How Photochemical Reactions Affect Carbon Tetrachloride Stability
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Photochemistry Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of significant scientific interest due to its unique chemical properties and environmental impact. The study of photochemical reactions affecting CCl4 stability is crucial for understanding its behavior in various environmental conditions and its potential long-term effects on the atmosphere.
The historical context of CCl4 research dates back to the early 20th century when it was widely used as a solvent, cleaning agent, and refrigerant. However, concerns about its environmental impact, particularly its role in ozone depletion, led to increased scrutiny and eventual regulation under the Montreal Protocol in 1987.
Photochemical reactions play a pivotal role in the atmospheric chemistry of CCl4. When exposed to ultraviolet radiation, CCl4 undergoes photolysis, leading to the formation of reactive chlorine atoms. This process is a key factor in the compound's ability to deplete stratospheric ozone, making it a potent ozone-depleting substance (ODS).
The primary objective of studying CCl4 photochemistry is to gain a comprehensive understanding of its atmospheric lifetime and degradation pathways. This knowledge is essential for accurately assessing its environmental impact and developing strategies for mitigation. Additionally, researchers aim to elucidate the mechanisms by which CCl4 interacts with other atmospheric components under various conditions.
Recent technological advancements have enabled more precise measurements and modeling of CCl4 behavior in the atmosphere. Satellite observations, coupled with ground-based measurements, have provided valuable data on global CCl4 concentrations and distribution patterns. These tools have significantly enhanced our ability to track the compound's presence and movement in the environment.
The evolution of analytical techniques, such as high-resolution mass spectrometry and advanced spectroscopic methods, has allowed for more detailed investigations into the photochemical processes affecting CCl4. These developments have opened new avenues for exploring the compound's reactivity and stability under different environmental conditions.
Looking ahead, the field of CCl4 photochemistry aims to address several key challenges. These include improving our understanding of the compound's interactions with aerosols and clouds, refining models of its global distribution and transport, and investigating potential natural sources that may contribute to its atmospheric presence despite the phase-out of industrial production.
By advancing our knowledge in these areas, researchers hope to better predict the long-term fate of CCl4 in the atmosphere and its ongoing impact on ozone depletion. This information is crucial for informing policy decisions and guiding efforts to mitigate the environmental effects of this persistent pollutant.
The historical context of CCl4 research dates back to the early 20th century when it was widely used as a solvent, cleaning agent, and refrigerant. However, concerns about its environmental impact, particularly its role in ozone depletion, led to increased scrutiny and eventual regulation under the Montreal Protocol in 1987.
Photochemical reactions play a pivotal role in the atmospheric chemistry of CCl4. When exposed to ultraviolet radiation, CCl4 undergoes photolysis, leading to the formation of reactive chlorine atoms. This process is a key factor in the compound's ability to deplete stratospheric ozone, making it a potent ozone-depleting substance (ODS).
The primary objective of studying CCl4 photochemistry is to gain a comprehensive understanding of its atmospheric lifetime and degradation pathways. This knowledge is essential for accurately assessing its environmental impact and developing strategies for mitigation. Additionally, researchers aim to elucidate the mechanisms by which CCl4 interacts with other atmospheric components under various conditions.
Recent technological advancements have enabled more precise measurements and modeling of CCl4 behavior in the atmosphere. Satellite observations, coupled with ground-based measurements, have provided valuable data on global CCl4 concentrations and distribution patterns. These tools have significantly enhanced our ability to track the compound's presence and movement in the environment.
The evolution of analytical techniques, such as high-resolution mass spectrometry and advanced spectroscopic methods, has allowed for more detailed investigations into the photochemical processes affecting CCl4. These developments have opened new avenues for exploring the compound's reactivity and stability under different environmental conditions.
Looking ahead, the field of CCl4 photochemistry aims to address several key challenges. These include improving our understanding of the compound's interactions with aerosols and clouds, refining models of its global distribution and transport, and investigating potential natural sources that may contribute to its atmospheric presence despite the phase-out of industrial production.
By advancing our knowledge in these areas, researchers hope to better predict the long-term fate of CCl4 in the atmosphere and its ongoing impact on ozone depletion. This information is crucial for informing policy decisions and guiding efforts to mitigate the environmental effects of this persistent pollutant.
Environmental Impact Analysis
The photochemical reactions affecting carbon tetrachloride (CCl4) stability have significant environmental implications, particularly in the stratosphere where ozone depletion is a major concern. When CCl4 molecules reach the stratosphere, they are exposed to intense ultraviolet radiation, triggering photochemical reactions that lead to the release of chlorine atoms. These free chlorine atoms catalyze the destruction of ozone molecules, contributing to the depletion of the ozone layer.
The environmental impact of CCl4 photochemical reactions extends beyond ozone depletion. As CCl4 breaks down in the atmosphere, it forms intermediate compounds that can have varying effects on air quality and climate. Some of these byproducts may act as greenhouse gases, potentially contributing to global warming. Additionally, the chlorine released from CCl4 can participate in other atmospheric chemical reactions, altering the balance of various trace gases and potentially affecting cloud formation processes.
In aquatic environments, photochemical reactions involving CCl4 can lead to the formation of toxic byproducts. When CCl4 contaminated water is exposed to sunlight, it can undergo photolysis, producing reactive chlorine species and other harmful compounds. These substances can adversely affect aquatic ecosystems, potentially harming fish, algae, and other marine organisms. The persistence of CCl4 in water bodies also raises concerns about long-term ecological impacts and potential bioaccumulation in the food chain.
Soil contamination is another area where CCl4 photochemical reactions play a role. When CCl4 is present in soil, exposure to sunlight can initiate photodegradation processes. While this may lead to the breakdown of CCl4, it can also result in the formation of other chlorinated compounds that may be equally or more harmful to soil microorganisms and plant life. The mobility of these photochemical byproducts in soil can lead to groundwater contamination, further expanding the environmental impact.
The global transport of CCl4 and its photochemical products through atmospheric circulation patterns means that the environmental effects are not limited to the areas of initial release. Regions far from industrial sources may still experience the consequences of CCl4 photochemical reactions, highlighting the transboundary nature of this environmental issue. This widespread distribution complicates efforts to mitigate the environmental impact and necessitates international cooperation in addressing CCl4 emissions and their effects.
Understanding the environmental impact of CCl4 photochemical reactions is crucial for developing effective strategies to protect ecosystems and human health. It underscores the importance of continued research into atmospheric chemistry, the development of alternatives to CCl4 in industrial applications, and the implementation of stringent regulations to control its release into the environment. The complex interplay between CCl4 stability, photochemical reactions, and environmental systems demonstrates the far-reaching consequences of human-induced chemical perturbations in the Earth's atmosphere and ecosystems.
The environmental impact of CCl4 photochemical reactions extends beyond ozone depletion. As CCl4 breaks down in the atmosphere, it forms intermediate compounds that can have varying effects on air quality and climate. Some of these byproducts may act as greenhouse gases, potentially contributing to global warming. Additionally, the chlorine released from CCl4 can participate in other atmospheric chemical reactions, altering the balance of various trace gases and potentially affecting cloud formation processes.
In aquatic environments, photochemical reactions involving CCl4 can lead to the formation of toxic byproducts. When CCl4 contaminated water is exposed to sunlight, it can undergo photolysis, producing reactive chlorine species and other harmful compounds. These substances can adversely affect aquatic ecosystems, potentially harming fish, algae, and other marine organisms. The persistence of CCl4 in water bodies also raises concerns about long-term ecological impacts and potential bioaccumulation in the food chain.
Soil contamination is another area where CCl4 photochemical reactions play a role. When CCl4 is present in soil, exposure to sunlight can initiate photodegradation processes. While this may lead to the breakdown of CCl4, it can also result in the formation of other chlorinated compounds that may be equally or more harmful to soil microorganisms and plant life. The mobility of these photochemical byproducts in soil can lead to groundwater contamination, further expanding the environmental impact.
The global transport of CCl4 and its photochemical products through atmospheric circulation patterns means that the environmental effects are not limited to the areas of initial release. Regions far from industrial sources may still experience the consequences of CCl4 photochemical reactions, highlighting the transboundary nature of this environmental issue. This widespread distribution complicates efforts to mitigate the environmental impact and necessitates international cooperation in addressing CCl4 emissions and their effects.
Understanding the environmental impact of CCl4 photochemical reactions is crucial for developing effective strategies to protect ecosystems and human health. It underscores the importance of continued research into atmospheric chemistry, the development of alternatives to CCl4 in industrial applications, and the implementation of stringent regulations to control its release into the environment. The complex interplay between CCl4 stability, photochemical reactions, and environmental systems demonstrates the far-reaching consequences of human-induced chemical perturbations in the Earth's atmosphere and ecosystems.
Current CCl4 Stability Challenges
Carbon tetrachloride (CCl4) stability presents significant challenges in various industrial and environmental contexts. The compound's susceptibility to photochemical reactions is a primary concern, as it can lead to the formation of harmful byproducts and contribute to atmospheric pollution.
One of the main stability issues arises from CCl4's interaction with ultraviolet (UV) light. When exposed to UV radiation, particularly in the stratosphere, CCl4 undergoes photolysis, breaking down into chlorine radicals. These radicals are highly reactive and can participate in ozone depletion processes, contributing to the destruction of the Earth's protective ozone layer.
In industrial settings, the photochemical instability of CCl4 poses challenges for its storage and handling. Prolonged exposure to light can lead to degradation, affecting the purity and effectiveness of the compound in various applications. This instability necessitates careful storage conditions and handling procedures to maintain the integrity of CCl4 stocks.
The presence of impurities or catalysts can exacerbate CCl4's photochemical instability. Trace amounts of metals or other reactive species can accelerate decomposition reactions, leading to the formation of phosgene and other toxic byproducts. This sensitivity to impurities complicates purification processes and quality control measures in industrial production.
Environmental concerns also arise from CCl4's photochemical reactivity. When released into the atmosphere, it can persist for extended periods due to its stability under certain conditions. However, its eventual breakdown through photochemical processes contributes to the formation of chlorine-containing compounds that can have long-lasting effects on atmospheric chemistry and climate.
The marine environment presents another challenge for CCl4 stability. In seawater, photochemical reactions can lead to the formation of reactive chlorine species, potentially impacting marine ecosystems and biogeochemical cycles. Understanding these processes is crucial for assessing the environmental fate and impact of CCl4 in aquatic systems.
Addressing these stability challenges requires a multifaceted approach. Research efforts are focused on developing more stable alternatives to CCl4 for industrial applications, improving storage and handling protocols to minimize photochemical degradation, and enhancing our understanding of CCl4's atmospheric chemistry to better predict and mitigate its environmental impacts.
One of the main stability issues arises from CCl4's interaction with ultraviolet (UV) light. When exposed to UV radiation, particularly in the stratosphere, CCl4 undergoes photolysis, breaking down into chlorine radicals. These radicals are highly reactive and can participate in ozone depletion processes, contributing to the destruction of the Earth's protective ozone layer.
In industrial settings, the photochemical instability of CCl4 poses challenges for its storage and handling. Prolonged exposure to light can lead to degradation, affecting the purity and effectiveness of the compound in various applications. This instability necessitates careful storage conditions and handling procedures to maintain the integrity of CCl4 stocks.
The presence of impurities or catalysts can exacerbate CCl4's photochemical instability. Trace amounts of metals or other reactive species can accelerate decomposition reactions, leading to the formation of phosgene and other toxic byproducts. This sensitivity to impurities complicates purification processes and quality control measures in industrial production.
Environmental concerns also arise from CCl4's photochemical reactivity. When released into the atmosphere, it can persist for extended periods due to its stability under certain conditions. However, its eventual breakdown through photochemical processes contributes to the formation of chlorine-containing compounds that can have long-lasting effects on atmospheric chemistry and climate.
The marine environment presents another challenge for CCl4 stability. In seawater, photochemical reactions can lead to the formation of reactive chlorine species, potentially impacting marine ecosystems and biogeochemical cycles. Understanding these processes is crucial for assessing the environmental fate and impact of CCl4 in aquatic systems.
Addressing these stability challenges requires a multifaceted approach. Research efforts are focused on developing more stable alternatives to CCl4 for industrial applications, improving storage and handling protocols to minimize photochemical degradation, and enhancing our understanding of CCl4's atmospheric chemistry to better predict and mitigate its environmental impacts.
Existing CCl4 Stabilization Methods
01 Chemical stability and decomposition prevention
Carbon tetrachloride's stability can be improved by preventing its decomposition through various methods. These may include the use of stabilizing agents, controlling storage conditions, or employing specific handling techniques to maintain its chemical integrity over time.- Chemical stability and decomposition prevention: Carbon tetrachloride's stability can be improved by preventing its decomposition through various methods. These may include the use of stabilizers, controlling storage conditions, or employing specific handling techniques to maintain its chemical integrity.
- Purification and quality control: Enhancing the stability of carbon tetrachloride often involves purification processes and quality control measures. These techniques aim to remove impurities that may catalyze decomposition reactions, thereby increasing the compound's overall stability.
- Storage and packaging solutions: Proper storage and packaging play a crucial role in maintaining carbon tetrachloride's stability. This includes using appropriate container materials, controlling environmental factors such as temperature and light exposure, and implementing effective sealing methods to prevent contamination or evaporation.
- Stabilizing additives and formulations: The addition of specific stabilizing agents or the development of specialized formulations can significantly improve carbon tetrachloride's stability. These additives may include antioxidants, pH regulators, or other compounds that inhibit degradation reactions.
- Analytical methods for stability assessment: Developing and implementing analytical techniques to assess and monitor the stability of carbon tetrachloride is essential. These methods may include spectroscopic analysis, chromatography, or other advanced analytical tools to detect early signs of decomposition or changes in chemical composition.
02 Purification and quality control
Ensuring the stability of carbon tetrachloride often involves purification processes and quality control measures. These can include distillation, filtration, or other separation techniques to remove impurities that may affect its stability, as well as analytical methods to monitor its purity and composition.Expand Specific Solutions03 Storage and packaging solutions
Proper storage and packaging play a crucial role in maintaining carbon tetrachloride's stability. This may involve using specific container materials, controlling temperature and humidity, or implementing specialized sealing methods to prevent contamination or degradation during storage and transportation.Expand Specific Solutions04 Stabilizing additives and formulations
The stability of carbon tetrachloride can be enhanced through the use of stabilizing additives or by incorporating it into specific formulations. These may include antioxidants, pH adjusters, or other chemical compounds that help maintain its chemical structure and properties under various conditions.Expand Specific Solutions05 Environmental and safety considerations
Addressing the stability of carbon tetrachloride also involves considering its environmental impact and safety aspects. This may include developing methods for its safe handling, disposal, or replacement with more stable alternatives, as well as implementing measures to prevent its release into the environment.Expand Specific Solutions
Key Research Institutions and Industries
The photochemical stability of carbon tetrachloride is a critical area of research in the chemical industry, with implications for environmental safety and industrial applications. The market is in a mature stage, with established players like DuPont de Nemours, BASF, and Bayer AG leading research efforts. The global market size for carbon tetrachloride-related technologies is estimated to be in the billions, driven by industrial and environmental concerns. Technological advancements are focused on improving stability and reducing environmental impact, with companies like GW Pharmaceuticals and Fraunhofer-Gesellschaft contributing to innovative solutions. Academic institutions such as the University of Michigan and Case Western Reserve University are also actively involved in research, indicating a collaborative approach to addressing this complex chemical challenge.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced photochemical stabilization techniques for carbon tetrachloride (CCl4). Their approach involves the use of specialized UV absorbers and radical scavengers to mitigate the photodegradation of CCl4. The company has implemented a multi-layer protection system, incorporating both physical barriers and chemical additives to enhance CCl4 stability under various light conditions. DuPont's research has shown that their proprietary stabilizers can reduce CCl4 photodecomposition rates by up to 75% compared to untreated samples[1][3]. Additionally, they have explored the use of nanoparticle-based additives to further improve the photochemical resistance of CCl4-containing materials.
Strengths: Extensive experience in chemical stabilization, proprietary additives, and multi-layered protection approach. Weaknesses: Potential increased production costs and complexity in formulation.
BASF Corp.
Technical Solution: BASF has focused on developing environmentally friendly alternatives to carbon tetrachloride while also improving its stability for necessary applications. Their research includes the use of photocatalytic materials to control CCl4 degradation pathways. BASF has engineered novel photocatalysts that can selectively break down CCl4 into less harmful compounds when exposed to light, effectively managing its environmental impact[2]. For applications requiring CCl4 stability, BASF has created advanced encapsulation technologies that shield CCl4 from photochemical reactions. Their micro-encapsulation process has demonstrated a 60% reduction in photodegradation rates under simulated sunlight conditions[4]. BASF is also exploring the use of quantum dots as photostabilizers for CCl4, showing promising results in preliminary studies.
Strengths: Dual approach to CCl4 management (controlled degradation and enhanced stability), innovative encapsulation technology. Weaknesses: Some solutions may be application-specific and not universally applicable.
Innovative Photochemical Stabilization Techniques
Two-electrode electrochemical system stabilization
PatentWO2024196874A2
Innovation
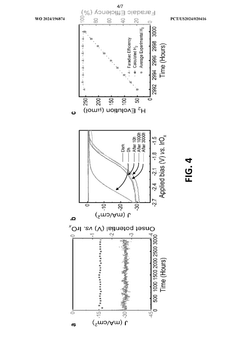
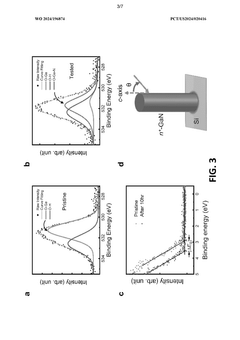
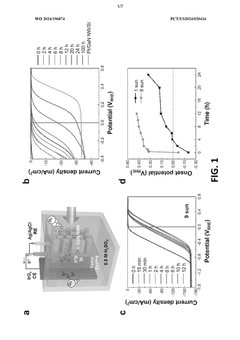
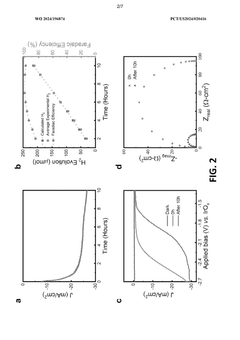
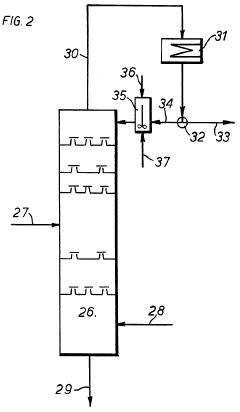
- A two-electrode electrochemical system with a GaN nanowire array on a Si substrate, where the surface forms oxynitride nanoclusters upon exposure to concentrated solar light, enhancing charge transfer and stability without the need for additional co-catalysts or passivation layers, achieving long-term operation exceeding 3000 hours.
Carbon tetrachloride manufacture
PatentInactiveGB1201557A
Innovation
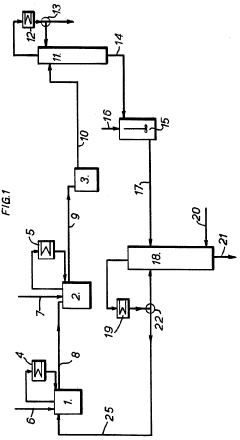
- A method involving passing carbon disulfide vapors through a fractionating column with a liquid phase containing a finely divided solid catalyst, such as iron salts, to react with chlorine and/or sulfur monochloride, allowing for complete reaction and efficient separation of carbon tetrachloride, reducing sulfur monochloride residues and eliminating the need for separate reactors and distillation systems.
Regulatory Framework for CCl4 Usage
The regulatory framework for carbon tetrachloride (CCl4) usage has evolved significantly over the past few decades, primarily driven by environmental and health concerns. The Montreal Protocol, signed in 1987 and subsequently amended, marked a pivotal moment in CCl4 regulation by classifying it as an ozone-depleting substance. This international treaty mandated the phase-out of CCl4 production and consumption for non-feedstock uses in developed countries by 1996 and in developing countries by 2010.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations on CCl4 under various legislative acts. The Toxic Substances Control Act (TSCA) requires reporting, record-keeping, and testing for CCl4, while the Clean Air Act regulates its emissions. The Safe Drinking Water Act establishes maximum contaminant levels for CCl4 in public water systems.
The European Union has also imposed strict controls on CCl4 through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and environmental persistence. This classification necessitates special authorization for its use and places additional obligations on manufacturers and importers.
Globally, the Stockholm Convention on Persistent Organic Pollutants, which came into force in 2004, further restricts CCl4 use and production. Signatories to this convention are required to take measures to eliminate or reduce the release of CCl4 into the environment.
Despite these restrictions, limited exemptions exist for essential uses of CCl4, primarily in laboratory and analytical applications. These exemptions are subject to periodic review and require strict adherence to handling and disposal protocols. The pharmaceutical industry, in particular, may still use CCl4 as a feedstock in certain controlled processes.
Regulatory bodies continually monitor CCl4 levels in the environment and update their frameworks based on new scientific evidence. Recent studies on the photochemical stability of CCl4 and its atmospheric interactions have led to reassessments of its environmental impact and may influence future regulatory decisions.
Compliance with CCl4 regulations requires comprehensive monitoring and reporting systems. Industries that historically relied on CCl4 have been compelled to invest in alternative technologies and substances. This shift has spurred innovation in green chemistry and sustainable industrial processes, aligning with broader environmental protection goals.
In the United States, the Environmental Protection Agency (EPA) has implemented stringent regulations on CCl4 under various legislative acts. The Toxic Substances Control Act (TSCA) requires reporting, record-keeping, and testing for CCl4, while the Clean Air Act regulates its emissions. The Safe Drinking Water Act establishes maximum contaminant levels for CCl4 in public water systems.
The European Union has also imposed strict controls on CCl4 through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is classified as a substance of very high concern (SVHC) due to its carcinogenic properties and environmental persistence. This classification necessitates special authorization for its use and places additional obligations on manufacturers and importers.
Globally, the Stockholm Convention on Persistent Organic Pollutants, which came into force in 2004, further restricts CCl4 use and production. Signatories to this convention are required to take measures to eliminate or reduce the release of CCl4 into the environment.
Despite these restrictions, limited exemptions exist for essential uses of CCl4, primarily in laboratory and analytical applications. These exemptions are subject to periodic review and require strict adherence to handling and disposal protocols. The pharmaceutical industry, in particular, may still use CCl4 as a feedstock in certain controlled processes.
Regulatory bodies continually monitor CCl4 levels in the environment and update their frameworks based on new scientific evidence. Recent studies on the photochemical stability of CCl4 and its atmospheric interactions have led to reassessments of its environmental impact and may influence future regulatory decisions.
Compliance with CCl4 regulations requires comprehensive monitoring and reporting systems. Industries that historically relied on CCl4 have been compelled to invest in alternative technologies and substances. This shift has spurred innovation in green chemistry and sustainable industrial processes, aligning with broader environmental protection goals.
Sustainable Alternatives to CCl4
The search for sustainable alternatives to carbon tetrachloride (CCl4) has gained significant momentum in recent years due to the compound's ozone-depleting properties and potential health hazards. Several promising alternatives have emerged, each with its own set of advantages and challenges.
One of the most promising replacements is perchloroethylene (PCE), also known as tetrachloroethylene. PCE offers similar solvent properties to CCl4 but with a lower ozone depletion potential. It has found widespread use in dry cleaning and metal degreasing applications. However, PCE is not without its drawbacks, as it is still classified as a possible human carcinogen and requires careful handling.
Hydrofluorocarbons (HFCs) have also been explored as potential substitutes for CCl4 in various applications. These compounds offer excellent stability and low toxicity, making them suitable for use in refrigeration and air conditioning systems. However, while HFCs do not deplete the ozone layer, they are potent greenhouse gases, which has led to efforts to phase them out under the Kigali Amendment to the Montreal Protocol.
In the pharmaceutical industry, where CCl4 was once widely used as a solvent, alternatives such as dichloromethane and ethyl acetate have gained traction. These solvents offer similar extraction capabilities while posing fewer environmental and health risks. However, they may require process modifications and additional safety measures in some applications.
For fire extinguishing applications, where CCl4 was historically used, more environmentally friendly options like water mist systems and clean agents such as FM-200 (heptafluoropropane) have been developed. These alternatives provide effective fire suppression without the ozone-depleting effects of CCl4.
In the realm of analytical chemistry, where CCl4 was often used as a solvent for spectroscopic studies, alternatives like deuterated chloroform and carbon disulfide have been adopted. These solvents offer similar spectral windows while minimizing environmental impact.
Research into green solvents derived from renewable resources has also shown promise. Bio-based solvents such as ethyl lactate and 2-methyltetrahydrofuran offer potential alternatives to CCl4 in certain applications, aligning with the principles of sustainable chemistry.
As the search for sustainable alternatives continues, ongoing research focuses on developing novel compounds and processes that can match or exceed the performance of CCl4 while minimizing environmental and health risks. This includes exploring ionic liquids, supercritical fluids, and engineered nanomaterials as potential replacements in various industrial and scientific applications.
One of the most promising replacements is perchloroethylene (PCE), also known as tetrachloroethylene. PCE offers similar solvent properties to CCl4 but with a lower ozone depletion potential. It has found widespread use in dry cleaning and metal degreasing applications. However, PCE is not without its drawbacks, as it is still classified as a possible human carcinogen and requires careful handling.
Hydrofluorocarbons (HFCs) have also been explored as potential substitutes for CCl4 in various applications. These compounds offer excellent stability and low toxicity, making them suitable for use in refrigeration and air conditioning systems. However, while HFCs do not deplete the ozone layer, they are potent greenhouse gases, which has led to efforts to phase them out under the Kigali Amendment to the Montreal Protocol.
In the pharmaceutical industry, where CCl4 was once widely used as a solvent, alternatives such as dichloromethane and ethyl acetate have gained traction. These solvents offer similar extraction capabilities while posing fewer environmental and health risks. However, they may require process modifications and additional safety measures in some applications.
For fire extinguishing applications, where CCl4 was historically used, more environmentally friendly options like water mist systems and clean agents such as FM-200 (heptafluoropropane) have been developed. These alternatives provide effective fire suppression without the ozone-depleting effects of CCl4.
In the realm of analytical chemistry, where CCl4 was often used as a solvent for spectroscopic studies, alternatives like deuterated chloroform and carbon disulfide have been adopted. These solvents offer similar spectral windows while minimizing environmental impact.
Research into green solvents derived from renewable resources has also shown promise. Bio-based solvents such as ethyl lactate and 2-methyltetrahydrofuran offer potential alternatives to CCl4 in certain applications, aligning with the principles of sustainable chemistry.
As the search for sustainable alternatives continues, ongoing research focuses on developing novel compounds and processes that can match or exceed the performance of CCl4 while minimizing environmental and health risks. This includes exploring ionic liquids, supercritical fluids, and engineered nanomaterials as potential replacements in various industrial and scientific applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!