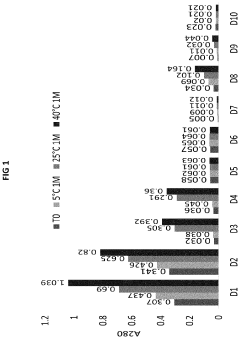
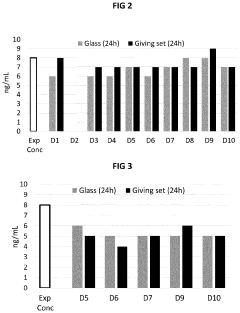
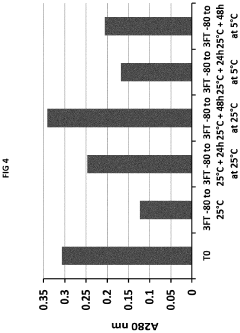
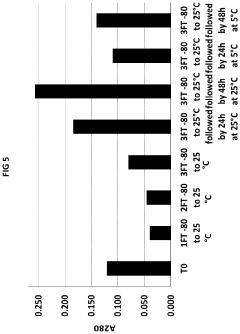
How Low-Temperature Conditions Affect Carbon Tetrachloride Stability
JUL 31, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Stability Background
Carbon tetrachloride (CCl4) is a synthetic chemical compound that has been widely used in various industrial applications, including as a solvent, cleaning agent, and refrigerant. Its stability under different environmental conditions, particularly at low temperatures, is of significant interest due to its potential impact on atmospheric chemistry and climate change.
The stability of CCl4 is primarily influenced by its molecular structure and the strength of its carbon-chlorine bonds. At room temperature, CCl4 is a colorless, non-flammable liquid with a sweet odor. It has a boiling point of 76.72°C (170.1°F) and a melting point of -22.92°C (-9.26°F). These physical properties contribute to its behavior and stability under various temperature conditions.
Historically, CCl4 was considered relatively stable under normal atmospheric conditions. However, research in the late 20th century revealed its role in ozone depletion, leading to its phaseout under the Montreal Protocol. This discovery highlighted the importance of understanding CCl4's stability and reactivity across different environmental scenarios, including low-temperature conditions.
The stability of CCl4 at low temperatures is particularly relevant in atmospheric chemistry. In the upper atmosphere, where temperatures can drop significantly, the behavior of CCl4 can change. Low temperatures can affect the kinetics of chemical reactions involving CCl4, potentially altering its decomposition rate and interaction with other atmospheric components.
One of the key factors influencing CCl4 stability at low temperatures is the reduced molecular kinetic energy. As temperature decreases, molecules move more slowly, which can affect the frequency and energy of collisions between CCl4 molecules and other reactive species. This reduction in molecular motion can potentially increase the stability of CCl4 by decreasing the likelihood of bond-breaking collisions.
However, the relationship between temperature and CCl4 stability is not straightforward. While lower temperatures generally reduce reaction rates, they can also lead to the formation of ice crystals or other particulates in the atmosphere. These particles can provide surfaces for heterogeneous reactions, potentially catalyzing the breakdown of CCl4 through mechanisms that are less prevalent at higher temperatures.
Understanding the stability of CCl4 under low-temperature conditions is crucial for accurately modeling its atmospheric lifetime and global distribution. This knowledge informs climate models, helps in assessing the long-term impact of historical CCl4 emissions, and guides strategies for monitoring and mitigating its environmental effects.
The stability of CCl4 is primarily influenced by its molecular structure and the strength of its carbon-chlorine bonds. At room temperature, CCl4 is a colorless, non-flammable liquid with a sweet odor. It has a boiling point of 76.72°C (170.1°F) and a melting point of -22.92°C (-9.26°F). These physical properties contribute to its behavior and stability under various temperature conditions.
Historically, CCl4 was considered relatively stable under normal atmospheric conditions. However, research in the late 20th century revealed its role in ozone depletion, leading to its phaseout under the Montreal Protocol. This discovery highlighted the importance of understanding CCl4's stability and reactivity across different environmental scenarios, including low-temperature conditions.
The stability of CCl4 at low temperatures is particularly relevant in atmospheric chemistry. In the upper atmosphere, where temperatures can drop significantly, the behavior of CCl4 can change. Low temperatures can affect the kinetics of chemical reactions involving CCl4, potentially altering its decomposition rate and interaction with other atmospheric components.
One of the key factors influencing CCl4 stability at low temperatures is the reduced molecular kinetic energy. As temperature decreases, molecules move more slowly, which can affect the frequency and energy of collisions between CCl4 molecules and other reactive species. This reduction in molecular motion can potentially increase the stability of CCl4 by decreasing the likelihood of bond-breaking collisions.
However, the relationship between temperature and CCl4 stability is not straightforward. While lower temperatures generally reduce reaction rates, they can also lead to the formation of ice crystals or other particulates in the atmosphere. These particles can provide surfaces for heterogeneous reactions, potentially catalyzing the breakdown of CCl4 through mechanisms that are less prevalent at higher temperatures.
Understanding the stability of CCl4 under low-temperature conditions is crucial for accurately modeling its atmospheric lifetime and global distribution. This knowledge informs climate models, helps in assessing the long-term impact of historical CCl4 emissions, and guides strategies for monitoring and mitigating its environmental effects.
Market Analysis
The market for carbon tetrachloride (CCl4) has undergone significant changes in recent decades due to environmental regulations and health concerns. However, the compound still finds applications in various industries, particularly in low-temperature conditions. The stability of CCl4 at low temperatures is crucial for its use in refrigeration, chemical synthesis, and as a solvent in laboratory settings.
In the refrigeration industry, CCl4 has historically been used as a refrigerant due to its excellent thermodynamic properties. While its use has been phased out in many countries due to its ozone-depleting potential, there remains a niche market for specialized low-temperature applications where alternatives are less effective. This segment, though small, continues to drive demand for CCl4 in certain regions.
The chemical synthesis sector represents another significant market for CCl4, especially in low-temperature reactions. Many organic synthesis processes require stable solvents at sub-zero temperatures, and CCl4's stability in these conditions makes it valuable for specific applications. The pharmaceutical and fine chemicals industries, in particular, continue to utilize CCl4 in controlled environments where its unique properties are beneficial.
Research laboratories and academic institutions form a steady market for CCl4, primarily for its use as a solvent in spectroscopic studies and other analytical techniques. The compound's transparency to infrared radiation and its stability at low temperatures make it ideal for certain types of spectroscopy and low-temperature experiments.
The global market for CCl4 has been declining due to regulatory pressures, but it has stabilized in recent years. The compound's use is now primarily limited to feedstock applications and essential laboratory uses. The market size for these applications is estimated to be in the range of several thousand metric tons annually, with the majority consumed in chemical manufacturing processes.
Geographically, the market for CCl4 is concentrated in regions with less stringent environmental regulations or where exemptions exist for specific uses. Developing countries in Asia and certain industrial sectors in North America and Europe account for the majority of current consumption. The market dynamics are heavily influenced by international agreements such as the Montreal Protocol, which has led to a significant reduction in CCl4 production and use globally.
Looking forward, the market for CCl4 in low-temperature applications is expected to remain stable but limited. Ongoing research into alternatives and the development of new technologies may further reduce demand in some sectors. However, the compound's unique properties ensure that it will likely maintain a presence in specialized applications where its stability at low temperatures provides distinct advantages over potential substitutes.
In the refrigeration industry, CCl4 has historically been used as a refrigerant due to its excellent thermodynamic properties. While its use has been phased out in many countries due to its ozone-depleting potential, there remains a niche market for specialized low-temperature applications where alternatives are less effective. This segment, though small, continues to drive demand for CCl4 in certain regions.
The chemical synthesis sector represents another significant market for CCl4, especially in low-temperature reactions. Many organic synthesis processes require stable solvents at sub-zero temperatures, and CCl4's stability in these conditions makes it valuable for specific applications. The pharmaceutical and fine chemicals industries, in particular, continue to utilize CCl4 in controlled environments where its unique properties are beneficial.
Research laboratories and academic institutions form a steady market for CCl4, primarily for its use as a solvent in spectroscopic studies and other analytical techniques. The compound's transparency to infrared radiation and its stability at low temperatures make it ideal for certain types of spectroscopy and low-temperature experiments.
The global market for CCl4 has been declining due to regulatory pressures, but it has stabilized in recent years. The compound's use is now primarily limited to feedstock applications and essential laboratory uses. The market size for these applications is estimated to be in the range of several thousand metric tons annually, with the majority consumed in chemical manufacturing processes.
Geographically, the market for CCl4 is concentrated in regions with less stringent environmental regulations or where exemptions exist for specific uses. Developing countries in Asia and certain industrial sectors in North America and Europe account for the majority of current consumption. The market dynamics are heavily influenced by international agreements such as the Montreal Protocol, which has led to a significant reduction in CCl4 production and use globally.
Looking forward, the market for CCl4 in low-temperature applications is expected to remain stable but limited. Ongoing research into alternatives and the development of new technologies may further reduce demand in some sectors. However, the compound's unique properties ensure that it will likely maintain a presence in specialized applications where its stability at low temperatures provides distinct advantages over potential substitutes.
Low-Temp Challenges
Carbon tetrachloride (CCl4) stability under low-temperature conditions presents significant challenges for various industrial applications and environmental concerns. The primary issue stems from the compound's physical and chemical properties, which can undergo substantial changes when exposed to cold environments.
One of the main challenges is the potential for phase transitions. As temperatures drop, CCl4 may undergo a liquid-to-solid phase change, which can cause problems in storage tanks, pipelines, and processing equipment. This solidification can lead to blockages, increased pressure, and potential damage to containment systems. Moreover, the expansion associated with freezing can cause structural stress on containers, potentially leading to leaks or ruptures.
Another critical challenge is the alteration of CCl4's chemical reactivity at low temperatures. While generally considered stable, carbon tetrachloride can become more susceptible to certain reactions in cold conditions. This increased reactivity may lead to unexpected degradation or the formation of byproducts, which can compromise the purity and effectiveness of the compound in its intended applications.
The volatility of CCl4 also changes with temperature, affecting its handling and containment. At lower temperatures, the vapor pressure decreases, which can impact processes that rely on the compound's gaseous state. This reduction in volatility may necessitate modifications to existing systems designed for room temperature operations, adding complexity and cost to industrial processes.
Furthermore, low temperatures can affect the solubility of CCl4 in various solvents, potentially leading to precipitation or separation in mixtures. This can be particularly problematic in applications where CCl4 is used as a solvent or in solution with other compounds. The altered solubility characteristics may require adjustments to formulations or processing methods to maintain desired product qualities.
The viscosity of carbon tetrachloride also increases as temperatures drop, which can impact its flow properties. This change in viscosity can affect pumping efficiency, heat transfer rates, and mixing processes, potentially leading to reduced productivity or increased energy consumption in industrial applications.
Lastly, the potential for condensation and moisture accumulation in cold environments poses a significant challenge. Water contamination can lead to the formation of hydrochloric acid through the hydrolysis of CCl4, which can cause corrosion in storage and handling equipment. This not only threatens the integrity of containment systems but also introduces safety hazards and potential environmental risks.
One of the main challenges is the potential for phase transitions. As temperatures drop, CCl4 may undergo a liquid-to-solid phase change, which can cause problems in storage tanks, pipelines, and processing equipment. This solidification can lead to blockages, increased pressure, and potential damage to containment systems. Moreover, the expansion associated with freezing can cause structural stress on containers, potentially leading to leaks or ruptures.
Another critical challenge is the alteration of CCl4's chemical reactivity at low temperatures. While generally considered stable, carbon tetrachloride can become more susceptible to certain reactions in cold conditions. This increased reactivity may lead to unexpected degradation or the formation of byproducts, which can compromise the purity and effectiveness of the compound in its intended applications.
The volatility of CCl4 also changes with temperature, affecting its handling and containment. At lower temperatures, the vapor pressure decreases, which can impact processes that rely on the compound's gaseous state. This reduction in volatility may necessitate modifications to existing systems designed for room temperature operations, adding complexity and cost to industrial processes.
Furthermore, low temperatures can affect the solubility of CCl4 in various solvents, potentially leading to precipitation or separation in mixtures. This can be particularly problematic in applications where CCl4 is used as a solvent or in solution with other compounds. The altered solubility characteristics may require adjustments to formulations or processing methods to maintain desired product qualities.
The viscosity of carbon tetrachloride also increases as temperatures drop, which can impact its flow properties. This change in viscosity can affect pumping efficiency, heat transfer rates, and mixing processes, potentially leading to reduced productivity or increased energy consumption in industrial applications.
Lastly, the potential for condensation and moisture accumulation in cold environments poses a significant challenge. Water contamination can lead to the formation of hydrochloric acid through the hydrolysis of CCl4, which can cause corrosion in storage and handling equipment. This not only threatens the integrity of containment systems but also introduces safety hazards and potential environmental risks.
Current Solutions
01 Chemical stabilization methods
Various chemical methods are employed to enhance the stability of carbon tetrachloride. These may include the addition of stabilizing agents, antioxidants, or other compounds that prevent decomposition or reaction with other substances. Such methods aim to extend the shelf life and maintain the purity of carbon tetrachloride during storage and use.- Chemical stability and decomposition prevention: Carbon tetrachloride's stability can be improved by preventing its decomposition. This involves using stabilizing agents or specific storage conditions to maintain its chemical integrity. Techniques may include adding inhibitors or controlling temperature and light exposure to minimize degradation.
- Purification and quality control: Enhancing the stability of carbon tetrachloride often requires purification processes and strict quality control measures. This may involve distillation, filtration, or other separation techniques to remove impurities that could catalyze decomposition. Regular testing and monitoring of the compound's purity are essential for maintaining its stability.
- Storage and handling considerations: Proper storage and handling practices are crucial for maintaining carbon tetrachloride stability. This includes using appropriate container materials, controlling storage temperature and humidity, and minimizing exposure to air and light. Specialized equipment and safety protocols may be necessary for handling this compound safely while preserving its stability.
- Stabilizing additives and formulations: The addition of specific stabilizing agents or the development of specialized formulations can significantly improve carbon tetrachloride stability. These additives may include antioxidants, UV absorbers, or other compounds that prevent or slow down degradation processes. The choice and concentration of stabilizers depend on the intended use and storage conditions of the carbon tetrachloride.
- Alternative compounds and substitutes: Due to environmental and health concerns associated with carbon tetrachloride, research has focused on developing more stable and safer alternatives. This includes identifying or synthesizing compounds with similar properties but improved stability and reduced toxicity. These substitutes aim to replace carbon tetrachloride in various applications while addressing stability issues.
02 Storage and handling techniques
Proper storage and handling techniques are crucial for maintaining the stability of carbon tetrachloride. This includes using appropriate containers, controlling temperature and humidity, and minimizing exposure to light and air. Specific guidelines for storage, transportation, and handling may be implemented to prevent degradation and ensure safety.Expand Specific Solutions03 Purification and quality control
Purification processes and quality control measures are essential for ensuring the stability of carbon tetrachloride. These may involve distillation, filtration, or other separation techniques to remove impurities that could affect stability. Regular testing and monitoring of purity levels and chemical properties are also important aspects of maintaining stability.Expand Specific Solutions04 Modification of molecular structure
Research into modifying the molecular structure of carbon tetrachloride or creating derivatives with improved stability properties. This may involve the synthesis of related compounds or the development of new formulations that retain the desired properties of carbon tetrachloride while enhancing its stability under various conditions.Expand Specific Solutions05 Environmental and safety considerations
Addressing environmental and safety concerns related to carbon tetrachloride stability. This includes developing safer alternatives, implementing pollution control measures, and creating protocols for proper disposal. Research in this area aims to balance the need for stability with environmental protection and human safety considerations.Expand Specific Solutions
Industry Leaders
The competition landscape for "How Low-Temperature Conditions Affect Carbon Tetrachloride Stability" is in a mature phase, with established players and ongoing research. The market size is moderate, driven by industrial and environmental applications. Technologically, the field is well-developed but still evolving. Key players like BASF, DuPont, and Bayer AG have extensive experience in chemical stability research. Academic institutions such as Central South University and Fudan University contribute to fundamental research. Specialized companies like Incisive Genetics and Cronus Research Labs may offer niche expertise. The involvement of diverse entities indicates ongoing interest in improving understanding and applications of carbon tetrachloride stability under low-temperature conditions.
DuPont de Nemours, Inc.
Technical Solution: DuPont has engineered a novel containment system for carbon tetrachloride storage and transport under low-temperature conditions. Their solution involves a multi-layered container design that incorporates thermal insulation and pressure regulation mechanisms. The innermost layer is coated with a proprietary material that resists CCl4 corrosion at low temperatures, while the outer layers provide thermal stability[3]. This system maintains CCl4 in a liquid state even at temperatures well below its normal freezing point, ensuring its stability and preventing phase changes that could compromise its chemical integrity[4].
Strengths: Enhances safety in CCl4 handling and transportation in cold climates. Weaknesses: Higher initial investment for specialized containment systems.
BASF Corp.
Technical Solution: BASF has developed innovative stabilization techniques for carbon tetrachloride under low-temperature conditions. Their approach involves the use of specialized additives that maintain the chemical's stability even at temperatures approaching its freezing point (-23°C). The company has implemented a proprietary blend of antioxidants and stabilizers that form a protective layer around CCl4 molecules, preventing degradation and maintaining its chemical properties[1]. This technology has been particularly useful in industrial refrigeration systems and cold-climate applications where CCl4 is used as a solvent or intermediate[2].
Strengths: Extends CCl4 usability in cold environments, reduces the need for frequent replacements. Weaknesses: May increase production costs, potential environmental concerns with additives.
Key Research Findings
Process of preparation of monobasic lead salt of 2,4 di nitro resorcinol using trichloro ethylene as an inert
PatentInactiveIN201821045441A
Innovation
- The process involves using trichloroethylene as an inert solvent media to replace carbon tetrachloride, involving nitrosation of resorcinol followed by alkaline oxidation and subsequent purification of 2,4-dinitroso resorcinol to produce monobasic lead salt of 2,4-di nitro resorcinol, which is then used in detonator compositions and fuze applications.
Antibody formulation diluent
PatentPendingUS20240092903A1
Innovation
- A diluent comprising a buffer and stabilizing or tonicity agents, such as Histidine, Tris, Citrate, Polysorbate 80, Arginine, and Lysine, is used to minimize antibody loss in infusion systems, maintaining stability and bioavailability by controlling pH and osmotic pressure, and is stable over various temperatures and storage conditions.
Environmental Impact
The environmental impact of carbon tetrachloride (CCl4) under low-temperature conditions is a critical concern due to its potential for long-term persistence and widespread distribution. At lower temperatures, CCl4 exhibits increased stability, which can lead to prolonged presence in various environmental compartments. This enhanced stability affects its transport, degradation, and accumulation patterns in ecosystems.
In aquatic environments, low temperatures reduce the rate of CCl4 hydrolysis and photolysis, extending its residence time in water bodies. This prolonged presence increases the risk of bioaccumulation in aquatic organisms and potential biomagnification through the food chain. Cold water conditions also affect the volatilization rate of CCl4, potentially leading to higher concentrations in surface waters and sediments.
Atmospheric transport of CCl4 is significantly influenced by low-temperature conditions. In colder regions, such as polar areas, CCl4 can persist for longer periods in the troposphere due to reduced photochemical degradation. This persistence allows for long-range transport, contributing to global distribution and potential impacts on remote ecosystems.
Soil environments are also affected by the increased stability of CCl4 at low temperatures. Reduced microbial activity and slower chemical reactions in cold soils can result in prolonged retention of CCl4 in soil matrices. This persistence may lead to increased risks of groundwater contamination through leaching processes, particularly in areas with permafrost or seasonally frozen soils.
The enhanced stability of CCl4 under low-temperature conditions has implications for global environmental policies and remediation strategies. It underscores the need for specialized approaches in cold climate regions to address CCl4 contamination and highlights the importance of considering temperature-dependent behavior in risk assessments and environmental modeling.
Furthermore, the interaction between CCl4 and other pollutants in cold environments may lead to synergistic effects, potentially exacerbating environmental impacts. This complex interplay necessitates comprehensive studies to fully understand the ecological consequences of CCl4 stability in low-temperature conditions across various environmental compartments.
In aquatic environments, low temperatures reduce the rate of CCl4 hydrolysis and photolysis, extending its residence time in water bodies. This prolonged presence increases the risk of bioaccumulation in aquatic organisms and potential biomagnification through the food chain. Cold water conditions also affect the volatilization rate of CCl4, potentially leading to higher concentrations in surface waters and sediments.
Atmospheric transport of CCl4 is significantly influenced by low-temperature conditions. In colder regions, such as polar areas, CCl4 can persist for longer periods in the troposphere due to reduced photochemical degradation. This persistence allows for long-range transport, contributing to global distribution and potential impacts on remote ecosystems.
Soil environments are also affected by the increased stability of CCl4 at low temperatures. Reduced microbial activity and slower chemical reactions in cold soils can result in prolonged retention of CCl4 in soil matrices. This persistence may lead to increased risks of groundwater contamination through leaching processes, particularly in areas with permafrost or seasonally frozen soils.
The enhanced stability of CCl4 under low-temperature conditions has implications for global environmental policies and remediation strategies. It underscores the need for specialized approaches in cold climate regions to address CCl4 contamination and highlights the importance of considering temperature-dependent behavior in risk assessments and environmental modeling.
Furthermore, the interaction between CCl4 and other pollutants in cold environments may lead to synergistic effects, potentially exacerbating environmental impacts. This complex interplay necessitates comprehensive studies to fully understand the ecological consequences of CCl4 stability in low-temperature conditions across various environmental compartments.
Safety Regulations
The safety regulations surrounding the use and handling of carbon tetrachloride under low-temperature conditions are crucial due to the compound's potential instability and hazardous nature. Regulatory bodies, such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA), have established strict guidelines to ensure the safe use of this chemical in various industrial and laboratory settings.
Under low-temperature conditions, carbon tetrachloride may exhibit altered physical properties and reactivity, necessitating specific safety measures. OSHA mandates that workers handling carbon tetrachloride in cold environments must be provided with appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and respiratory protection. The agency also requires employers to conduct regular risk assessments and provide comprehensive training on the proper handling and storage of the compound at low temperatures.
The EPA has set stringent regulations regarding the storage and transportation of carbon tetrachloride, particularly when exposed to cold conditions. These regulations include requirements for specialized containment systems, temperature-controlled storage facilities, and regular monitoring of storage conditions. Additionally, the EPA enforces strict reporting requirements for any accidental releases or spills of carbon tetrachloride, with specific protocols for low-temperature incidents.
International regulations, such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) program, also address the use of carbon tetrachloride under various conditions. REACH requires manufacturers and importers to assess and manage the risks associated with the substance, including its behavior at low temperatures, and to communicate this information to downstream users.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication for carbon tetrachloride, including specific precautionary statements related to its use in cold environments. This system ensures consistent safety information across different countries and regions.
In the context of laboratory safety, organizations such as the American Chemical Society (ACS) have developed guidelines for the safe handling of carbon tetrachloride at low temperatures. These guidelines emphasize the importance of proper ventilation, the use of fume hoods, and the implementation of spill containment measures specifically designed for cold conditions.
Regulatory bodies also mandate regular safety audits and inspections for facilities handling carbon tetrachloride, with particular attention paid to low-temperature storage and processing areas. These audits aim to ensure compliance with safety regulations and identify potential risks associated with the compound's stability under cold conditions.
Under low-temperature conditions, carbon tetrachloride may exhibit altered physical properties and reactivity, necessitating specific safety measures. OSHA mandates that workers handling carbon tetrachloride in cold environments must be provided with appropriate personal protective equipment (PPE), including chemical-resistant gloves, goggles, and respiratory protection. The agency also requires employers to conduct regular risk assessments and provide comprehensive training on the proper handling and storage of the compound at low temperatures.
The EPA has set stringent regulations regarding the storage and transportation of carbon tetrachloride, particularly when exposed to cold conditions. These regulations include requirements for specialized containment systems, temperature-controlled storage facilities, and regular monitoring of storage conditions. Additionally, the EPA enforces strict reporting requirements for any accidental releases or spills of carbon tetrachloride, with specific protocols for low-temperature incidents.
International regulations, such as the European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) program, also address the use of carbon tetrachloride under various conditions. REACH requires manufacturers and importers to assess and manage the risks associated with the substance, including its behavior at low temperatures, and to communicate this information to downstream users.
The United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard communication for carbon tetrachloride, including specific precautionary statements related to its use in cold environments. This system ensures consistent safety information across different countries and regions.
In the context of laboratory safety, organizations such as the American Chemical Society (ACS) have developed guidelines for the safe handling of carbon tetrachloride at low temperatures. These guidelines emphasize the importance of proper ventilation, the use of fume hoods, and the implementation of spill containment measures specifically designed for cold conditions.
Regulatory bodies also mandate regular safety audits and inspections for facilities handling carbon tetrachloride, with particular attention paid to low-temperature storage and processing areas. These audits aim to ensure compliance with safety regulations and identify potential risks associated with the compound's stability under cold conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!