Exploring Carbon Tetrachloride Degradation Pathways in Water Bodies
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Degradation Background and Objectives
Carbon tetrachloride (CCl4) degradation in water bodies has been a subject of significant environmental concern and scientific research for decades. This persistent organic pollutant, once widely used in various industrial applications, has become a global environmental issue due to its toxicity, long-term stability, and potential for bioaccumulation. The historical use of CCl4 in refrigerants, fire extinguishers, and as a solvent has led to its widespread distribution in aquatic ecosystems worldwide.
The evolution of CCl4 degradation research has closely followed the growing awareness of its environmental impact. Initial studies in the 1970s and 1980s focused primarily on identifying the presence and distribution of CCl4 in water bodies. As analytical techniques improved, researchers began to investigate the natural attenuation processes and potential degradation pathways of CCl4 in aquatic environments.
The technological progression in this field has been marked by significant milestones. The development of advanced chromatographic and spectroscopic methods enabled more accurate detection and quantification of CCl4 and its degradation products. Isotope fractionation techniques emerged as powerful tools for tracing the fate of CCl4 in complex environmental matrices. More recently, molecular biology approaches have shed light on the microbial communities involved in CCl4 degradation.
The primary objective of current research in CCl4 degradation is to develop comprehensive understanding of the various pathways through which this compound breaks down in water bodies. This includes elucidating both abiotic and biotic degradation mechanisms, identifying key intermediates and end products, and quantifying the rates of these processes under different environmental conditions.
Another crucial aim is to assess the potential for enhancing natural attenuation processes or implementing active remediation strategies. This involves exploring innovative technologies such as in situ chemical reduction, advanced oxidation processes, and bioaugmentation with specialized microbial consortia. The ultimate goal is to develop cost-effective and environmentally friendly methods for accelerating CCl4 degradation in contaminated water bodies.
Furthermore, researchers are striving to understand the broader ecological implications of CCl4 degradation. This includes investigating the potential formation of toxic byproducts, the impact on aquatic ecosystems, and the long-term fate of degradation products in the environment. By addressing these objectives, scientists aim to provide a solid foundation for risk assessment and informed decision-making in environmental management and remediation efforts.
The evolution of CCl4 degradation research has closely followed the growing awareness of its environmental impact. Initial studies in the 1970s and 1980s focused primarily on identifying the presence and distribution of CCl4 in water bodies. As analytical techniques improved, researchers began to investigate the natural attenuation processes and potential degradation pathways of CCl4 in aquatic environments.
The technological progression in this field has been marked by significant milestones. The development of advanced chromatographic and spectroscopic methods enabled more accurate detection and quantification of CCl4 and its degradation products. Isotope fractionation techniques emerged as powerful tools for tracing the fate of CCl4 in complex environmental matrices. More recently, molecular biology approaches have shed light on the microbial communities involved in CCl4 degradation.
The primary objective of current research in CCl4 degradation is to develop comprehensive understanding of the various pathways through which this compound breaks down in water bodies. This includes elucidating both abiotic and biotic degradation mechanisms, identifying key intermediates and end products, and quantifying the rates of these processes under different environmental conditions.
Another crucial aim is to assess the potential for enhancing natural attenuation processes or implementing active remediation strategies. This involves exploring innovative technologies such as in situ chemical reduction, advanced oxidation processes, and bioaugmentation with specialized microbial consortia. The ultimate goal is to develop cost-effective and environmentally friendly methods for accelerating CCl4 degradation in contaminated water bodies.
Furthermore, researchers are striving to understand the broader ecological implications of CCl4 degradation. This includes investigating the potential formation of toxic byproducts, the impact on aquatic ecosystems, and the long-term fate of degradation products in the environment. By addressing these objectives, scientists aim to provide a solid foundation for risk assessment and informed decision-making in environmental management and remediation efforts.
Environmental Impact and Remediation Demand
Carbon tetrachloride (CCl4) contamination in water bodies poses significant environmental and health risks, necessitating urgent remediation efforts. The persistent nature of CCl4 in aquatic ecosystems has led to widespread concern among environmental scientists and policymakers. Its presence in water sources can lead to long-term ecological damage and potential human health hazards through exposure to contaminated drinking water.
The environmental impact of CCl4 is multifaceted, affecting both aquatic life and surrounding ecosystems. In water bodies, CCl4 can disrupt the natural balance of microbial communities, potentially altering nutrient cycles and food webs. Fish and other aquatic organisms may suffer from chronic exposure, leading to reduced populations and biodiversity loss. Furthermore, CCl4 can accumulate in sediments, creating a long-term source of contamination that continues to affect water quality even after initial remediation efforts.
The demand for effective remediation strategies is driven by both environmental protection goals and public health concerns. Regulatory agencies worldwide have established strict guidelines for CCl4 levels in drinking water and environmental waters, reflecting the urgency of addressing this contaminant. In the United States, for instance, the Environmental Protection Agency (EPA) has set a maximum contaminant level goal of zero for CCl4 in drinking water, underscoring the importance of complete removal.
Remediation efforts face several challenges, including the chemical stability of CCl4 and its tendency to form dense non-aqueous phase liquids (DNAPLs) in groundwater. Traditional pump-and-treat methods have shown limited effectiveness, prompting research into more innovative approaches. In-situ remediation techniques, such as chemical reduction, bioremediation, and thermal treatment, are being explored as potentially more efficient and cost-effective solutions.
The global market for water remediation technologies is expanding rapidly, driven in part by the need to address CCl4 and similar contaminants. This growth is fueled by increasing environmental regulations, public awareness of water quality issues, and technological advancements in treatment methods. Developing countries, in particular, are seeing a surge in demand for water remediation services as they grapple with industrial pollution and the need to ensure safe drinking water supplies.
As research continues to uncover the full extent of CCl4 contamination and its impacts, the demand for remediation is expected to grow. This presents both challenges and opportunities for environmental technology firms, water treatment companies, and research institutions. Collaborative efforts between academia, industry, and government agencies are likely to play a crucial role in developing and implementing effective strategies for CCl4 degradation in water bodies, ultimately contributing to improved environmental quality and public health outcomes.
The environmental impact of CCl4 is multifaceted, affecting both aquatic life and surrounding ecosystems. In water bodies, CCl4 can disrupt the natural balance of microbial communities, potentially altering nutrient cycles and food webs. Fish and other aquatic organisms may suffer from chronic exposure, leading to reduced populations and biodiversity loss. Furthermore, CCl4 can accumulate in sediments, creating a long-term source of contamination that continues to affect water quality even after initial remediation efforts.
The demand for effective remediation strategies is driven by both environmental protection goals and public health concerns. Regulatory agencies worldwide have established strict guidelines for CCl4 levels in drinking water and environmental waters, reflecting the urgency of addressing this contaminant. In the United States, for instance, the Environmental Protection Agency (EPA) has set a maximum contaminant level goal of zero for CCl4 in drinking water, underscoring the importance of complete removal.
Remediation efforts face several challenges, including the chemical stability of CCl4 and its tendency to form dense non-aqueous phase liquids (DNAPLs) in groundwater. Traditional pump-and-treat methods have shown limited effectiveness, prompting research into more innovative approaches. In-situ remediation techniques, such as chemical reduction, bioremediation, and thermal treatment, are being explored as potentially more efficient and cost-effective solutions.
The global market for water remediation technologies is expanding rapidly, driven in part by the need to address CCl4 and similar contaminants. This growth is fueled by increasing environmental regulations, public awareness of water quality issues, and technological advancements in treatment methods. Developing countries, in particular, are seeing a surge in demand for water remediation services as they grapple with industrial pollution and the need to ensure safe drinking water supplies.
As research continues to uncover the full extent of CCl4 contamination and its impacts, the demand for remediation is expected to grow. This presents both challenges and opportunities for environmental technology firms, water treatment companies, and research institutions. Collaborative efforts between academia, industry, and government agencies are likely to play a crucial role in developing and implementing effective strategies for CCl4 degradation in water bodies, ultimately contributing to improved environmental quality and public health outcomes.
Current Challenges in CCl4 Degradation
The degradation of carbon tetrachloride (CCl4) in water bodies presents several significant challenges that hinder effective remediation efforts. One of the primary obstacles is the high chemical stability of CCl4, which makes it resistant to natural degradation processes. This stability is attributed to the strong carbon-chlorine bonds, requiring substantial energy input for cleavage.
Another major challenge is the low solubility of CCl4 in water, which limits its bioavailability for microbial degradation. This property also contributes to the formation of dense non-aqueous phase liquids (DNAPLs) in contaminated sites, making it difficult to locate and treat the source of pollution effectively.
The anaerobic conditions prevalent in many water bodies further complicate CCl4 degradation. While anaerobic degradation pathways exist, they often lead to the formation of more toxic intermediates, such as chloroform and dichloromethane. These byproducts can pose additional environmental and health risks, necessitating careful monitoring and management of degradation processes.
The presence of co-contaminants in water bodies can also interfere with CCl4 degradation. Competitive inhibition or preferential degradation of other pollutants may occur, reducing the efficiency of CCl4 removal. Additionally, the complex interactions between CCl4 and various environmental factors, such as pH, temperature, and the presence of natural organic matter, can significantly influence degradation rates and pathways.
A significant technical challenge lies in developing effective in situ remediation technologies for CCl4 contamination. Current methods, such as pump-and-treat systems, often have limited success due to the aforementioned challenges. Advanced oxidation processes and reductive dechlorination techniques show promise but face obstacles in field-scale implementation and long-term sustainability.
The accurate detection and quantification of CCl4 and its degradation products in water bodies also present challenges. Conventional analytical methods may not be sensitive enough to detect low concentrations, while more advanced techniques can be costly and time-consuming. This limitation hampers the assessment of degradation progress and the effectiveness of remediation strategies.
Lastly, the potential for long-range transport of CCl4 through groundwater and surface water systems poses challenges for containment and treatment. The persistence of CCl4 in the environment means that contamination can spread over large areas, making it difficult to identify and address all sources of pollution effectively.
Another major challenge is the low solubility of CCl4 in water, which limits its bioavailability for microbial degradation. This property also contributes to the formation of dense non-aqueous phase liquids (DNAPLs) in contaminated sites, making it difficult to locate and treat the source of pollution effectively.
The anaerobic conditions prevalent in many water bodies further complicate CCl4 degradation. While anaerobic degradation pathways exist, they often lead to the formation of more toxic intermediates, such as chloroform and dichloromethane. These byproducts can pose additional environmental and health risks, necessitating careful monitoring and management of degradation processes.
The presence of co-contaminants in water bodies can also interfere with CCl4 degradation. Competitive inhibition or preferential degradation of other pollutants may occur, reducing the efficiency of CCl4 removal. Additionally, the complex interactions between CCl4 and various environmental factors, such as pH, temperature, and the presence of natural organic matter, can significantly influence degradation rates and pathways.
A significant technical challenge lies in developing effective in situ remediation technologies for CCl4 contamination. Current methods, such as pump-and-treat systems, often have limited success due to the aforementioned challenges. Advanced oxidation processes and reductive dechlorination techniques show promise but face obstacles in field-scale implementation and long-term sustainability.
The accurate detection and quantification of CCl4 and its degradation products in water bodies also present challenges. Conventional analytical methods may not be sensitive enough to detect low concentrations, while more advanced techniques can be costly and time-consuming. This limitation hampers the assessment of degradation progress and the effectiveness of remediation strategies.
Lastly, the potential for long-range transport of CCl4 through groundwater and surface water systems poses challenges for containment and treatment. The persistence of CCl4 in the environment means that contamination can spread over large areas, making it difficult to identify and address all sources of pollution effectively.
Existing CCl4 Degradation Techniques
01 Biological degradation methods
Utilizing microorganisms or enzymes to break down carbon tetrachloride in contaminated environments. This approach involves the use of specific bacteria or fungi that can metabolize the compound, converting it into less harmful substances. The process can be enhanced by optimizing environmental conditions such as temperature, pH, and nutrient availability.- Biological degradation methods: Utilizing microorganisms or enzymes to break down carbon tetrachloride in contaminated environments. This approach involves the use of specific bacteria or fungi that can metabolize the compound, converting it into less harmful substances. The process can be enhanced by optimizing environmental conditions such as temperature, pH, and nutrient availability.
- Chemical reduction techniques: Employing chemical reducing agents to transform carbon tetrachloride into less toxic compounds. This method often involves the use of zero-valent metals, such as iron, or other reducing agents that can break the carbon-chlorine bonds. The process can be carried out in various environmental matrices, including soil and groundwater.
- Advanced oxidation processes: Utilizing advanced oxidation techniques to degrade carbon tetrachloride. These methods typically involve the generation of highly reactive species such as hydroxyl radicals, which can rapidly oxidize the contaminant. Techniques may include UV irradiation, ozonation, or the use of hydrogen peroxide in combination with catalysts.
- Thermal decomposition methods: Applying heat to break down carbon tetrachloride into simpler, less harmful compounds. This approach can involve incineration, pyrolysis, or other high-temperature treatments. The process may be conducted in specialized reactors or in situ for contaminated soil remediation.
- Catalytic degradation processes: Using catalysts to facilitate the breakdown of carbon tetrachloride under milder conditions. This method can involve heterogeneous or homogeneous catalysts that promote the cleavage of carbon-chlorine bonds. The process can be combined with other degradation techniques to enhance efficiency and reduce energy requirements.
02 Chemical reduction techniques
Employing chemical reducing agents to transform carbon tetrachloride into less toxic compounds. This method often involves the use of zero-valent metals, such as iron, or other reducing agents that can break the carbon-chlorine bonds. The process can be carried out in various environmental matrices, including soil and groundwater.Expand Specific Solutions03 Advanced oxidation processes
Utilizing advanced oxidation techniques to degrade carbon tetrachloride. These methods typically involve the generation of highly reactive species such as hydroxyl radicals, which can rapidly oxidize the compound. Techniques may include UV irradiation, ozonation, or the use of hydrogen peroxide in combination with catalysts.Expand Specific Solutions04 Thermal decomposition methods
Applying heat to break down carbon tetrachloride into simpler, less harmful compounds. This approach can involve various techniques such as incineration, pyrolysis, or plasma treatment. The high temperatures cause the molecule to dissociate, often resulting in the formation of carbon dioxide and hydrochloric acid.Expand Specific Solutions05 Catalytic degradation processes
Using catalysts to facilitate the breakdown of carbon tetrachloride under milder conditions. This method often involves the use of metal-based catalysts or nanoparticles that can promote the cleavage of carbon-chlorine bonds. The process can be carried out in both gas and liquid phases, depending on the specific application and catalyst used.Expand Specific Solutions
Key Players in CCl4 Remediation Research
The exploration of carbon tetrachloride degradation pathways in water bodies is currently in a developing stage, with growing interest due to environmental concerns. The market size for this research area is expanding as regulations on water quality become stricter globally. Technologically, the field is progressing, with various approaches being investigated. Companies like Jiangsu Lee & Man Chemical and Ion Exchange (India) Ltd. are actively involved in water treatment solutions, while research institutions such as Beijing University of Chemical Technology, Arizona State University, and Nanjing University are contributing to the advancement of degradation techniques. The competitive landscape is diverse, with both industrial and academic players working towards innovative solutions for this environmental challenge.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed an innovative approach for carbon tetrachloride (CCl4) degradation in water bodies using advanced oxidation processes (AOPs). Their method combines UV irradiation with hydrogen peroxide (H2O2) to generate highly reactive hydroxyl radicals. These radicals effectively break down CCl4 molecules into less harmful compounds. The university's research has shown that this AOP technique can achieve up to 95% CCl4 removal efficiency within 60 minutes of treatment[1]. Additionally, they have explored the use of nano-scale zero-valent iron (nZVI) particles as a catalyst to enhance the degradation process, resulting in improved reaction kinetics and higher overall removal rates[2].
Strengths: High removal efficiency, relatively quick treatment time, and potential for catalyst enhancement. Weaknesses: Energy-intensive UV irradiation, potential formation of toxic intermediates, and scalability challenges for large water bodies.
Nanjing University
Technical Solution: Nanjing University has pioneered a novel approach to CCl4 degradation in water using a combination of electrochemical and biological methods. Their system employs a bioelectrochemical reactor where electroactive microorganisms are cultivated on the cathode. These microorganisms catalyze the reductive dechlorination of CCl4 when an external voltage is applied. The process has demonstrated a CCl4 removal efficiency of up to 98% under optimized conditions[3]. Furthermore, the university has integrated this technology with a membrane filtration system to enhance the overall water treatment process, allowing for the simultaneous removal of degradation byproducts and other contaminants[4].
Strengths: High removal efficiency, potential for simultaneous treatment of multiple contaminants, and lower chemical input compared to traditional methods. Weaknesses: Complexity of maintaining optimal microbial communities, potential for electrode fouling, and higher initial setup costs.
Innovative CCl4 Degradation Mechanisms
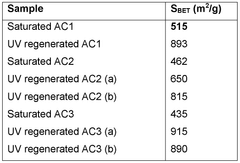
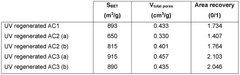
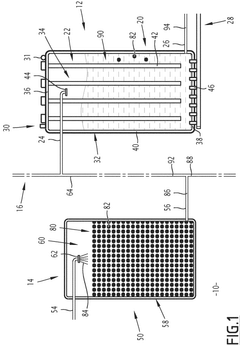
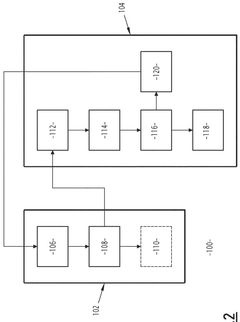
Water treatment method with regeneration of activated carbon particles by UV light
PatentWO2025088011A1
Innovation
- A single-step water treatment method that regenerates activated carbon particles by exposing them to UV light, utilizing the particles as photocatalysts to produce radical species that decompose organic molecules, thereby eliminating the need for additional reactive agents or high-temperature treatments.
Bioremediation method
PatentActiveUS20120070882A1
Innovation
- Enhancing the contaminated site with organic carbon sources like lactose and B-12-fortified brewer's yeast, nitrogen, phosphorus, and macro/micronutrients, including elemental sulfur and potassium oxide, to create bio-available hydrogen, which supports the growth of Dehaloccoides bacteria, facilitating effective cVOC degradation.
Regulatory Framework for CCl4 Remediation
The regulatory framework for Carbon Tetrachloride (CCl4) remediation is a complex and evolving landscape that encompasses international, national, and local regulations. At the global level, the Montreal Protocol on Substances that Deplete the Ozone Layer, which came into effect in 1989, has been instrumental in phasing out the production and consumption of CCl4. This international treaty has led to a significant reduction in CCl4 emissions worldwide, although its presence in water bodies remains a concern due to historical contamination and ongoing industrial processes.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating CCl4 remediation. The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), also known as Superfund, provides a federal framework for cleaning up contaminated sites, including those affected by CCl4. The EPA has established a maximum contaminant level (MCL) for CCl4 in drinking water at 0.005 mg/L, reflecting the agency's commitment to protecting public health from this toxic substance.
The Resource Conservation and Recovery Act (RCRA) further supports CCl4 remediation efforts by regulating the generation, transportation, treatment, storage, and disposal of hazardous waste. Under RCRA, CCl4 is classified as a hazardous waste, necessitating strict handling and disposal procedures to prevent environmental contamination.
At the state level, regulations may vary, with some states imposing more stringent standards than federal requirements. For instance, California's Proposition 65 requires businesses to provide warnings about significant exposures to chemicals that cause cancer, birth defects, or other reproductive harm, including CCl4.
Internationally, the European Union has implemented the Water Framework Directive, which aims to achieve good qualitative and quantitative status of all water bodies. This directive indirectly addresses CCl4 contamination by setting environmental quality standards for priority substances in surface waters.
The regulatory landscape also includes guidelines and best practices developed by environmental agencies and professional organizations. These documents often provide detailed methodologies for site assessment, remediation techniques, and long-term monitoring of CCl4-contaminated sites.
As scientific understanding of CCl4 degradation pathways in water bodies advances, regulatory frameworks are expected to evolve. This may lead to the development of more targeted and effective remediation strategies, as well as the potential for risk-based approaches that consider site-specific conditions and exposure pathways.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating CCl4 remediation. The Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), also known as Superfund, provides a federal framework for cleaning up contaminated sites, including those affected by CCl4. The EPA has established a maximum contaminant level (MCL) for CCl4 in drinking water at 0.005 mg/L, reflecting the agency's commitment to protecting public health from this toxic substance.
The Resource Conservation and Recovery Act (RCRA) further supports CCl4 remediation efforts by regulating the generation, transportation, treatment, storage, and disposal of hazardous waste. Under RCRA, CCl4 is classified as a hazardous waste, necessitating strict handling and disposal procedures to prevent environmental contamination.
At the state level, regulations may vary, with some states imposing more stringent standards than federal requirements. For instance, California's Proposition 65 requires businesses to provide warnings about significant exposures to chemicals that cause cancer, birth defects, or other reproductive harm, including CCl4.
Internationally, the European Union has implemented the Water Framework Directive, which aims to achieve good qualitative and quantitative status of all water bodies. This directive indirectly addresses CCl4 contamination by setting environmental quality standards for priority substances in surface waters.
The regulatory landscape also includes guidelines and best practices developed by environmental agencies and professional organizations. These documents often provide detailed methodologies for site assessment, remediation techniques, and long-term monitoring of CCl4-contaminated sites.
As scientific understanding of CCl4 degradation pathways in water bodies advances, regulatory frameworks are expected to evolve. This may lead to the development of more targeted and effective remediation strategies, as well as the potential for risk-based approaches that consider site-specific conditions and exposure pathways.
Ecological Implications of CCl4 Degradation
The degradation of carbon tetrachloride (CCl4) in water bodies has significant ecological implications that extend beyond the immediate chemical reactions. As CCl4 breaks down, it produces a variety of intermediate compounds and end products, each with its own potential impact on aquatic ecosystems.
One of the primary concerns is the formation of chloroform (CHCl3) as a byproduct of CCl4 degradation. Chloroform is known to be toxic to many aquatic organisms, potentially causing harm to fish, invertebrates, and microorganisms. This toxicity can lead to disruptions in the food chain and overall ecosystem balance.
The degradation process also releases chloride ions into the water, which can affect the salinity of freshwater systems. Increased salinity can stress aquatic plants and animals that are not adapted to higher salt concentrations, potentially altering species composition and biodiversity in affected areas.
Carbon dioxide (CO2) is another end product of CCl4 degradation. While CO2 is a natural component of aquatic systems, excessive amounts can lead to acidification of water bodies. This change in pH can have far-reaching effects on aquatic life, particularly on organisms with calcium carbonate shells or skeletons, such as mollusks and certain plankton species.
The presence of CCl4 and its degradation products can also impact microbial communities in water bodies. Some microorganisms may develop the ability to use CCl4 as a carbon source, potentially leading to shifts in microbial population dynamics. This could have cascading effects on nutrient cycling and other ecosystem processes mediated by microbes.
Furthermore, the persistence of CCl4 in sediments can create long-term sources of contamination. As sediments are disturbed or as conditions change, CCl4 can be re-released into the water column, prolonging its ecological impact and complicating remediation efforts.
The bioaccumulation of CCl4 and its degradation products in aquatic organisms is another significant concern. As these compounds move up the food chain, they can become concentrated in higher trophic levels, potentially affecting top predators and posing risks to human health through consumption of contaminated fish or other aquatic resources.
In conclusion, the ecological implications of CCl4 degradation in water bodies are complex and multifaceted. They encompass direct toxicity effects, changes in water chemistry, alterations to microbial communities, and potential long-term impacts on ecosystem structure and function. Understanding these implications is crucial for developing effective strategies to mitigate the environmental impact of CCl4 contamination and protect aquatic ecosystems.
One of the primary concerns is the formation of chloroform (CHCl3) as a byproduct of CCl4 degradation. Chloroform is known to be toxic to many aquatic organisms, potentially causing harm to fish, invertebrates, and microorganisms. This toxicity can lead to disruptions in the food chain and overall ecosystem balance.
The degradation process also releases chloride ions into the water, which can affect the salinity of freshwater systems. Increased salinity can stress aquatic plants and animals that are not adapted to higher salt concentrations, potentially altering species composition and biodiversity in affected areas.
Carbon dioxide (CO2) is another end product of CCl4 degradation. While CO2 is a natural component of aquatic systems, excessive amounts can lead to acidification of water bodies. This change in pH can have far-reaching effects on aquatic life, particularly on organisms with calcium carbonate shells or skeletons, such as mollusks and certain plankton species.
The presence of CCl4 and its degradation products can also impact microbial communities in water bodies. Some microorganisms may develop the ability to use CCl4 as a carbon source, potentially leading to shifts in microbial population dynamics. This could have cascading effects on nutrient cycling and other ecosystem processes mediated by microbes.
Furthermore, the persistence of CCl4 in sediments can create long-term sources of contamination. As sediments are disturbed or as conditions change, CCl4 can be re-released into the water column, prolonging its ecological impact and complicating remediation efforts.
The bioaccumulation of CCl4 and its degradation products in aquatic organisms is another significant concern. As these compounds move up the food chain, they can become concentrated in higher trophic levels, potentially affecting top predators and posing risks to human health through consumption of contaminated fish or other aquatic resources.
In conclusion, the ecological implications of CCl4 degradation in water bodies are complex and multifaceted. They encompass direct toxicity effects, changes in water chemistry, alterations to microbial communities, and potential long-term impacts on ecosystem structure and function. Understanding these implications is crucial for developing effective strategies to mitigate the environmental impact of CCl4 contamination and protect aquatic ecosystems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!