focus on applied technologies for oxidant management.
University of South Alabama
Technical Solution: The University of South Alabama has conducted extensive research on the mechanisms of carbon tetrachloride (CCl4) toxicity, with a particular emphasis on the role of reactive oxygen species (ROS). Their studies have revealed that CCl4-induced ROS generation leads to lipid peroxidation and protein oxidation, ultimately resulting in hepatocellular damage[13]. To combat these effects, the research team has developed a multi-pronged approach. Firstly, they have explored the use of natural antioxidants, such as polyphenols and flavonoids, which have shown promising results in scavenging ROS and reducing oxidative stress in CCl4-exposed liver cells[14]. Secondly, the university's scientists have investigated the potential of modulating cellular antioxidant defense systems, particularly through the activation of the Nrf2-Keap1 pathway, which regulates the expression of numerous cytoprotective genes[15]. Additionally, they have studied the role of mitochondrial dysfunction in CCl4 toxicity and have developed strategies to preserve mitochondrial integrity and function under oxidative stress conditions.
Strengths: Utilization of natural compounds with potentially fewer side effects; Focus on enhancing endogenous antioxidant defenses; Comprehensive approach addressing multiple aspects of CCl4-induced oxidative stress. Weaknesses: Natural antioxidants may have limited bioavailability and efficacy compared to synthetic compounds; Potential for variability in the potency of natural extracts.
Vertex Pharmaceuticals, Inc.
Technical Solution: Vertex Pharmaceuticals has developed a novel approach to mitigate carbon tetrachloride (CCl4) toxicity by targeting reactive oxygen species (ROS) production. Their strategy involves the use of small molecule inhibitors that specifically block the cytochrome P450 enzymes responsible for CCl4 metabolism and subsequent ROS generation[1]. This approach not only reduces the formation of trichloromethyl radicals but also decreases lipid peroxidation and oxidative stress in hepatocytes. The company has also explored the potential of antioxidant therapy, incorporating a proprietary blend of antioxidants that can effectively scavenge ROS and protect cellular components from oxidative damage[3]. Additionally, Vertex has investigated the role of mitochondrial dysfunction in CCl4-induced toxicity and developed compounds that can preserve mitochondrial integrity and function under oxidative stress conditions[5].
Strengths: Targeted approach to prevent ROS formation at the source; Comprehensive strategy addressing multiple aspects of CCl4 toxicity; Potential for broader applications in other chemical-induced liver injuries. Weaknesses: May not fully address secondary mechanisms of CCl4 toxicity; Potential for drug-drug interactions due to cytochrome P450 inhibition.
Critical Findings on ROS-Mediated CCl4 Toxicity
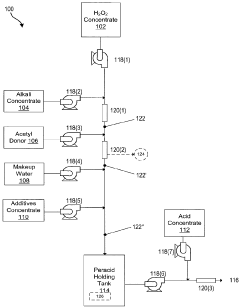
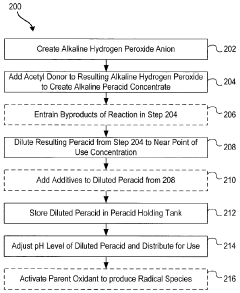
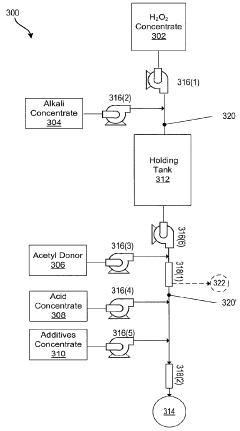
Systems and methods for generation of reactive oxygen species and applications thereof
PatentActiveCA2900460C
Innovation
- Development of formulations that generate reactive oxygen species in situ through pH adjustments, temperature changes, irradiation, or addition of precursors, allowing for the combination of different reactive species to enhance activity and stability, and the use of electrochemical methods to produce reactive oxygen species formulations that are active for extended periods without excessive hydrogen peroxide residual.
Nucleotide sequences and corresponding polypeptides conferring modulated growth rate and biomass in plants grown in saline and oxidative conditions
PatentActiveUS20230132139A1
Innovation
- Introduction of transgenic plants with modulated levels of tolerance to salinity and oxidative stress through the expression of recombinant DNA molecules encoding specific polypeptides, which are introduced into plant cells using exogenous nucleic acids linked to regulatory regions, enhancing their ability to withstand stress conditions.
Regulatory Framework for CCl4 Usage and Exposure
The regulatory framework for carbon tetrachloride (CCl4) usage and exposure has evolved significantly over the past few decades, reflecting growing concerns about its toxicity and environmental impact. In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations under the Toxic Substances Control Act (TSCA) and the Clean Air Act to control CCl4 production, use, and disposal.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for CCl4 in workplace settings. The current PEL is set at 10 ppm as an 8-hour time-weighted average, with a ceiling limit of 25 ppm. These limits aim to protect workers from the acute and chronic effects of CCl4 exposure, including liver and kidney damage.
Internationally, the Montreal Protocol on Substances that Deplete the Ozone Layer has played a crucial role in phasing out CCl4 production and consumption. As a result, global production of CCl4 has decreased significantly since the 1990s. However, atmospheric concentrations remain higher than expected, suggesting ongoing emissions from unreported sources or legacy contamination.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to assess and manage the risks associated with CCl4. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and environmental persistence.
In developing countries, regulatory frameworks for CCl4 are often less comprehensive or poorly enforced. This disparity in global regulations has led to concerns about the potential for CCl4 production and use to shift to regions with less stringent controls, highlighting the need for harmonized international standards.
Recent scientific evidence on the role of reactive oxygen species in CCl4 toxicity has prompted regulatory bodies to reassess exposure limits and risk assessment methodologies. The EPA and other agencies are now considering incorporating mechanistic data on oxidative stress into their regulatory decision-making processes for CCl4 and similar compounds.
As research continues to elucidate the complex mechanisms of CCl4 toxicity, regulatory frameworks are likely to evolve further. Future regulations may focus on more targeted interventions based on specific cellular and molecular pathways involved in CCl4-induced oxidative damage, potentially leading to more effective risk management strategies and improved public health outcomes.
The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for CCl4 in workplace settings. The current PEL is set at 10 ppm as an 8-hour time-weighted average, with a ceiling limit of 25 ppm. These limits aim to protect workers from the acute and chronic effects of CCl4 exposure, including liver and kidney damage.
Internationally, the Montreal Protocol on Substances that Deplete the Ozone Layer has played a crucial role in phasing out CCl4 production and consumption. As a result, global production of CCl4 has decreased significantly since the 1990s. However, atmospheric concentrations remain higher than expected, suggesting ongoing emissions from unreported sources or legacy contamination.
The European Union has implemented REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulations, which require manufacturers and importers to assess and manage the risks associated with CCl4. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and environmental persistence.
In developing countries, regulatory frameworks for CCl4 are often less comprehensive or poorly enforced. This disparity in global regulations has led to concerns about the potential for CCl4 production and use to shift to regions with less stringent controls, highlighting the need for harmonized international standards.
Recent scientific evidence on the role of reactive oxygen species in CCl4 toxicity has prompted regulatory bodies to reassess exposure limits and risk assessment methodologies. The EPA and other agencies are now considering incorporating mechanistic data on oxidative stress into their regulatory decision-making processes for CCl4 and similar compounds.
As research continues to elucidate the complex mechanisms of CCl4 toxicity, regulatory frameworks are likely to evolve further. Future regulations may focus on more targeted interventions based on specific cellular and molecular pathways involved in CCl4-induced oxidative damage, potentially leading to more effective risk management strategies and improved public health outcomes.
Antioxidant Strategies for CCl4 Toxicity Mitigation
Antioxidant strategies play a crucial role in mitigating carbon tetrachloride (CCl4) toxicity by counteracting the harmful effects of reactive oxygen species (ROS) generated during CCl4 metabolism. These strategies can be broadly categorized into endogenous and exogenous approaches, each targeting different aspects of the oxidative stress cascade.
Endogenous antioxidant systems, including enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), form the first line of defense against CCl4-induced oxidative damage. Enhancing the activity and expression of these enzymes through genetic modulation or pharmacological induction has shown promising results in experimental models of CCl4 toxicity.
Non-enzymatic antioxidants, such as glutathione (GSH), also play a vital role in cellular defense. Strategies aimed at increasing GSH levels, either through direct supplementation or by enhancing its synthesis, have demonstrated significant protective effects against CCl4-induced liver injury.
Exogenous antioxidants, including vitamins C and E, carotenoids, and polyphenols, offer another avenue for combating CCl4 toxicity. These compounds can directly scavenge free radicals, chelate metal ions involved in ROS generation, and support the regeneration of endogenous antioxidants.
Plant-derived antioxidants have gained considerable attention due to their diverse bioactive compounds and potential synergistic effects. Extracts from various plants, such as silymarin from milk thistle and curcumin from turmeric, have shown hepatoprotective effects against CCl4-induced damage in preclinical studies.
Nanotechnology-based approaches are emerging as innovative strategies for antioxidant delivery. Nanocarriers loaded with antioxidants can enhance bioavailability, improve targeting to affected tissues, and provide sustained release, potentially increasing the efficacy of antioxidant interventions in CCl4 toxicity.
Combination therapies that target multiple aspects of the oxidative stress pathway have shown promise in recent research. These approaches may include combining different classes of antioxidants or pairing antioxidants with agents that modulate cellular signaling pathways involved in the oxidative stress response.
While antioxidant strategies have demonstrated significant potential in mitigating CCl4 toxicity, it is important to note that the optimal approach may vary depending on the specific context of exposure and individual factors. Future research should focus on developing personalized antioxidant interventions and exploring novel delivery methods to maximize therapeutic efficacy while minimizing potential side effects.
Endogenous antioxidant systems, including enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), form the first line of defense against CCl4-induced oxidative damage. Enhancing the activity and expression of these enzymes through genetic modulation or pharmacological induction has shown promising results in experimental models of CCl4 toxicity.
Non-enzymatic antioxidants, such as glutathione (GSH), also play a vital role in cellular defense. Strategies aimed at increasing GSH levels, either through direct supplementation or by enhancing its synthesis, have demonstrated significant protective effects against CCl4-induced liver injury.
Exogenous antioxidants, including vitamins C and E, carotenoids, and polyphenols, offer another avenue for combating CCl4 toxicity. These compounds can directly scavenge free radicals, chelate metal ions involved in ROS generation, and support the regeneration of endogenous antioxidants.
Plant-derived antioxidants have gained considerable attention due to their diverse bioactive compounds and potential synergistic effects. Extracts from various plants, such as silymarin from milk thistle and curcumin from turmeric, have shown hepatoprotective effects against CCl4-induced damage in preclinical studies.
Nanotechnology-based approaches are emerging as innovative strategies for antioxidant delivery. Nanocarriers loaded with antioxidants can enhance bioavailability, improve targeting to affected tissues, and provide sustained release, potentially increasing the efficacy of antioxidant interventions in CCl4 toxicity.
Combination therapies that target multiple aspects of the oxidative stress pathway have shown promise in recent research. These approaches may include combining different classes of antioxidants or pairing antioxidants with agents that modulate cellular signaling pathways involved in the oxidative stress response.
While antioxidant strategies have demonstrated significant potential in mitigating CCl4 toxicity, it is important to note that the optimal approach may vary depending on the specific context of exposure and individual factors. Future research should focus on developing personalized antioxidant interventions and exploring novel delivery methods to maximize therapeutic efficacy while minimizing potential side effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!