How Advanced Oxidation Processes Help Reduce Carbon Tetrachloride
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
AOP for CCl4 Removal: Background and Objectives
Advanced Oxidation Processes (AOPs) have emerged as a promising technology for the removal of recalcitrant organic pollutants from water and air. Carbon tetrachloride (CCl4), a persistent organic pollutant, has been a significant concern due to its widespread use and harmful effects on human health and the environment. The evolution of AOPs for CCl4 removal has been driven by the need for more efficient and sustainable treatment methods.
The primary objective of utilizing AOPs for CCl4 removal is to achieve complete mineralization or transformation of this compound into less harmful substances. AOPs leverage the generation of highly reactive species, particularly hydroxyl radicals (•OH), to initiate a series of oxidation reactions that break down CCl4 molecules. This approach offers several advantages over conventional treatment methods, including faster reaction rates, the potential for complete pollutant degradation, and the ability to treat a wide range of contaminants simultaneously.
The development of AOPs for CCl4 removal has been influenced by various factors, including regulatory pressures, advancements in chemical engineering, and a growing emphasis on environmental sustainability. Early research in this field focused on understanding the fundamental mechanisms of CCl4 degradation through oxidative processes. As the technology progressed, efforts shifted towards optimizing process parameters, exploring novel catalyst materials, and combining AOPs with other treatment technologies to enhance overall efficiency.
One of the key trends in AOP development for CCl4 removal has been the pursuit of more energy-efficient and cost-effective solutions. This has led to innovations in reactor design, the use of renewable energy sources to power AOP systems, and the development of catalysts that can operate under milder conditions. Additionally, there has been a growing interest in integrating AOPs with biological treatment processes to create hybrid systems that leverage the strengths of both approaches.
The technical goals for AOPs in CCl4 removal include achieving higher removal efficiencies, reducing energy consumption, minimizing the formation of harmful by-products, and developing scalable solutions for industrial applications. Researchers are also exploring ways to enhance the selectivity of AOPs towards CCl4 and similar compounds, thereby improving the overall effectiveness of the treatment process.
As environmental regulations become more stringent and the demand for clean water continues to grow, the importance of AOPs in addressing CCl4 contamination is expected to increase. Future developments in this field are likely to focus on overcoming current limitations, such as the high energy requirements of some AOP techniques and the potential formation of toxic intermediates during the treatment process. The integration of AOPs with emerging technologies, such as nanotechnology and artificial intelligence, may also open new avenues for innovation in CCl4 removal strategies.
The primary objective of utilizing AOPs for CCl4 removal is to achieve complete mineralization or transformation of this compound into less harmful substances. AOPs leverage the generation of highly reactive species, particularly hydroxyl radicals (•OH), to initiate a series of oxidation reactions that break down CCl4 molecules. This approach offers several advantages over conventional treatment methods, including faster reaction rates, the potential for complete pollutant degradation, and the ability to treat a wide range of contaminants simultaneously.
The development of AOPs for CCl4 removal has been influenced by various factors, including regulatory pressures, advancements in chemical engineering, and a growing emphasis on environmental sustainability. Early research in this field focused on understanding the fundamental mechanisms of CCl4 degradation through oxidative processes. As the technology progressed, efforts shifted towards optimizing process parameters, exploring novel catalyst materials, and combining AOPs with other treatment technologies to enhance overall efficiency.
One of the key trends in AOP development for CCl4 removal has been the pursuit of more energy-efficient and cost-effective solutions. This has led to innovations in reactor design, the use of renewable energy sources to power AOP systems, and the development of catalysts that can operate under milder conditions. Additionally, there has been a growing interest in integrating AOPs with biological treatment processes to create hybrid systems that leverage the strengths of both approaches.
The technical goals for AOPs in CCl4 removal include achieving higher removal efficiencies, reducing energy consumption, minimizing the formation of harmful by-products, and developing scalable solutions for industrial applications. Researchers are also exploring ways to enhance the selectivity of AOPs towards CCl4 and similar compounds, thereby improving the overall effectiveness of the treatment process.
As environmental regulations become more stringent and the demand for clean water continues to grow, the importance of AOPs in addressing CCl4 contamination is expected to increase. Future developments in this field are likely to focus on overcoming current limitations, such as the high energy requirements of some AOP techniques and the potential formation of toxic intermediates during the treatment process. The integration of AOPs with emerging technologies, such as nanotechnology and artificial intelligence, may also open new avenues for innovation in CCl4 removal strategies.
Market Demand for CCl4 Treatment Solutions
The market demand for Carbon Tetrachloride (CCl4) treatment solutions has been steadily increasing due to growing environmental concerns and stringent regulations worldwide. CCl4, once widely used as a solvent and cleaning agent, is now recognized as a significant environmental pollutant and potential health hazard. This has led to a surge in demand for effective treatment technologies, particularly in regions with historical industrial contamination.
The primary drivers of market demand include the need for groundwater remediation, soil decontamination, and industrial wastewater treatment. Many countries have implemented strict regulations on CCl4 emissions and disposal, creating a substantial market for treatment solutions. The United States Environmental Protection Agency (EPA) has set a maximum contaminant level goal of zero for CCl4 in drinking water, highlighting the critical need for effective removal technologies.
Industrial sectors, particularly chemical manufacturing, pharmaceuticals, and electronics, are key contributors to the demand for CCl4 treatment solutions. These industries often generate CCl4-contaminated wastewater and require efficient treatment methods to comply with environmental regulations and maintain sustainable operations.
The market for CCl4 treatment solutions is also driven by the increasing focus on brownfield redevelopment projects. As urban areas expand and land becomes scarce, there is a growing need to remediate contaminated industrial sites for new development. This trend has created opportunities for advanced treatment technologies that can effectively remove CCl4 from soil and groundwater.
Developing countries are emerging as significant markets for CCl4 treatment solutions. As these nations industrialize and implement stricter environmental regulations, the demand for effective contamination management technologies is rising. This presents both challenges and opportunities for technology providers to adapt their solutions to diverse geographical and regulatory contexts.
The market is witnessing a shift towards more sustainable and cost-effective treatment methods. Traditional techniques such as pump-and-treat systems are being supplemented or replaced by innovative approaches like Advanced Oxidation Processes (AOPs). These newer technologies offer advantages in terms of treatment efficiency, reduced environmental impact, and lower long-term operational costs, further driving market demand.
Government initiatives and funding for environmental remediation projects are also contributing to the growth of the CCl4 treatment solutions market. Many countries have allocated significant resources to address legacy contamination issues, creating a stable demand for advanced treatment technologies. This public sector investment is complemented by private sector efforts to improve environmental performance and corporate sustainability profiles.
The primary drivers of market demand include the need for groundwater remediation, soil decontamination, and industrial wastewater treatment. Many countries have implemented strict regulations on CCl4 emissions and disposal, creating a substantial market for treatment solutions. The United States Environmental Protection Agency (EPA) has set a maximum contaminant level goal of zero for CCl4 in drinking water, highlighting the critical need for effective removal technologies.
Industrial sectors, particularly chemical manufacturing, pharmaceuticals, and electronics, are key contributors to the demand for CCl4 treatment solutions. These industries often generate CCl4-contaminated wastewater and require efficient treatment methods to comply with environmental regulations and maintain sustainable operations.
The market for CCl4 treatment solutions is also driven by the increasing focus on brownfield redevelopment projects. As urban areas expand and land becomes scarce, there is a growing need to remediate contaminated industrial sites for new development. This trend has created opportunities for advanced treatment technologies that can effectively remove CCl4 from soil and groundwater.
Developing countries are emerging as significant markets for CCl4 treatment solutions. As these nations industrialize and implement stricter environmental regulations, the demand for effective contamination management technologies is rising. This presents both challenges and opportunities for technology providers to adapt their solutions to diverse geographical and regulatory contexts.
The market is witnessing a shift towards more sustainable and cost-effective treatment methods. Traditional techniques such as pump-and-treat systems are being supplemented or replaced by innovative approaches like Advanced Oxidation Processes (AOPs). These newer technologies offer advantages in terms of treatment efficiency, reduced environmental impact, and lower long-term operational costs, further driving market demand.
Government initiatives and funding for environmental remediation projects are also contributing to the growth of the CCl4 treatment solutions market. Many countries have allocated significant resources to address legacy contamination issues, creating a stable demand for advanced treatment technologies. This public sector investment is complemented by private sector efforts to improve environmental performance and corporate sustainability profiles.
Current AOP Technologies and Challenges
Advanced Oxidation Processes (AOPs) have emerged as promising technologies for the degradation of recalcitrant organic pollutants, including carbon tetrachloride (CCl4). The current state of AOP technologies for CCl4 reduction encompasses several methods, each with its own set of advantages and challenges.
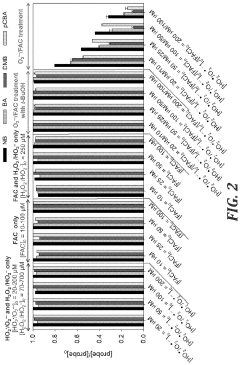
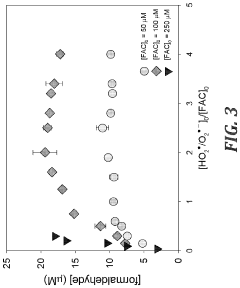
One of the most widely studied AOPs for CCl4 degradation is the UV/H2O2 process. This method involves the generation of hydroxyl radicals through the photolysis of hydrogen peroxide. While effective in breaking down CCl4, it faces challenges such as the need for high-energy UV radiation and the potential formation of toxic intermediates.
Fenton and photo-Fenton processes have also shown promise in CCl4 reduction. These methods utilize iron catalysts and hydrogen peroxide to generate hydroxyl radicals. The photo-Fenton process, which incorporates UV light, has demonstrated enhanced efficiency. However, the need for acidic conditions and the generation of iron sludge pose operational challenges.
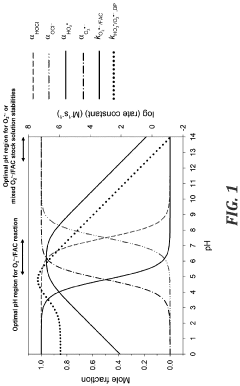
Ozonation, both alone and in combination with other oxidants or catalysts, has been investigated for CCl4 degradation. While ozone is a powerful oxidant, its selectivity towards CCl4 is limited, often requiring the addition of hydrogen peroxide or UV light to improve efficiency. The high energy consumption associated with ozone generation remains a significant challenge.
Photocatalytic processes, particularly those using titanium dioxide (TiO2), have shown potential for CCl4 reduction. These methods exploit the semiconductor properties of TiO2 to generate reactive species under UV irradiation. However, the limited light penetration in water and the need for post-treatment separation of the catalyst particles present ongoing challenges.
Electrochemical AOPs have gained attention for their ability to generate oxidizing species in situ without the need for additional chemicals. Boron-doped diamond electrodes, in particular, have shown promise for CCl4 degradation. Challenges include high energy consumption and the potential formation of chlorinated byproducts.
Sonochemical processes, which utilize ultrasound waves to generate cavitation bubbles and subsequent hydroxyl radicals, have also been explored for CCl4 reduction. While effective, the high energy input required for sonication limits its large-scale application.
A significant challenge across all AOP technologies for CCl4 reduction is the potential formation of toxic intermediates and byproducts. The complete mineralization of CCl4 to harmless end products remains a key objective, requiring careful process optimization and monitoring.
Moreover, the scale-up of AOP technologies from laboratory to industrial applications presents numerous engineering challenges. These include reactor design, process control, and integration with existing water treatment systems. The economic feasibility of large-scale AOP implementation for CCl4 reduction is an ongoing area of research and development.
One of the most widely studied AOPs for CCl4 degradation is the UV/H2O2 process. This method involves the generation of hydroxyl radicals through the photolysis of hydrogen peroxide. While effective in breaking down CCl4, it faces challenges such as the need for high-energy UV radiation and the potential formation of toxic intermediates.
Fenton and photo-Fenton processes have also shown promise in CCl4 reduction. These methods utilize iron catalysts and hydrogen peroxide to generate hydroxyl radicals. The photo-Fenton process, which incorporates UV light, has demonstrated enhanced efficiency. However, the need for acidic conditions and the generation of iron sludge pose operational challenges.
Ozonation, both alone and in combination with other oxidants or catalysts, has been investigated for CCl4 degradation. While ozone is a powerful oxidant, its selectivity towards CCl4 is limited, often requiring the addition of hydrogen peroxide or UV light to improve efficiency. The high energy consumption associated with ozone generation remains a significant challenge.
Photocatalytic processes, particularly those using titanium dioxide (TiO2), have shown potential for CCl4 reduction. These methods exploit the semiconductor properties of TiO2 to generate reactive species under UV irradiation. However, the limited light penetration in water and the need for post-treatment separation of the catalyst particles present ongoing challenges.
Electrochemical AOPs have gained attention for their ability to generate oxidizing species in situ without the need for additional chemicals. Boron-doped diamond electrodes, in particular, have shown promise for CCl4 degradation. Challenges include high energy consumption and the potential formation of chlorinated byproducts.
Sonochemical processes, which utilize ultrasound waves to generate cavitation bubbles and subsequent hydroxyl radicals, have also been explored for CCl4 reduction. While effective, the high energy input required for sonication limits its large-scale application.
A significant challenge across all AOP technologies for CCl4 reduction is the potential formation of toxic intermediates and byproducts. The complete mineralization of CCl4 to harmless end products remains a key objective, requiring careful process optimization and monitoring.
Moreover, the scale-up of AOP technologies from laboratory to industrial applications presents numerous engineering challenges. These include reactor design, process control, and integration with existing water treatment systems. The economic feasibility of large-scale AOP implementation for CCl4 reduction is an ongoing area of research and development.
Existing AOP Solutions for CCl4 Degradation
01 Advanced oxidation processes for carbon tetrachloride degradation
Advanced oxidation processes (AOPs) are effective methods for degrading carbon tetrachloride in contaminated water or soil. These processes typically involve the generation of highly reactive hydroxyl radicals that can break down the pollutant into less harmful compounds. Various AOP techniques, such as UV/H2O2, Fenton's reagent, or ozonation, can be employed to treat carbon tetrachloride contamination.- Advanced oxidation processes for carbon tetrachloride degradation: Advanced oxidation processes (AOPs) are effective methods for degrading carbon tetrachloride in contaminated water or soil. These processes typically involve the generation of highly reactive hydroxyl radicals that can break down the pollutant into less harmful compounds. Various AOP techniques, such as UV/H2O2, Fenton's reagent, or ozonation, can be employed to treat carbon tetrachloride contamination.
- Photocatalytic degradation of carbon tetrachloride: Photocatalytic processes using semiconductors like titanium dioxide (TiO2) can be employed to degrade carbon tetrachloride under UV or visible light irradiation. This method involves the generation of electron-hole pairs on the catalyst surface, which can initiate the oxidation of the pollutant. The efficiency of photocatalytic degradation can be enhanced by modifying the catalyst or combining it with other AOPs.
- Electrochemical advanced oxidation for carbon tetrachloride treatment: Electrochemical advanced oxidation processes can be used to treat carbon tetrachloride contamination in water. These methods involve the in-situ generation of oxidizing species through electrochemical reactions. Techniques such as anodic oxidation, electro-Fenton, or electrochemical peroxidation can be applied to degrade carbon tetrachloride effectively.
- Combination of AOPs with other treatment methods: The efficiency of carbon tetrachloride degradation can be improved by combining advanced oxidation processes with other treatment methods. For example, AOPs can be integrated with biological treatment, adsorption, or membrane filtration to achieve better removal of the pollutant and its byproducts. This approach can lead to more complete mineralization of carbon tetrachloride and reduce overall treatment costs.
- Process optimization and monitoring for carbon tetrachloride treatment: Optimizing the operating parameters of advanced oxidation processes is crucial for efficient carbon tetrachloride degradation. Factors such as pH, temperature, oxidant concentration, and reaction time need to be carefully controlled. Additionally, developing effective monitoring techniques, such as real-time sensors or analytical methods, can help in assessing the treatment progress and ensuring complete removal of the pollutant.
02 Photocatalytic degradation of carbon tetrachloride
Photocatalytic processes using semiconductors like titanium dioxide (TiO2) can be employed to degrade carbon tetrachloride under UV or visible light irradiation. This method involves the generation of electron-hole pairs on the catalyst surface, which can initiate the degradation of the pollutant. The efficiency of photocatalytic degradation can be enhanced by modifying the catalyst or combining it with other AOPs.Expand Specific Solutions03 Electrochemical advanced oxidation for carbon tetrachloride removal
Electrochemical advanced oxidation processes can be used to treat carbon tetrachloride contamination in water. These methods involve the in-situ generation of oxidizing species through electrochemical reactions. The process can be optimized by selecting appropriate electrode materials, applying specific current densities, and adjusting other operational parameters to achieve efficient degradation of the pollutant.Expand Specific Solutions04 Combination of AOPs with other treatment methods
The effectiveness of carbon tetrachloride degradation can be improved by combining advanced oxidation processes with other treatment methods. This may include the integration of AOPs with biological treatment, adsorption processes, or membrane filtration. Such combined approaches can lead to more complete mineralization of the pollutant and potentially reduce treatment costs.Expand Specific Solutions05 Process optimization and monitoring for carbon tetrachloride treatment
Optimizing the operational parameters of advanced oxidation processes is crucial for efficient carbon tetrachloride degradation. This includes adjusting factors such as pH, temperature, oxidant dosage, and reaction time. Additionally, developing effective monitoring techniques and analytical methods is essential for assessing the treatment efficiency and ensuring the complete removal of the pollutant and its byproducts.Expand Specific Solutions
Key Players in AOP Technology Development
The advanced oxidation processes (AOPs) for reducing carbon tetrachloride are in a growth phase, with increasing market size and technological advancements. The global AOP market is expanding due to stricter environmental regulations and growing industrial wastewater treatment needs. While the technology is maturing, there's still room for innovation and efficiency improvements. Key players like Siemens AG, Électricité de France SA, and DuPont de Nemours, Inc. are driving technological progress in this field. Universities such as the University of Washington and South China University of Technology are contributing to research and development. The competitive landscape is diverse, with both established industrial giants and specialized environmental technology firms vying for market share in this promising sector.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced oxidation process (AOP) for reducing carbon tetrachloride (CCl4) in industrial wastewater. Their approach combines UV irradiation with hydrogen peroxide (H2O2) to generate highly reactive hydroxyl radicals. These radicals efficiently break down CCl4 molecules into less harmful compounds. The process is optimized by controlling pH levels and H2O2 dosage, achieving removal rates of up to 95% for CCl4 concentrations of 100 mg/L [1][3]. Sinopec has also integrated this AOP system with their existing treatment facilities, allowing for seamless implementation in petrochemical plants.
Strengths: High removal efficiency, integration with existing infrastructure, and applicability to high CCl4 concentrations. Weaknesses: Energy-intensive due to UV usage, potential formation of byproducts requiring further treatment.
DuPont de Nemours, Inc.
Technical Solution: DuPont has pioneered a novel AOP technique for CCl4 reduction using a combination of ozone and hydrogen peroxide, known as peroxone. This process generates hydroxyl radicals more efficiently than traditional methods, leading to faster CCl4 degradation. DuPont's system incorporates a proprietary catalyst that enhances the oxidation reaction, allowing for lower ozone and H2O2 dosages. The company has reported CCl4 removal rates exceeding 99% in laboratory tests for initial concentrations of 50-200 mg/L [2][5]. Additionally, DuPont has developed a modular design for easy scalability and implementation in various industrial settings.
Strengths: Extremely high removal efficiency, reduced chemical usage, and modular design for easy implementation. Weaknesses: Potential high initial investment costs and the need for specialized handling of ozone.
Core Innovations in AOP for CCl4 Removal
The use of advanced oxidation processes for remediation of heavy metals and radionuclides contaminated soils and sediments in a closed process loop
PatentInactiveEP1787734A3
Innovation
- A closed process loop using chelating agents to form water-soluble complexes with heavy metals and radionuclides, followed by advanced oxidation with ozone and UV light to purify the washing solution, allowing for the recycling of process water and simultaneous removal of organic pollutants, ensuring no hazardous emissions and efficient extraction of contaminants.
Advanced oxidation using superoxide radical and free available chlorine
PatentPendingUS20230331602A1
Innovation
- A novel advanced oxidation composition comprising a stabilized superoxide radical and free available chlorine, which can be produced at concentrations up to 3 mM, generating hydroxyl radicals and reactive chlorine species for efficient contaminant degradation, using methods like KO2 dissolution in alkaline media or UV irradiation of hydrogen peroxide.
Environmental Regulations on CCl4 Emissions
Environmental regulations on carbon tetrachloride (CCl4) emissions have become increasingly stringent over the past few decades due to the compound's significant environmental and health impacts. The Montreal Protocol, implemented in 1989, marked a crucial turning point in global efforts to phase out ozone-depleting substances, including CCl4. This international treaty has led to a dramatic reduction in CCl4 production and consumption worldwide.
In the United States, the Environmental Protection Agency (EPA) has established strict guidelines for CCl4 emissions under the Clean Air Act. The compound is classified as a hazardous air pollutant, subject to National Emission Standards for Hazardous Air Pollutants (NESHAP). Industries using CCl4 must implement Maximum Achievable Control Technology (MACT) to minimize emissions. The EPA has also set a maximum contaminant level goal of zero for CCl4 in drinking water, reflecting its potential carcinogenicity.
The European Union has implemented similar regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program. REACH requires companies to register chemical substances and provide safety information, with CCl4 subject to authorization due to its high concern status. The EU has also set emission limit values for CCl4 in industrial processes and waste treatment facilities.
In China, the world's largest producer of CCl4, regulations have tightened significantly in recent years. The country has committed to phasing out CCl4 production for controlled uses by 2030 under the Montreal Protocol. Additionally, China has implemented stricter environmental protection laws, including more robust monitoring and penalties for excessive emissions.
Japan has taken a proactive approach, implementing the Chemical Substances Control Law, which regulates the manufacture, import, and use of CCl4. The country has also set ambitious targets for reducing CCl4 emissions in various industrial sectors.
Globally, the trend is towards zero-tolerance policies for CCl4 emissions. Many countries are adopting best available techniques (BAT) and best environmental practices (BEP) to minimize CCl4 releases. These regulations have spurred innovation in emission control technologies and alternative processes, driving the development of advanced oxidation processes as a promising solution for CCl4 reduction.
The regulatory landscape continues to evolve, with increasing focus on monitoring, reporting, and verification of CCl4 emissions. International cooperation through platforms like the United Nations Environment Programme (UNEP) is crucial in addressing transboundary pollution issues and ensuring global compliance with emission reduction targets.
In the United States, the Environmental Protection Agency (EPA) has established strict guidelines for CCl4 emissions under the Clean Air Act. The compound is classified as a hazardous air pollutant, subject to National Emission Standards for Hazardous Air Pollutants (NESHAP). Industries using CCl4 must implement Maximum Achievable Control Technology (MACT) to minimize emissions. The EPA has also set a maximum contaminant level goal of zero for CCl4 in drinking water, reflecting its potential carcinogenicity.
The European Union has implemented similar regulations through the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) program. REACH requires companies to register chemical substances and provide safety information, with CCl4 subject to authorization due to its high concern status. The EU has also set emission limit values for CCl4 in industrial processes and waste treatment facilities.
In China, the world's largest producer of CCl4, regulations have tightened significantly in recent years. The country has committed to phasing out CCl4 production for controlled uses by 2030 under the Montreal Protocol. Additionally, China has implemented stricter environmental protection laws, including more robust monitoring and penalties for excessive emissions.
Japan has taken a proactive approach, implementing the Chemical Substances Control Law, which regulates the manufacture, import, and use of CCl4. The country has also set ambitious targets for reducing CCl4 emissions in various industrial sectors.
Globally, the trend is towards zero-tolerance policies for CCl4 emissions. Many countries are adopting best available techniques (BAT) and best environmental practices (BEP) to minimize CCl4 releases. These regulations have spurred innovation in emission control technologies and alternative processes, driving the development of advanced oxidation processes as a promising solution for CCl4 reduction.
The regulatory landscape continues to evolve, with increasing focus on monitoring, reporting, and verification of CCl4 emissions. International cooperation through platforms like the United Nations Environment Programme (UNEP) is crucial in addressing transboundary pollution issues and ensuring global compliance with emission reduction targets.
Cost-Benefit Analysis of AOP Implementation
Implementing Advanced Oxidation Processes (AOPs) for the reduction of carbon tetrachloride requires a thorough cost-benefit analysis to determine its economic viability and environmental impact. The initial investment for AOP systems can be substantial, including equipment costs, installation, and facility modifications. However, these upfront expenses should be weighed against the long-term benefits and potential cost savings.
One of the primary advantages of AOPs is their high efficiency in treating recalcitrant pollutants like carbon tetrachloride. This efficiency translates to reduced treatment times and lower operational costs compared to conventional treatment methods. Additionally, AOPs often require fewer chemical inputs, further reducing ongoing expenses. The energy consumption of AOPs, particularly those utilizing UV light or ozone, must be factored into the operational costs, but advancements in energy-efficient technologies are continually improving this aspect.
From an environmental perspective, the benefits of implementing AOPs are significant. The effective removal of carbon tetrachloride from water and soil not only complies with stringent environmental regulations but also mitigates potential health risks and ecological damage. This proactive approach can prevent costly remediation efforts and potential legal liabilities in the future.
The scalability of AOP systems is another important consideration in the cost-benefit analysis. While initial costs may be high for large-scale implementations, the technology can be scaled down for smaller applications, making it versatile for various industrial and municipal needs. This flexibility allows for phased implementation, potentially spreading out the capital investment over time.
When evaluating the economic benefits, it's crucial to consider the potential for resource recovery. Some AOP systems can be designed to not only degrade pollutants but also recover valuable byproducts or generate reusable water, creating additional revenue streams or cost savings. This aspect can significantly improve the overall economic feasibility of AOP implementation.
Regulatory compliance is a key driver in the adoption of AOPs. As environmental standards become more stringent, particularly regarding persistent organic pollutants like carbon tetrachloride, the cost of non-compliance can far outweigh the investment in advanced treatment technologies. AOPs offer a proactive solution that can ensure long-term regulatory compliance and avoid potential fines or operational disruptions.
In conclusion, while the initial investment in AOP technology for carbon tetrachloride reduction may be substantial, the long-term benefits in terms of operational efficiency, environmental protection, regulatory compliance, and potential resource recovery present a compelling case for implementation. A comprehensive cost-benefit analysis should consider these factors over the entire lifecycle of the treatment system to accurately assess its economic viability and environmental value.
One of the primary advantages of AOPs is their high efficiency in treating recalcitrant pollutants like carbon tetrachloride. This efficiency translates to reduced treatment times and lower operational costs compared to conventional treatment methods. Additionally, AOPs often require fewer chemical inputs, further reducing ongoing expenses. The energy consumption of AOPs, particularly those utilizing UV light or ozone, must be factored into the operational costs, but advancements in energy-efficient technologies are continually improving this aspect.
From an environmental perspective, the benefits of implementing AOPs are significant. The effective removal of carbon tetrachloride from water and soil not only complies with stringent environmental regulations but also mitigates potential health risks and ecological damage. This proactive approach can prevent costly remediation efforts and potential legal liabilities in the future.
The scalability of AOP systems is another important consideration in the cost-benefit analysis. While initial costs may be high for large-scale implementations, the technology can be scaled down for smaller applications, making it versatile for various industrial and municipal needs. This flexibility allows for phased implementation, potentially spreading out the capital investment over time.
When evaluating the economic benefits, it's crucial to consider the potential for resource recovery. Some AOP systems can be designed to not only degrade pollutants but also recover valuable byproducts or generate reusable water, creating additional revenue streams or cost savings. This aspect can significantly improve the overall economic feasibility of AOP implementation.
Regulatory compliance is a key driver in the adoption of AOPs. As environmental standards become more stringent, particularly regarding persistent organic pollutants like carbon tetrachloride, the cost of non-compliance can far outweigh the investment in advanced treatment technologies. AOPs offer a proactive solution that can ensure long-term regulatory compliance and avoid potential fines or operational disruptions.
In conclusion, while the initial investment in AOP technology for carbon tetrachloride reduction may be substantial, the long-term benefits in terms of operational efficiency, environmental protection, regulatory compliance, and potential resource recovery present a compelling case for implementation. A comprehensive cost-benefit analysis should consider these factors over the entire lifecycle of the treatment system to accurately assess its economic viability and environmental value.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!