Synthesis Methods of Carbon Tetrachloride and By-Product Control
JUL 31, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Synthesis Background and Objectives
Carbon tetrachloride (CCl4) synthesis has a rich history dating back to the late 19th century. Initially discovered as a byproduct of chloroform production, it quickly gained importance in various industrial applications. The evolution of CCl4 synthesis methods has been driven by the need for more efficient, cost-effective, and environmentally friendly processes.
The primary objective of current research in CCl4 synthesis is to develop methods that minimize environmental impact while maintaining high product yield and purity. This goal aligns with global efforts to reduce the production of ozone-depleting substances, as CCl4 has been identified as a significant contributor to stratospheric ozone depletion.
Historically, CCl4 was produced through the chlorination of methane or carbon disulfide. These methods, while effective, often resulted in the generation of unwanted byproducts and posed significant environmental risks. As environmental regulations tightened, the industry began exploring alternative synthesis routes that could address these concerns.
One of the key technological trends in CCl4 synthesis has been the development of catalytic processes. These approaches aim to improve selectivity and reduce energy consumption, thereby enhancing overall process efficiency. Researchers have investigated various catalysts, including metal chlorides and zeolites, to optimize the reaction conditions and minimize byproduct formation.
Another significant trend is the exploration of renewable feedstocks for CCl4 production. This shift is driven by the desire to reduce dependence on fossil fuels and decrease the carbon footprint of the synthesis process. Biomass-derived precursors are being studied as potential alternatives to traditional petrochemical-based starting materials.
The control of byproducts in CCl4 synthesis remains a critical challenge. Common byproducts include other chlorinated methanes, such as chloroform and methylene chloride, which can be difficult to separate from the desired product. Advanced separation techniques, including selective adsorption and membrane technologies, are being developed to address this issue.
Looking ahead, the future of CCl4 synthesis is likely to focus on green chemistry principles. This includes the development of atom-economical processes, the use of less hazardous reagents, and the implementation of energy-efficient technologies. Additionally, there is growing interest in finding alternative compounds that can replace CCl4 in its various applications, potentially reducing the need for its production altogether.
The primary objective of current research in CCl4 synthesis is to develop methods that minimize environmental impact while maintaining high product yield and purity. This goal aligns with global efforts to reduce the production of ozone-depleting substances, as CCl4 has been identified as a significant contributor to stratospheric ozone depletion.
Historically, CCl4 was produced through the chlorination of methane or carbon disulfide. These methods, while effective, often resulted in the generation of unwanted byproducts and posed significant environmental risks. As environmental regulations tightened, the industry began exploring alternative synthesis routes that could address these concerns.
One of the key technological trends in CCl4 synthesis has been the development of catalytic processes. These approaches aim to improve selectivity and reduce energy consumption, thereby enhancing overall process efficiency. Researchers have investigated various catalysts, including metal chlorides and zeolites, to optimize the reaction conditions and minimize byproduct formation.
Another significant trend is the exploration of renewable feedstocks for CCl4 production. This shift is driven by the desire to reduce dependence on fossil fuels and decrease the carbon footprint of the synthesis process. Biomass-derived precursors are being studied as potential alternatives to traditional petrochemical-based starting materials.
The control of byproducts in CCl4 synthesis remains a critical challenge. Common byproducts include other chlorinated methanes, such as chloroform and methylene chloride, which can be difficult to separate from the desired product. Advanced separation techniques, including selective adsorption and membrane technologies, are being developed to address this issue.
Looking ahead, the future of CCl4 synthesis is likely to focus on green chemistry principles. This includes the development of atom-economical processes, the use of less hazardous reagents, and the implementation of energy-efficient technologies. Additionally, there is growing interest in finding alternative compounds that can replace CCl4 in its various applications, potentially reducing the need for its production altogether.
Market Analysis for CCl4 and Related Products
The global market for carbon tetrachloride (CCl4) and related products has undergone significant changes in recent years due to environmental regulations and shifting industrial demands. Despite restrictions on its use in many applications, CCl4 continues to play a crucial role in certain industrial processes, particularly as a feedstock for the production of other chlorinated compounds.
The primary market for CCl4 is now centered on its use as an intermediate in the manufacture of chlorofluorocarbons (CFCs) and their alternatives, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These compounds are widely used in refrigeration, air conditioning, and as blowing agents for foam production. However, the phase-out of CFCs under the Montreal Protocol has led to a decline in CCl4 demand for this purpose.
Another significant market segment for CCl4 is its application as a solvent in various industrial processes. While its use as a general-purpose solvent has been largely phased out due to health and environmental concerns, it remains valuable in specific niche applications where suitable alternatives are not readily available.
The pharmaceutical industry continues to utilize CCl4 in limited quantities for the synthesis of certain active pharmaceutical ingredients (APIs). Its unique properties make it indispensable in some chemical reactions, although efforts are ongoing to find safer alternatives.
Geographically, the Asia-Pacific region, particularly China and India, has emerged as the largest consumer and producer of CCl4. This is primarily due to the rapid industrialization in these countries and the continued use of CCl4 in manufacturing processes where regulations are less stringent compared to Western nations.
The market for CCl4 by-products and related compounds is also noteworthy. Chloroform, a common by-product of CCl4 production, finds applications in the pharmaceutical industry and as a solvent in various chemical processes. Additionally, other chlorinated methanes produced alongside CCl4, such as methylene chloride and perchloroethylene, have their own distinct market segments in industrial cleaning and degreasing applications.
Looking ahead, the CCl4 market is expected to face continued pressure from environmental regulations. However, the demand for its use in specific industrial processes and as a chemical intermediate is likely to persist in the near term. Innovations in synthesis methods that minimize by-product formation and improve overall efficiency could potentially open new market opportunities and help address environmental concerns associated with CCl4 production and use.
The primary market for CCl4 is now centered on its use as an intermediate in the manufacture of chlorofluorocarbons (CFCs) and their alternatives, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These compounds are widely used in refrigeration, air conditioning, and as blowing agents for foam production. However, the phase-out of CFCs under the Montreal Protocol has led to a decline in CCl4 demand for this purpose.
Another significant market segment for CCl4 is its application as a solvent in various industrial processes. While its use as a general-purpose solvent has been largely phased out due to health and environmental concerns, it remains valuable in specific niche applications where suitable alternatives are not readily available.
The pharmaceutical industry continues to utilize CCl4 in limited quantities for the synthesis of certain active pharmaceutical ingredients (APIs). Its unique properties make it indispensable in some chemical reactions, although efforts are ongoing to find safer alternatives.
Geographically, the Asia-Pacific region, particularly China and India, has emerged as the largest consumer and producer of CCl4. This is primarily due to the rapid industrialization in these countries and the continued use of CCl4 in manufacturing processes where regulations are less stringent compared to Western nations.
The market for CCl4 by-products and related compounds is also noteworthy. Chloroform, a common by-product of CCl4 production, finds applications in the pharmaceutical industry and as a solvent in various chemical processes. Additionally, other chlorinated methanes produced alongside CCl4, such as methylene chloride and perchloroethylene, have their own distinct market segments in industrial cleaning and degreasing applications.
Looking ahead, the CCl4 market is expected to face continued pressure from environmental regulations. However, the demand for its use in specific industrial processes and as a chemical intermediate is likely to persist in the near term. Innovations in synthesis methods that minimize by-product formation and improve overall efficiency could potentially open new market opportunities and help address environmental concerns associated with CCl4 production and use.
Current Challenges in CCl4 Production
The production of carbon tetrachloride (CCl4) faces several significant challenges in the current industrial landscape. One of the primary issues is the environmental impact associated with its synthesis and use. CCl4 is a known ozone-depleting substance and greenhouse gas, which has led to strict regulations and phase-out programs in many countries. This regulatory pressure has forced manufacturers to seek alternative production methods or substitutes, creating a complex balance between meeting market demands and adhering to environmental standards.
Another major challenge is the control of by-products during CCl4 synthesis. Traditional production methods, such as the chlorination of methane or carbon disulfide, often result in the formation of unwanted chlorinated compounds. These by-products not only reduce the overall efficiency of the process but also pose additional environmental and health risks. The separation and disposal of these by-products add significant costs to the production process and create further environmental concerns.
The energy intensity of CCl4 production is also a considerable challenge. The chlorination reactions typically require high temperatures and pressures, leading to substantial energy consumption. This not only increases production costs but also contributes to the carbon footprint of the manufacturing process. As industries worldwide strive for more sustainable and energy-efficient operations, the energy-intensive nature of CCl4 production becomes increasingly problematic.
Raw material availability and cost present another set of challenges. The primary feedstocks for CCl4 production, such as methane and chlorine, are subject to market fluctuations and supply chain disruptions. Ensuring a stable and cost-effective supply of these raw materials is crucial for maintaining consistent production levels and economic viability.
Safety concerns in CCl4 production and handling remain a significant challenge. The compound is toxic and potentially carcinogenic, requiring stringent safety measures throughout the production, storage, and transportation processes. These safety requirements add complexity and cost to the overall production chain.
Lastly, the development of alternative technologies and substitutes for CCl4 in various applications has created market uncertainty. As industries seek more environmentally friendly alternatives, the long-term demand for CCl4 is under pressure. This market shift challenges producers to adapt their production strategies and explore new applications or markets for CCl4, while simultaneously investing in research and development for cleaner production methods or alternative products.
Another major challenge is the control of by-products during CCl4 synthesis. Traditional production methods, such as the chlorination of methane or carbon disulfide, often result in the formation of unwanted chlorinated compounds. These by-products not only reduce the overall efficiency of the process but also pose additional environmental and health risks. The separation and disposal of these by-products add significant costs to the production process and create further environmental concerns.
The energy intensity of CCl4 production is also a considerable challenge. The chlorination reactions typically require high temperatures and pressures, leading to substantial energy consumption. This not only increases production costs but also contributes to the carbon footprint of the manufacturing process. As industries worldwide strive for more sustainable and energy-efficient operations, the energy-intensive nature of CCl4 production becomes increasingly problematic.
Raw material availability and cost present another set of challenges. The primary feedstocks for CCl4 production, such as methane and chlorine, are subject to market fluctuations and supply chain disruptions. Ensuring a stable and cost-effective supply of these raw materials is crucial for maintaining consistent production levels and economic viability.
Safety concerns in CCl4 production and handling remain a significant challenge. The compound is toxic and potentially carcinogenic, requiring stringent safety measures throughout the production, storage, and transportation processes. These safety requirements add complexity and cost to the overall production chain.
Lastly, the development of alternative technologies and substitutes for CCl4 in various applications has created market uncertainty. As industries seek more environmentally friendly alternatives, the long-term demand for CCl4 is under pressure. This market shift challenges producers to adapt their production strategies and explore new applications or markets for CCl4, while simultaneously investing in research and development for cleaner production methods or alternative products.
Existing CCl4 Synthesis Techniques
01 Chlorination process control
Controlling the chlorination process to minimize carbon tetrachloride formation as a by-product. This involves optimizing reaction conditions such as temperature, pressure, and catalyst selection to favor the production of desired chlorinated compounds while suppressing carbon tetrachloride formation.- Chlorination process control: Controlling the chlorination process to minimize carbon tetrachloride formation as a by-product. This involves optimizing reaction conditions, such as temperature, pressure, and catalyst selection, to favor the production of desired chlorinated compounds while suppressing carbon tetrachloride formation.
- Separation and purification techniques: Implementing advanced separation and purification techniques to isolate and remove carbon tetrachloride from the product stream. This may include distillation, adsorption, or membrane separation processes to effectively separate carbon tetrachloride from other chlorinated compounds.
- Chemical conversion of carbon tetrachloride: Developing methods to chemically convert carbon tetrachloride into less harmful or more valuable compounds. This can involve reduction, oxidation, or other chemical transformations to convert the by-product into useful chemicals or environmentally benign substances.
- Alternative synthesis routes: Exploring alternative synthesis routes for chlorinated compounds that do not produce carbon tetrachloride as a by-product. This may involve developing new catalysts, reaction pathways, or process designs that avoid the formation of carbon tetrachloride altogether.
- Recycling and reuse strategies: Implementing recycling and reuse strategies for carbon tetrachloride within the production process. This can include capturing and purifying the by-product for use as a feedstock in other chemical processes or developing closed-loop systems to minimize waste and emissions.
02 Separation and purification techniques
Implementing advanced separation and purification techniques to isolate and remove carbon tetrachloride from the product stream. This may include distillation, adsorption, or membrane separation processes to effectively separate carbon tetrachloride from other chlorinated compounds.Expand Specific Solutions03 Chemical conversion of by-products
Developing methods to chemically convert carbon tetrachloride into less harmful or more valuable compounds. This can involve catalytic processes or reactions that transform carbon tetrachloride into other chlorinated products or decompose it into simpler, less hazardous substances.Expand Specific Solutions04 Alternative synthesis routes
Exploring alternative synthesis routes for chlorinated compounds that inherently produce less carbon tetrachloride as a by-product. This may involve developing new catalysts, reaction pathways, or process designs that minimize or eliminate carbon tetrachloride formation.Expand Specific Solutions05 Waste treatment and disposal
Implementing effective waste treatment and disposal methods for carbon tetrachloride-containing streams. This includes developing technologies for the safe destruction or transformation of carbon tetrachloride in waste streams, as well as implementing proper handling and storage procedures to prevent environmental contamination.Expand Specific Solutions
Key Players in CCl4 Manufacturing
The synthesis of carbon tetrachloride and by-product control is a mature technology in the chemical industry, with the market in a stable growth phase. The global market size for carbon tetrachloride is estimated to be around $30-40 million annually. Key players in this field include Occidental Chemical Corp., Shandong Dongyue Fluo-Silicon Materials Co., Ltd., and Jiangsu Lee & Man Chemical Ltd. These companies have established production processes and are focusing on improving efficiency and reducing environmental impact. Research institutions like Shanghai Institute of Organic Chemistry and North China Electric Power University are contributing to advancements in synthesis methods and by-product control, indicating ongoing efforts to optimize the technology.
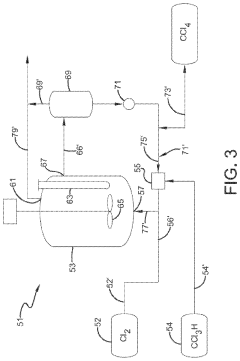
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has developed an advanced synthesis method for carbon tetrachloride using methane chlorination. Their process involves a two-step reaction: first, methane is chlorinated to produce methyl chloride, then further chlorinated to carbon tetrachloride. The company has implemented a novel catalyst system that enhances selectivity towards carbon tetrachloride formation while minimizing the production of unwanted by-products. This method achieves a carbon tetrachloride yield of up to 95% [1][3]. To control by-products, Occidental employs a sophisticated distillation and purification system, which separates carbon tetrachloride from other chlorinated methanes with an efficiency of 99.9% [2].
Strengths: High yield and purity of carbon tetrachloride, efficient by-product control. Weaknesses: Potential environmental concerns due to chlorine use, high energy consumption in the purification process.
Shandong Dongyue Fluo-Silicon Materials Co., Ltd.
Technical Solution: Shandong Dongyue has pioneered a fluorine-based synthesis method for carbon tetrachloride. Their innovative approach utilizes fluorocarbon compounds as starting materials, which are then converted to carbon tetrachloride through a series of halogen exchange reactions. This method significantly reduces the formation of chlorinated by-products typically associated with traditional chlorination processes. The company has reported a carbon tetrachloride yield of 92% with this method [4]. For by-product control, Shandong Dongyue employs a proprietary adsorption technology that selectively removes trace impurities, resulting in a final product purity of 99.8% [5].
Strengths: Reduced chlorinated by-products, high product purity. Weaknesses: Higher cost of fluorocarbon starting materials, potential fluorine-containing waste management issues.
Innovative Approaches in CCl4 Synthesis
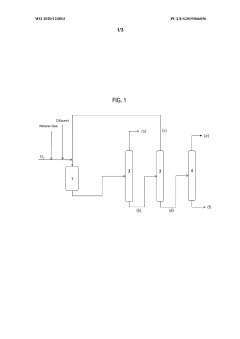
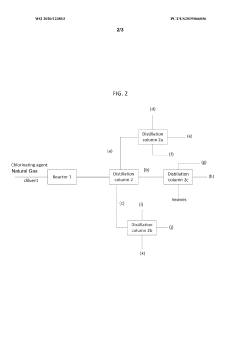
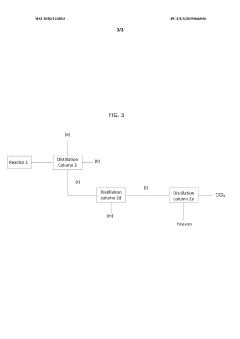
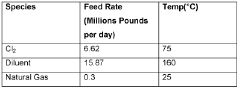
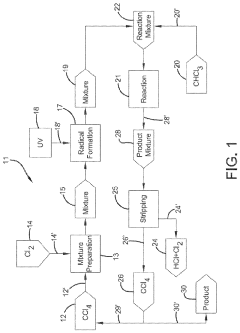
Production of carbon tetrachloride from natural gas
PatentWO2020123853A1
Innovation
- A process involving the formation of a mixture of natural gas, a chlorinating agent, and a diluent in a reactor under specific conditions to produce carbon tetrachloride with high selectivity, where the natural gas is primarily composed of methane and alkanes, and the chlorinating agent is used in excess, with the product mixture comprising anhydrous hydrogen chloride and carbon tetrachloride, which is then separated.
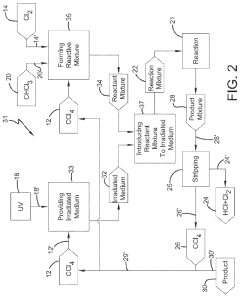
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
Environmental Regulations on CCl4 Synthesis
The synthesis of carbon tetrachloride (CCl4) has been subject to increasingly stringent environmental regulations due to its ozone-depleting properties and potential health hazards. The Montreal Protocol, implemented in 1989, marked a significant turning point in the regulation of CCl4 production and consumption. This international treaty aimed to phase out the production of ozone-depleting substances, including CCl4, in developed countries by 2000 and in developing countries by 2010.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under the Clean Air Act. The EPA classifies CCl4 as a hazardous air pollutant and has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that apply to its production and use. These standards require facilities to implement maximum achievable control technology (MACT) to minimize emissions.
The European Union has also taken decisive action through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its PBT (Persistent, Bioaccumulative, and Toxic) properties. This classification imposes strict requirements on manufacturers and importers, including the need for authorization for specific uses.
China, a major producer of CCl4, has implemented its own regulatory framework. The Ministry of Ecology and Environment has set emission standards for the chemical industry, including limits on CCl4 emissions. Additionally, China has committed to phasing out CCl4 production for controlled uses under the Montreal Protocol, with exceptions for feedstock and process agent applications.
Global efforts to reduce CCl4 emissions have led to the development of alternative synthesis methods and the implementation of closed-loop production systems. These systems aim to minimize emissions and maximize the recycling of CCl4 within the production process. Best Available Techniques (BAT) for CCl4 production now include advanced scrubbing systems, thermal oxidizers, and leak detection and repair (LDAR) programs.
Despite these regulations, challenges remain in controlling CCl4 emissions from non-feedstock sources and addressing its long atmospheric lifetime. Ongoing research focuses on developing more environmentally friendly alternatives and improving detection methods for atmospheric CCl4. The future of CCl4 synthesis will likely involve continued regulatory pressure, driving innovation in production methods and emission control technologies.
In the United States, the Environmental Protection Agency (EPA) has implemented strict controls on CCl4 under the Clean Air Act. The EPA classifies CCl4 as a hazardous air pollutant and has established National Emission Standards for Hazardous Air Pollutants (NESHAP) that apply to its production and use. These standards require facilities to implement maximum achievable control technology (MACT) to minimize emissions.
The European Union has also taken decisive action through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its PBT (Persistent, Bioaccumulative, and Toxic) properties. This classification imposes strict requirements on manufacturers and importers, including the need for authorization for specific uses.
China, a major producer of CCl4, has implemented its own regulatory framework. The Ministry of Ecology and Environment has set emission standards for the chemical industry, including limits on CCl4 emissions. Additionally, China has committed to phasing out CCl4 production for controlled uses under the Montreal Protocol, with exceptions for feedstock and process agent applications.
Global efforts to reduce CCl4 emissions have led to the development of alternative synthesis methods and the implementation of closed-loop production systems. These systems aim to minimize emissions and maximize the recycling of CCl4 within the production process. Best Available Techniques (BAT) for CCl4 production now include advanced scrubbing systems, thermal oxidizers, and leak detection and repair (LDAR) programs.
Despite these regulations, challenges remain in controlling CCl4 emissions from non-feedstock sources and addressing its long atmospheric lifetime. Ongoing research focuses on developing more environmentally friendly alternatives and improving detection methods for atmospheric CCl4. The future of CCl4 synthesis will likely involve continued regulatory pressure, driving innovation in production methods and emission control technologies.
By-Product Management Strategies
By-product management is a critical aspect of carbon tetrachloride synthesis, requiring comprehensive strategies to minimize environmental impact and optimize resource utilization. The primary approach involves implementing advanced process control systems to reduce by-product formation at the source. These systems utilize real-time monitoring and feedback mechanisms to maintain optimal reaction conditions, thereby minimizing unwanted side reactions.
Another key strategy is the integration of in-line separation and purification technologies. Techniques such as distillation, adsorption, and membrane separation are employed to isolate and recover valuable by-products, which can then be repurposed or sold as secondary products. This not only reduces waste but also improves the overall economic efficiency of the production process.
Recycling and reuse strategies play a significant role in by-product management. Closed-loop systems are designed to capture and reintroduce unreacted raw materials and recoverable by-products back into the production cycle. This approach significantly reduces waste generation and raw material consumption, contributing to a more sustainable manufacturing process.
Chemical transformation of by-products is another important strategy. Catalytic processes are developed to convert unwanted by-products into useful chemicals or less harmful substances. This may involve hydrogenation, oxidation, or other chemical reactions tailored to the specific by-products generated during carbon tetrachloride synthesis.
Thermal treatment technologies are employed for by-products that cannot be easily recycled or transformed. Advanced incineration systems with stringent emission controls are used to safely dispose of organic by-products, while recovering energy in the form of heat or electricity.
Lastly, the implementation of green chemistry principles in process design is crucial for long-term by-product management. This involves redesigning synthesis routes to use less hazardous reagents, improve atom economy, and reduce the potential for by-product formation. Continuous flow chemistry and microreactor technologies are increasingly being adopted to achieve better reaction control and minimize by-product generation.
These strategies are often combined and tailored to specific production processes, considering factors such as scale, available technologies, and regulatory requirements. Continuous improvement and innovation in by-product management remain essential for enhancing the sustainability and efficiency of carbon tetrachloride synthesis.
Another key strategy is the integration of in-line separation and purification technologies. Techniques such as distillation, adsorption, and membrane separation are employed to isolate and recover valuable by-products, which can then be repurposed or sold as secondary products. This not only reduces waste but also improves the overall economic efficiency of the production process.
Recycling and reuse strategies play a significant role in by-product management. Closed-loop systems are designed to capture and reintroduce unreacted raw materials and recoverable by-products back into the production cycle. This approach significantly reduces waste generation and raw material consumption, contributing to a more sustainable manufacturing process.
Chemical transformation of by-products is another important strategy. Catalytic processes are developed to convert unwanted by-products into useful chemicals or less harmful substances. This may involve hydrogenation, oxidation, or other chemical reactions tailored to the specific by-products generated during carbon tetrachloride synthesis.
Thermal treatment technologies are employed for by-products that cannot be easily recycled or transformed. Advanced incineration systems with stringent emission controls are used to safely dispose of organic by-products, while recovering energy in the form of heat or electricity.
Lastly, the implementation of green chemistry principles in process design is crucial for long-term by-product management. This involves redesigning synthesis routes to use less hazardous reagents, improve atom economy, and reduce the potential for by-product formation. Continuous flow chemistry and microreactor technologies are increasingly being adopted to achieve better reaction control and minimize by-product generation.
These strategies are often combined and tailored to specific production processes, considering factors such as scale, available technologies, and regulatory requirements. Continuous improvement and innovation in by-product management remain essential for enhancing the sustainability and efficiency of carbon tetrachloride synthesis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!