The Role of Carbon Tetrachloride in Organic Synthesis Reactions
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 in Organic Synthesis: Background and Objectives
Carbon tetrachloride (CCl4) has played a significant role in organic synthesis reactions since its discovery in the mid-19th century. This versatile compound has been widely utilized as a solvent, reagent, and intermediate in various chemical processes. Its unique properties, including high stability, non-flammability, and excellent solvating power, have made it an attractive choice for numerous applications in organic chemistry.
The evolution of CCl4 in organic synthesis can be traced back to its initial use as a non-polar solvent for extraction and purification processes. As researchers delved deeper into its reactivity, they discovered its potential as a source of chlorine atoms in radical reactions. This led to the development of numerous synthetic methodologies involving CCl4, particularly in the areas of chlorination, radical addition, and carbon-carbon bond formation.
One of the most notable applications of CCl4 in organic synthesis is the Appel reaction, discovered in 1975. This reaction utilizes CCl4 in combination with triphenylphosphine to convert alcohols into alkyl chlorides, providing a valuable tool for functional group interconversion. The Appel reaction has since become a cornerstone in many synthetic strategies, enabling the preparation of complex organic molecules and natural products.
The use of CCl4 in free radical reactions has also been extensively explored. Its ability to generate trichloromethyl radicals under various conditions has been exploited in the synthesis of chlorinated compounds, as well as in the initiation of radical polymerization processes. These applications have found widespread use in both academic research and industrial settings.
However, the recognition of CCl4's ozone-depleting properties and potential health hazards has led to a shift in research focus. In recent years, there has been a growing emphasis on developing alternative reagents and methodologies that can replace CCl4 in organic synthesis while maintaining or improving reaction efficiency and selectivity.
The primary objectives of current research in this field are twofold. First, there is a concerted effort to fully understand the mechanistic aspects of CCl4-mediated reactions, aiming to optimize existing processes and uncover new reactivity patterns. Second, researchers are actively seeking environmentally friendly alternatives that can mimic the reactivity of CCl4 without its associated drawbacks.
As we move forward, the challenge lies in balancing the undeniable synthetic utility of CCl4 with the pressing need for sustainable and safe chemical processes. This necessitates a comprehensive reevaluation of CCl4's role in organic synthesis, exploring novel approaches that can maintain its synthetic versatility while addressing environmental and safety concerns.
The evolution of CCl4 in organic synthesis can be traced back to its initial use as a non-polar solvent for extraction and purification processes. As researchers delved deeper into its reactivity, they discovered its potential as a source of chlorine atoms in radical reactions. This led to the development of numerous synthetic methodologies involving CCl4, particularly in the areas of chlorination, radical addition, and carbon-carbon bond formation.
One of the most notable applications of CCl4 in organic synthesis is the Appel reaction, discovered in 1975. This reaction utilizes CCl4 in combination with triphenylphosphine to convert alcohols into alkyl chlorides, providing a valuable tool for functional group interconversion. The Appel reaction has since become a cornerstone in many synthetic strategies, enabling the preparation of complex organic molecules and natural products.
The use of CCl4 in free radical reactions has also been extensively explored. Its ability to generate trichloromethyl radicals under various conditions has been exploited in the synthesis of chlorinated compounds, as well as in the initiation of radical polymerization processes. These applications have found widespread use in both academic research and industrial settings.
However, the recognition of CCl4's ozone-depleting properties and potential health hazards has led to a shift in research focus. In recent years, there has been a growing emphasis on developing alternative reagents and methodologies that can replace CCl4 in organic synthesis while maintaining or improving reaction efficiency and selectivity.
The primary objectives of current research in this field are twofold. First, there is a concerted effort to fully understand the mechanistic aspects of CCl4-mediated reactions, aiming to optimize existing processes and uncover new reactivity patterns. Second, researchers are actively seeking environmentally friendly alternatives that can mimic the reactivity of CCl4 without its associated drawbacks.
As we move forward, the challenge lies in balancing the undeniable synthetic utility of CCl4 with the pressing need for sustainable and safe chemical processes. This necessitates a comprehensive reevaluation of CCl4's role in organic synthesis, exploring novel approaches that can maintain its synthetic versatility while addressing environmental and safety concerns.
Market Demand Analysis for CCl4 in Chemical Industry
Carbon tetrachloride (CCl4) has long been a significant component in various industrial processes, particularly in organic synthesis reactions. However, its market demand in the chemical industry has undergone substantial changes over the past few decades due to environmental concerns and regulatory restrictions.
The global market for CCl4 has experienced a sharp decline since the 1980s when it was widely used as a solvent, cleaning agent, and refrigerant. This decline was primarily driven by the Montreal Protocol, which identified CCl4 as an ozone-depleting substance. As a result, many countries phased out its production and consumption for non-feedstock uses.
Despite these restrictions, there remains a niche market demand for CCl4 in specific organic synthesis reactions. The chemical industry continues to utilize CCl4 as a reagent in the production of certain pharmaceuticals, agrochemicals, and specialty chemicals. Its unique properties, such as its ability to act as a chlorinating agent and its inertness in many reactions, make it valuable in select synthetic processes.
The pharmaceutical sector represents a significant portion of the current demand for CCl4 in organic synthesis. It is used in the production of various active pharmaceutical ingredients (APIs) and intermediates. The agrochemical industry also maintains a demand for CCl4, particularly in the synthesis of certain pesticides and herbicides.
Market analysis indicates that the Asia-Pacific region, especially China and India, has emerged as the primary consumer of CCl4 for chemical synthesis. This is largely due to the rapid growth of their pharmaceutical and agrochemical industries, coupled with less stringent environmental regulations compared to Western countries.
However, the overall market trend for CCl4 in organic synthesis is one of gradual decline. This is driven by increasing environmental awareness, stricter regulations, and the development of alternative synthetic routes that avoid the use of ozone-depleting substances. Many chemical companies are investing in research to find safer and more sustainable alternatives to CCl4 in their synthesis processes.
The future market demand for CCl4 in the chemical industry is expected to continue its downward trajectory. As more countries implement stricter environmental policies and as alternative technologies become more cost-effective, the reliance on CCl4 in organic synthesis is likely to diminish further. However, it may retain its significance in certain specialized applications where suitable alternatives have not yet been developed or where its use remains critical to the synthesis process.
The global market for CCl4 has experienced a sharp decline since the 1980s when it was widely used as a solvent, cleaning agent, and refrigerant. This decline was primarily driven by the Montreal Protocol, which identified CCl4 as an ozone-depleting substance. As a result, many countries phased out its production and consumption for non-feedstock uses.
Despite these restrictions, there remains a niche market demand for CCl4 in specific organic synthesis reactions. The chemical industry continues to utilize CCl4 as a reagent in the production of certain pharmaceuticals, agrochemicals, and specialty chemicals. Its unique properties, such as its ability to act as a chlorinating agent and its inertness in many reactions, make it valuable in select synthetic processes.
The pharmaceutical sector represents a significant portion of the current demand for CCl4 in organic synthesis. It is used in the production of various active pharmaceutical ingredients (APIs) and intermediates. The agrochemical industry also maintains a demand for CCl4, particularly in the synthesis of certain pesticides and herbicides.
Market analysis indicates that the Asia-Pacific region, especially China and India, has emerged as the primary consumer of CCl4 for chemical synthesis. This is largely due to the rapid growth of their pharmaceutical and agrochemical industries, coupled with less stringent environmental regulations compared to Western countries.
However, the overall market trend for CCl4 in organic synthesis is one of gradual decline. This is driven by increasing environmental awareness, stricter regulations, and the development of alternative synthetic routes that avoid the use of ozone-depleting substances. Many chemical companies are investing in research to find safer and more sustainable alternatives to CCl4 in their synthesis processes.
The future market demand for CCl4 in the chemical industry is expected to continue its downward trajectory. As more countries implement stricter environmental policies and as alternative technologies become more cost-effective, the reliance on CCl4 in organic synthesis is likely to diminish further. However, it may retain its significance in certain specialized applications where suitable alternatives have not yet been developed or where its use remains critical to the synthesis process.
Current Status and Challenges of CCl4 Usage
Carbon tetrachloride (CCl4) has long been recognized as a versatile reagent in organic synthesis reactions. However, its current status and usage face significant challenges due to environmental and health concerns. The Montreal Protocol, implemented in 1989, has led to a global phase-out of CCl4 production and consumption, drastically reducing its availability for industrial and research purposes.
Despite these restrictions, CCl4 continues to play a role in certain specialized organic synthesis reactions. Its unique properties, such as its ability to act as a radical initiator and its inertness in many reaction conditions, make it valuable in specific applications. However, the scientific community has been actively seeking alternatives to reduce reliance on this compound.
One of the primary challenges in CCl4 usage is its ozone-depleting potential. As a result, strict regulations govern its production, distribution, and use in laboratories and industrial settings. This has led to increased costs and limited accessibility, forcing researchers to explore alternative reagents or develop new synthetic methodologies that avoid CCl4 altogether.
The toxicity of CCl4 presents another significant challenge. Exposure to CCl4 can cause severe liver and kidney damage, as well as potential carcinogenic effects. Consequently, stringent safety measures are required when handling this compound, including the use of specialized equipment and protective gear. These safety requirements further complicate its use in both research and industrial settings.
In response to these challenges, the scientific community has made considerable efforts to develop greener alternatives and more sustainable synthetic methods. Many researchers have focused on replacing CCl4 with less harmful halogenated solvents or exploring entirely new reaction pathways that eliminate the need for such compounds.
Despite these efforts, CCl4 remains irreplaceable in certain niche applications. For instance, it continues to be used in some radical-mediated reactions and as a solvent in specific spectroscopic techniques. In these cases, researchers must carefully weigh the benefits against the environmental and health risks, often implementing rigorous containment and disposal protocols.
The current status of CCl4 usage in organic synthesis is characterized by a delicate balance between its unique chemical properties and the pressing need for safer, more environmentally friendly alternatives. As regulations continue to tighten and awareness of environmental issues grows, the challenge of finding suitable replacements for CCl4 in organic synthesis reactions remains a priority for chemists and chemical engineers worldwide.
Despite these restrictions, CCl4 continues to play a role in certain specialized organic synthesis reactions. Its unique properties, such as its ability to act as a radical initiator and its inertness in many reaction conditions, make it valuable in specific applications. However, the scientific community has been actively seeking alternatives to reduce reliance on this compound.
One of the primary challenges in CCl4 usage is its ozone-depleting potential. As a result, strict regulations govern its production, distribution, and use in laboratories and industrial settings. This has led to increased costs and limited accessibility, forcing researchers to explore alternative reagents or develop new synthetic methodologies that avoid CCl4 altogether.
The toxicity of CCl4 presents another significant challenge. Exposure to CCl4 can cause severe liver and kidney damage, as well as potential carcinogenic effects. Consequently, stringent safety measures are required when handling this compound, including the use of specialized equipment and protective gear. These safety requirements further complicate its use in both research and industrial settings.
In response to these challenges, the scientific community has made considerable efforts to develop greener alternatives and more sustainable synthetic methods. Many researchers have focused on replacing CCl4 with less harmful halogenated solvents or exploring entirely new reaction pathways that eliminate the need for such compounds.
Despite these efforts, CCl4 remains irreplaceable in certain niche applications. For instance, it continues to be used in some radical-mediated reactions and as a solvent in specific spectroscopic techniques. In these cases, researchers must carefully weigh the benefits against the environmental and health risks, often implementing rigorous containment and disposal protocols.
The current status of CCl4 usage in organic synthesis is characterized by a delicate balance between its unique chemical properties and the pressing need for safer, more environmentally friendly alternatives. As regulations continue to tighten and awareness of environmental issues grows, the challenge of finding suitable replacements for CCl4 in organic synthesis reactions remains a priority for chemists and chemical engineers worldwide.
Current Methodologies Utilizing CCl4
01 Production and purification methods
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification methods: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications in chemical processes: Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in organic synthesis, extraction processes, and as a raw material for the production of other chlorinated compounds.
- Environmental and safety considerations: Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.
- Historical industrial uses: Carbon tetrachloride has been historically used in various industrial applications, including as a cleaning agent, fire extinguishing agent, and in the production of refrigerants. Patents describe these applications and related equipment or processes.
- Analytical and research applications: Carbon tetrachloride is used in analytical chemistry and research settings. Patents describe its use in spectroscopic techniques, as a standard in chemical analysis, and in studying chemical reactions and molecular structures.
02 Applications in chemical processes
Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It plays a role in organic synthesis, extraction procedures, and as a raw material for the production of other chlorinated compounds.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on alternatives to carbon tetrachloride, proper handling and disposal methods, and techniques for detecting and monitoring its presence in various environments.Expand Specific Solutions04 Historical industrial uses
Carbon tetrachloride has been used historically in various industrial applications, including as a cleaning agent, fire extinguishing agent, and in the production of refrigerants. Many of these uses have been phased out due to environmental concerns.Expand Specific Solutions05 Analytical and research applications
Carbon tetrachloride is used in analytical chemistry and research settings. It serves as a solvent in spectroscopy, a medium for chemical reactions, and a standard in various analytical techniques.Expand Specific Solutions
Key Players in CCl4 Production and Application
The competitive landscape for carbon tetrachloride in organic synthesis reactions is characterized by a mature market with established players and moderate growth potential. The global market size is estimated to be in the range of several hundred million dollars annually. Technologically, the field is well-developed, with companies like Occidental Chemical Corp., LOTTE Fine Chemical, and Shin-Etsu Chemical leading in production capabilities. However, environmental and safety concerns have led to increased research into alternatives, with academic institutions like Zhejiang University and Shanghai Institute of Organic Chemistry contributing to innovation. Emerging players such as Kiverdi are exploring novel approaches to carbon-based chemistry, potentially disrupting traditional synthesis methods.
Evonik Operations GmbH
Technical Solution: Evonik Operations GmbH has taken a unique approach to the use of carbon tetrachloride in organic synthesis reactions. They have developed a microreactor technology that allows for the controlled use of CCl4 in continuous flow processes[13]. This method significantly reduces the overall quantity of CCl4 required while improving reaction efficiency. Evonik has also explored the use of supercritical carbon dioxide as a replacement for CCl4 in certain chlorination reactions, leveraging their expertise in high-pressure chemistry[15]. Additionally, they have invested in research on photochemical alternatives to CCl4-mediated reactions, aiming to harness the power of light to drive challenging transformations without the need for harmful reagents[17].
Strengths: Advanced microreactor technology, expertise in alternative reaction media. Weaknesses: High initial investment costs for new technologies, potential limitations in reaction scope.
Shanghai Institute of Organic Chemistry
Technical Solution: The Shanghai Institute of Organic Chemistry has conducted extensive research on the role of carbon tetrachloride in organic synthesis reactions. They have pioneered the use of CCl4 in conjunction with visible-light photoredox catalysis to achieve challenging C-H functionalization reactions[7]. This approach enables the activation of traditionally unreactive C-H bonds under mild conditions. The institute has also explored the use of CCl4 in asymmetric synthesis, developing novel chiral catalysts that can induce high levels of enantioselectivity in chlorination reactions[9]. Their work has significantly expanded the synthetic utility of CCl4 while addressing concerns about its toxicity through the development of low-concentration, highly efficient reaction systems[11].
Strengths: Cutting-edge research in photoredox catalysis and asymmetric synthesis, focus on green chemistry principles. Weaknesses: Primarily academic research, potential challenges in industrial scale-up.
Innovative CCl4-Based Reaction Mechanisms
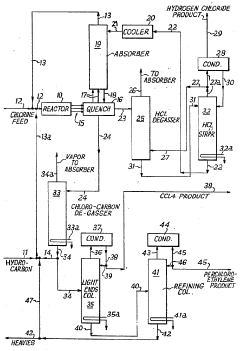
Production of carbon tetrachloride
PatentInactiveUS3697610A
Innovation
- A process involving the reaction of carbon with chlorine at elevated temperatures and pressures, using carbonized wood or coal, with reactivity enhancement through air and chlorine treatment, allowing for continuous production and high yields of carbon tetrachloride with minimal byproduct formation.
Production of carbon tetrachloride and perchloroethylene
PatentInactiveGB1009356A
Innovation
- A vapor phase process involving the thermal chlorination of hydrocarbons or chlorohydrocarbons with chlorine at elevated temperatures, followed by quenching with an aqueous medium to separate and recycle organic phases, allowing for the controlled production of carbon tetrachloride and perchloroethylene as co-products with adjustable ratios by varying reaction conditions.
Environmental Impact and Regulations
Carbon tetrachloride, once widely used in organic synthesis reactions, has been subject to increasing environmental scrutiny and regulatory control due to its significant environmental impact. The compound's ozone-depleting properties have led to its phase-out under the Montreal Protocol, a global agreement aimed at protecting the Earth's ozone layer.
The environmental concerns surrounding carbon tetrachloride stem from its high global warming potential and long atmospheric lifetime. When released into the atmosphere, it can persist for decades, contributing to the depletion of stratospheric ozone. This ozone depletion increases the amount of harmful ultraviolet radiation reaching the Earth's surface, posing risks to human health and ecosystems.
In response to these environmental threats, many countries have implemented strict regulations on the production, use, and disposal of carbon tetrachloride. The United States Environmental Protection Agency (EPA) has classified it as a hazardous air pollutant and has banned its use in consumer products. The European Union has also imposed severe restrictions on its use, limiting it to essential laboratory and analytical applications.
The regulatory landscape for carbon tetrachloride continues to evolve, with ongoing efforts to further reduce its emissions and find suitable alternatives for its remaining applications. Research institutions and chemical companies are actively developing environmentally friendly substitutes that can replicate the compound's useful properties in organic synthesis without the associated environmental risks.
Despite these restrictions, carbon tetrachloride still finds limited use in some industrial processes and as a feedstock for the production of other chemicals. However, these applications are subject to stringent controls and reporting requirements to minimize environmental release. The chemical industry has been compelled to invest in improved containment systems, waste treatment technologies, and more efficient production processes to comply with these regulations.
The case of carbon tetrachloride serves as a prime example of how environmental concerns can drive significant changes in chemical use and regulation. It highlights the ongoing challenge of balancing the benefits of chemical compounds in scientific and industrial applications with the need to protect the environment and human health. As a result, the organic synthesis community has been forced to adapt, developing new methodologies and exploring alternative reagents that offer similar reactivity with reduced environmental impact.
The environmental concerns surrounding carbon tetrachloride stem from its high global warming potential and long atmospheric lifetime. When released into the atmosphere, it can persist for decades, contributing to the depletion of stratospheric ozone. This ozone depletion increases the amount of harmful ultraviolet radiation reaching the Earth's surface, posing risks to human health and ecosystems.
In response to these environmental threats, many countries have implemented strict regulations on the production, use, and disposal of carbon tetrachloride. The United States Environmental Protection Agency (EPA) has classified it as a hazardous air pollutant and has banned its use in consumer products. The European Union has also imposed severe restrictions on its use, limiting it to essential laboratory and analytical applications.
The regulatory landscape for carbon tetrachloride continues to evolve, with ongoing efforts to further reduce its emissions and find suitable alternatives for its remaining applications. Research institutions and chemical companies are actively developing environmentally friendly substitutes that can replicate the compound's useful properties in organic synthesis without the associated environmental risks.
Despite these restrictions, carbon tetrachloride still finds limited use in some industrial processes and as a feedstock for the production of other chemicals. However, these applications are subject to stringent controls and reporting requirements to minimize environmental release. The chemical industry has been compelled to invest in improved containment systems, waste treatment technologies, and more efficient production processes to comply with these regulations.
The case of carbon tetrachloride serves as a prime example of how environmental concerns can drive significant changes in chemical use and regulation. It highlights the ongoing challenge of balancing the benefits of chemical compounds in scientific and industrial applications with the need to protect the environment and human health. As a result, the organic synthesis community has been forced to adapt, developing new methodologies and exploring alternative reagents that offer similar reactivity with reduced environmental impact.
Alternative Solvents and Green Chemistry Approaches
The shift towards alternative solvents and green chemistry approaches in organic synthesis reactions represents a significant paradigm shift in the field. This transition is driven by the growing awareness of environmental concerns and the need for more sustainable practices in chemical processes. As the use of carbon tetrachloride becomes increasingly restricted due to its ozone-depleting properties and potential health hazards, researchers are exploring a wide range of alternative solvents and methodologies.
One of the primary focuses in this area is the development of bio-based solvents derived from renewable resources. These include solvents such as ethyl lactate, 2-methyltetrahydrofuran, and cyrene, which offer lower toxicity profiles and reduced environmental impact compared to traditional petrochemical-based solvents. These bio-based alternatives often exhibit similar or even enhanced performance in organic synthesis reactions, making them viable replacements for carbon tetrachloride in many applications.
Water has emerged as a particularly promising green solvent for organic synthesis. While traditionally considered incompatible with many organic reactions, advances in surfactant technology and the development of water-compatible catalysts have expanded the scope of aqueous organic chemistry. This approach not only eliminates the need for harmful organic solvents but also often leads to improved reaction rates and selectivities.
Ionic liquids represent another class of alternative solvents gaining traction in organic synthesis. These low-melting-point salts offer unique properties such as negligible vapor pressure, high thermal stability, and the ability to dissolve a wide range of organic and inorganic compounds. Their customizable nature allows for the fine-tuning of solvent properties to optimize reaction conditions, potentially surpassing the performance of traditional solvents like carbon tetrachloride.
The concept of solvent-free reactions has also gained significant attention in the green chemistry community. These approaches eliminate the need for solvents altogether, reducing waste generation and simplifying purification processes. Techniques such as mechanochemistry, where reactions are induced by mechanical forces, and neat reactions, where reagents are mixed without any solvent, are becoming increasingly popular in organic synthesis.
Supercritical fluids, particularly supercritical carbon dioxide (scCO2), offer another environmentally benign alternative to traditional organic solvents. The unique properties of scCO2, such as its tunable density and solvating power, make it suitable for a wide range of organic transformations. Its use can lead to enhanced reaction rates, improved selectivities, and easier product separation compared to conventional solvent systems.
As the field of green chemistry continues to evolve, researchers are also exploring novel reaction media such as deep eutectic solvents, switchable solvents, and fluorous solvents. These innovative approaches aim to combine the benefits of traditional organic solvents with improved environmental profiles and enhanced reaction control.
One of the primary focuses in this area is the development of bio-based solvents derived from renewable resources. These include solvents such as ethyl lactate, 2-methyltetrahydrofuran, and cyrene, which offer lower toxicity profiles and reduced environmental impact compared to traditional petrochemical-based solvents. These bio-based alternatives often exhibit similar or even enhanced performance in organic synthesis reactions, making them viable replacements for carbon tetrachloride in many applications.
Water has emerged as a particularly promising green solvent for organic synthesis. While traditionally considered incompatible with many organic reactions, advances in surfactant technology and the development of water-compatible catalysts have expanded the scope of aqueous organic chemistry. This approach not only eliminates the need for harmful organic solvents but also often leads to improved reaction rates and selectivities.
Ionic liquids represent another class of alternative solvents gaining traction in organic synthesis. These low-melting-point salts offer unique properties such as negligible vapor pressure, high thermal stability, and the ability to dissolve a wide range of organic and inorganic compounds. Their customizable nature allows for the fine-tuning of solvent properties to optimize reaction conditions, potentially surpassing the performance of traditional solvents like carbon tetrachloride.
The concept of solvent-free reactions has also gained significant attention in the green chemistry community. These approaches eliminate the need for solvents altogether, reducing waste generation and simplifying purification processes. Techniques such as mechanochemistry, where reactions are induced by mechanical forces, and neat reactions, where reagents are mixed without any solvent, are becoming increasingly popular in organic synthesis.
Supercritical fluids, particularly supercritical carbon dioxide (scCO2), offer another environmentally benign alternative to traditional organic solvents. The unique properties of scCO2, such as its tunable density and solvating power, make it suitable for a wide range of organic transformations. Its use can lead to enhanced reaction rates, improved selectivities, and easier product separation compared to conventional solvent systems.
As the field of green chemistry continues to evolve, researchers are also exploring novel reaction media such as deep eutectic solvents, switchable solvents, and fluorous solvents. These innovative approaches aim to combine the benefits of traditional organic solvents with improved environmental profiles and enhanced reaction control.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!