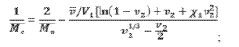Case Study: Hydrogel Use for Soft Tissue Repair — Design, Animal Data and Translational Notes
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Technology Evolution and Objectives
Hydrogel technology has evolved significantly over the past several decades, transforming from simple cross-linked polymer networks to sophisticated biomaterials with tunable properties. The journey began in the 1960s with the development of basic hydrophilic networks, primarily used in contact lenses and simple wound dressings. By the 1980s, researchers had begun exploring hydrogels for drug delivery applications, recognizing their potential to control release kinetics through network design.
The 1990s marked a pivotal shift toward biomedical applications, with pioneering work in tissue engineering scaffolds. During this period, scientists developed the first generation of biodegradable hydrogels, enabling temporary support structures that could be gradually replaced by native tissue. The early 2000s saw the emergence of stimuli-responsive "smart" hydrogels, capable of changing their properties in response to environmental cues such as pH, temperature, or specific biomolecules.
The past decade has witnessed remarkable advancements in hydrogel design for soft tissue repair specifically. Injectable hydrogels that can be administered minimally invasively and subsequently form robust networks in situ have gained significant attention. Concurrently, the integration of bioactive molecules, growth factors, and cell-adhesion motifs has enhanced the biocompatibility and regenerative capacity of these materials.
Current research trends focus on developing hydrogels with mechanical properties that closely mimic those of native soft tissues. This biomimetic approach aims to provide appropriate mechanical support while facilitating cellular infiltration and tissue integration. Additionally, there is growing interest in hydrogels that can respond dynamically to the healing process, gradually transferring mechanical load to newly formed tissue as regeneration progresses.
The primary objectives for hydrogel technology in soft tissue repair include achieving precise control over degradation kinetics to match the rate of new tissue formation, enhancing mechanical strength without compromising biocompatibility, and improving integration with surrounding tissues. Researchers are also working toward hydrogels that can modulate the inflammatory response to promote healing rather than fibrosis.
Looking forward, the field is moving toward personalized hydrogel formulations tailored to specific patient needs and tissue types. Advanced manufacturing techniques, including 3D bioprinting, are enabling the creation of complex hydrogel structures with spatially controlled properties. The ultimate goal remains the development of "off-the-shelf" hydrogel products that can effectively restore function to damaged soft tissues while minimizing the need for donor tissues or complex surgical interventions.
The 1990s marked a pivotal shift toward biomedical applications, with pioneering work in tissue engineering scaffolds. During this period, scientists developed the first generation of biodegradable hydrogels, enabling temporary support structures that could be gradually replaced by native tissue. The early 2000s saw the emergence of stimuli-responsive "smart" hydrogels, capable of changing their properties in response to environmental cues such as pH, temperature, or specific biomolecules.
The past decade has witnessed remarkable advancements in hydrogel design for soft tissue repair specifically. Injectable hydrogels that can be administered minimally invasively and subsequently form robust networks in situ have gained significant attention. Concurrently, the integration of bioactive molecules, growth factors, and cell-adhesion motifs has enhanced the biocompatibility and regenerative capacity of these materials.
Current research trends focus on developing hydrogels with mechanical properties that closely mimic those of native soft tissues. This biomimetic approach aims to provide appropriate mechanical support while facilitating cellular infiltration and tissue integration. Additionally, there is growing interest in hydrogels that can respond dynamically to the healing process, gradually transferring mechanical load to newly formed tissue as regeneration progresses.
The primary objectives for hydrogel technology in soft tissue repair include achieving precise control over degradation kinetics to match the rate of new tissue formation, enhancing mechanical strength without compromising biocompatibility, and improving integration with surrounding tissues. Researchers are also working toward hydrogels that can modulate the inflammatory response to promote healing rather than fibrosis.
Looking forward, the field is moving toward personalized hydrogel formulations tailored to specific patient needs and tissue types. Advanced manufacturing techniques, including 3D bioprinting, are enabling the creation of complex hydrogel structures with spatially controlled properties. The ultimate goal remains the development of "off-the-shelf" hydrogel products that can effectively restore function to damaged soft tissues while minimizing the need for donor tissues or complex surgical interventions.
Market Analysis for Soft Tissue Repair Solutions
The global soft tissue repair market is experiencing significant growth, valued at approximately $16.5 billion in 2022 and projected to reach $25.6 billion by 2028, with a compound annual growth rate of 7.6%. This expansion is primarily driven by the increasing prevalence of sports-related injuries, rising geriatric population, and growing awareness about advanced treatment options.
Hydrogel-based solutions represent one of the fastest-growing segments within this market, with an estimated growth rate of 9.2% annually. Their unique properties—biocompatibility, tunable mechanical characteristics, and ability to mimic native tissue environments—position them as ideal candidates for soft tissue repair applications. The demand is particularly strong in orthopedic and sports medicine sectors, where soft tissue injuries account for over 45% of all musculoskeletal injuries treated annually.
Regional analysis reveals North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to improving healthcare infrastructure, increasing disposable income, and growing adoption of advanced medical technologies in countries like China, Japan, and India.
By application segment, tendon and ligament repair constitutes the largest market share (32%), followed by hernia repair (28%), cardiovascular repair (18%), and other applications (22%). The preference for minimally invasive procedures is reshaping market dynamics, with products supporting such approaches experiencing higher demand growth compared to traditional solutions.
End-user analysis indicates hospitals remain the primary market (65%), followed by ambulatory surgical centers (25%) and specialty clinics (10%). However, ambulatory surgical centers are showing the fastest growth rate due to cost advantages and shorter patient recovery times.
Key market drivers include the rising incidence of sports injuries (approximately 8.6 million sports-related injuries occur annually in the US alone), growing prevalence of chronic diseases affecting soft tissues, and increasing adoption of regenerative medicine approaches. Additionally, the shift toward value-based healthcare models is creating demand for solutions that demonstrate superior clinical outcomes and cost-effectiveness.
Market restraints include high product costs, stringent regulatory approval processes, and limited reimbursement policies in certain regions. The average development timeline for new hydrogel-based products ranges from 5-7 years, with regulatory approval adding another 1-3 years depending on the jurisdiction.
Hydrogel-based solutions represent one of the fastest-growing segments within this market, with an estimated growth rate of 9.2% annually. Their unique properties—biocompatibility, tunable mechanical characteristics, and ability to mimic native tissue environments—position them as ideal candidates for soft tissue repair applications. The demand is particularly strong in orthopedic and sports medicine sectors, where soft tissue injuries account for over 45% of all musculoskeletal injuries treated annually.
Regional analysis reveals North America currently dominates the market with approximately 38% share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to improving healthcare infrastructure, increasing disposable income, and growing adoption of advanced medical technologies in countries like China, Japan, and India.
By application segment, tendon and ligament repair constitutes the largest market share (32%), followed by hernia repair (28%), cardiovascular repair (18%), and other applications (22%). The preference for minimally invasive procedures is reshaping market dynamics, with products supporting such approaches experiencing higher demand growth compared to traditional solutions.
End-user analysis indicates hospitals remain the primary market (65%), followed by ambulatory surgical centers (25%) and specialty clinics (10%). However, ambulatory surgical centers are showing the fastest growth rate due to cost advantages and shorter patient recovery times.
Key market drivers include the rising incidence of sports injuries (approximately 8.6 million sports-related injuries occur annually in the US alone), growing prevalence of chronic diseases affecting soft tissues, and increasing adoption of regenerative medicine approaches. Additionally, the shift toward value-based healthcare models is creating demand for solutions that demonstrate superior clinical outcomes and cost-effectiveness.
Market restraints include high product costs, stringent regulatory approval processes, and limited reimbursement policies in certain regions. The average development timeline for new hydrogel-based products ranges from 5-7 years, with regulatory approval adding another 1-3 years depending on the jurisdiction.
Current Hydrogel Technologies and Challenges
Hydrogels represent a significant advancement in biomaterials for soft tissue repair, offering unique properties that mimic natural tissue environments. Currently, hydrogels are classified into several categories based on their composition: natural polymer-based (collagen, hyaluronic acid, fibrin), synthetic polymer-based (polyethylene glycol, polyvinyl alcohol), and hybrid hydrogels combining both natural and synthetic components. Each category presents distinct advantages and limitations in tissue engineering applications.
Natural polymer-based hydrogels excel in biocompatibility and cell recognition, providing excellent environments for cell adhesion and proliferation. However, they often suffer from batch-to-batch variability, limited mechanical strength, and rapid degradation rates that may not align with tissue regeneration timelines. These limitations have restricted their application in load-bearing tissue repairs despite their superior biological performance.
Synthetic hydrogels offer greater control over mechanical properties, degradation rates, and chemical functionalization. Their production processes yield more consistent results, making them attractive for standardized medical applications. Nevertheless, they typically lack the bioactive signals necessary for optimal cell-material interactions, potentially limiting their efficacy in promoting tissue integration and regeneration without additional modifications.
A significant challenge in current hydrogel technologies lies in achieving appropriate mechanical properties that match target tissues while maintaining biocompatibility. Soft tissues exhibit complex viscoelastic behaviors and mechanical heterogeneity that are difficult to replicate with existing hydrogel systems. This mechanical mismatch can lead to integration failures and compromised functional outcomes in clinical applications.
Controlled degradation represents another major hurdle. Ideal hydrogels should degrade at rates that complement new tissue formation—neither too rapidly, which compromises structural support, nor too slowly, which impedes tissue remodeling. Current technologies struggle to achieve this delicate balance, particularly in heterogeneous tissue environments where degradation requirements may vary spatially.
Vascularization remains perhaps the most critical challenge for hydrogel-based tissue engineering. Without adequate blood vessel formation, cells within larger constructs suffer from hypoxia and nutrient deficiency, limiting the size and functionality of engineered tissues. Despite various approaches incorporating angiogenic factors or prevascularization strategies, creating functional vascular networks within hydrogels remains elusive.
Immunomodulatory properties of hydrogels present both opportunities and challenges. While some immune responses are beneficial for tissue regeneration, excessive inflammation can lead to fibrosis and impaired healing. Current hydrogel technologies lack sophisticated control over immune cell recruitment and activation, often resulting in suboptimal healing environments.
Translational barriers further complicate hydrogel implementation in clinical settings. Scalable manufacturing processes, sterilization methods that preserve hydrogel properties, shelf stability, and regulatory approval pathways all present significant hurdles for moving promising laboratory developments into clinical practice.
Natural polymer-based hydrogels excel in biocompatibility and cell recognition, providing excellent environments for cell adhesion and proliferation. However, they often suffer from batch-to-batch variability, limited mechanical strength, and rapid degradation rates that may not align with tissue regeneration timelines. These limitations have restricted their application in load-bearing tissue repairs despite their superior biological performance.
Synthetic hydrogels offer greater control over mechanical properties, degradation rates, and chemical functionalization. Their production processes yield more consistent results, making them attractive for standardized medical applications. Nevertheless, they typically lack the bioactive signals necessary for optimal cell-material interactions, potentially limiting their efficacy in promoting tissue integration and regeneration without additional modifications.
A significant challenge in current hydrogel technologies lies in achieving appropriate mechanical properties that match target tissues while maintaining biocompatibility. Soft tissues exhibit complex viscoelastic behaviors and mechanical heterogeneity that are difficult to replicate with existing hydrogel systems. This mechanical mismatch can lead to integration failures and compromised functional outcomes in clinical applications.
Controlled degradation represents another major hurdle. Ideal hydrogels should degrade at rates that complement new tissue formation—neither too rapidly, which compromises structural support, nor too slowly, which impedes tissue remodeling. Current technologies struggle to achieve this delicate balance, particularly in heterogeneous tissue environments where degradation requirements may vary spatially.
Vascularization remains perhaps the most critical challenge for hydrogel-based tissue engineering. Without adequate blood vessel formation, cells within larger constructs suffer from hypoxia and nutrient deficiency, limiting the size and functionality of engineered tissues. Despite various approaches incorporating angiogenic factors or prevascularization strategies, creating functional vascular networks within hydrogels remains elusive.
Immunomodulatory properties of hydrogels present both opportunities and challenges. While some immune responses are beneficial for tissue regeneration, excessive inflammation can lead to fibrosis and impaired healing. Current hydrogel technologies lack sophisticated control over immune cell recruitment and activation, often resulting in suboptimal healing environments.
Translational barriers further complicate hydrogel implementation in clinical settings. Scalable manufacturing processes, sterilization methods that preserve hydrogel properties, shelf stability, and regulatory approval pathways all present significant hurdles for moving promising laboratory developments into clinical practice.
Current Hydrogel Design Approaches
01 Hydrogel compositions for soft tissue repair
Specialized hydrogel compositions can be formulated specifically for soft tissue repair applications. These hydrogels typically contain biocompatible polymers that provide structural support while promoting tissue regeneration. The compositions may include natural polymers like collagen or hyaluronic acid, synthetic polymers, or combinations thereof. These hydrogels create a scaffold that supports cell migration, proliferation, and integration with surrounding tissues, facilitating the repair process.- Hydrogel compositions for soft tissue repair: Specialized hydrogel compositions can be formulated specifically for soft tissue repair applications. These hydrogels typically contain biocompatible polymers that provide structural support while promoting tissue regeneration. The compositions may include natural polymers like collagen or hyaluronic acid, synthetic polymers, or combinations thereof. These hydrogels create a scaffold that supports cell migration, proliferation, and integration with surrounding tissues, facilitating effective soft tissue repair.
- Injectable hydrogel systems for minimally invasive procedures: Injectable hydrogel systems allow for minimally invasive delivery to soft tissue repair sites. These systems typically exist as flowable solutions that can be injected through needles or catheters, after which they undergo in situ gelation in response to physiological conditions, temperature changes, or crosslinking agents. This approach enables precise placement of the hydrogel at the defect site, conforming to irregular tissue geometries while minimizing surgical trauma and reducing recovery time for patients.
- Bioactive hydrogels with growth factors and therapeutic agents: Bioactive hydrogels incorporate growth factors, stem cells, or therapeutic agents to enhance soft tissue regeneration. These advanced formulations can deliver bioactive molecules in a controlled manner, stimulating cell proliferation, angiogenesis, and extracellular matrix production. The hydrogel matrix protects these bioactive components from degradation while providing sustained release over time, creating an optimal microenvironment for tissue healing and regeneration.
- Mechanically tuned hydrogels matching tissue properties: Hydrogels with mechanically tuned properties can be designed to match the specific characteristics of the target soft tissue. By adjusting crosslinking density, polymer concentration, or incorporating reinforcing components, these hydrogels can mimic the elasticity, strength, and viscoelastic behavior of native tissues. This mechanical matching is crucial for proper tissue function, reducing stress shielding effects, and ensuring appropriate cellular responses during the repair process.
- Adhesive hydrogels for improved tissue integration: Adhesive hydrogels contain functional groups that enable strong bonding to surrounding tissues, improving integration and preventing displacement. These bioadhesive properties can be achieved through various mechanisms, including chemical bonding, physical entanglement, or biomimetic approaches inspired by natural adhesives. The enhanced tissue-material interface reduces gaps between the hydrogel and native tissue, promoting better cellular migration across boundaries and more effective tissue repair outcomes.
02 Injectable hydrogel systems for minimally invasive repair
Injectable hydrogel systems offer minimally invasive approaches for soft tissue repair. These systems transition from a liquid state during injection to a gel state in situ, conforming to irregular tissue defects. The injectable nature allows for precise delivery to the target site without requiring extensive surgical procedures. Some formulations include temperature-responsive or chemically crosslinking components that solidify under physiological conditions, providing immediate structural support while promoting tissue regeneration.Expand Specific Solutions03 Bioactive hydrogels with growth factors for enhanced healing
Bioactive hydrogels incorporate growth factors and other bioactive molecules to enhance the healing process in soft tissue repair. These specialized formulations can deliver controlled release of therapeutic agents directly to the injury site, stimulating cell proliferation, angiogenesis, and tissue regeneration. The hydrogel matrix protects the bioactive components from degradation while maintaining their activity over extended periods, resulting in more effective tissue repair outcomes compared to conventional approaches.Expand Specific Solutions04 Composite hydrogel scaffolds with reinforcing elements
Composite hydrogel scaffolds incorporate reinforcing elements to enhance mechanical properties for soft tissue repair applications. These reinforcements may include nanofibers, microparticles, or secondary polymer networks that improve strength, elasticity, and durability while maintaining biocompatibility. The composite structure mimics the hierarchical organization of natural tissues, providing better mechanical match to the target tissue while supporting cellular infiltration and tissue integration. These advanced scaffolds are particularly useful for load-bearing soft tissue applications.Expand Specific Solutions05 Stimuli-responsive hydrogels for adaptive tissue repair
Stimuli-responsive hydrogels change their properties in response to external triggers such as pH, temperature, or electrical signals, enabling adaptive tissue repair strategies. These smart materials can undergo controlled swelling, degradation, or mechanical changes to accommodate the dynamic nature of the healing process. Some formulations respond to biological cues within the wound environment, activating therapeutic functions precisely when needed. This responsive behavior allows for temporal control over the repair process, potentially improving outcomes in complex soft tissue injuries.Expand Specific Solutions
Leading Companies and Research Institutions
The hydrogel soft tissue repair market is currently in a growth phase, characterized by increasing clinical applications and technological advancements. The competitive landscape features established pharmaceutical companies like Genzyme, Allergan, and Ethicon Endo-Surgery alongside emerging specialized players such as SentryX BV and Prolynx LLC. Academic institutions including MIT, Harvard, and several Chinese universities are driving innovation through fundamental research. The market is witnessing a transition from basic research to clinical applications, with companies developing proprietary hydrogel formulations for specific tissue repair applications. Technical challenges remain in optimizing biocompatibility, mechanical properties, and controlled drug delivery, with collaborative efforts between industry and academia accelerating translation to clinical practice.
Genzyme Ltd
Technical Solution: Genzyme has developed advanced biopolymer-based hydrogels specifically engineered for soft tissue repair applications. Their flagship technology utilizes modified hyaluronic acid (HA) combined with proprietary crosslinking chemistry to create injectable hydrogels with tissue-specific mechanical properties[2]. Genzyme's approach incorporates controlled degradation profiles through the use of enzyme-sensitive crosslinks, allowing gradual replacement by native tissue over time[4]. Their hydrogels feature a unique porous microstructure that facilitates cellular infiltration and neovascularization, critical factors for successful tissue integration. Genzyme has conducted extensive preclinical studies demonstrating superior biocompatibility and reduced foreign body response compared to conventional materials[7]. Their translational strategy includes comprehensive toxicology studies and manufacturing processes compliant with pharmaceutical-grade standards. Recent innovations include the development of composite hydrogels incorporating bioactive molecules to enhance tissue regeneration outcomes in challenging applications such as cartilage repair.
Strengths: Pharmaceutical-grade manufacturing capabilities ensuring consistent product quality; extensive clinical experience in related biomaterials; strong regulatory expertise facilitating market approval. Weaknesses: Higher production costs compared to simpler hydrogel systems; relatively slow degradation profiles may limit applications requiring rapid tissue remodeling; limited customization options for specific surgical scenarios.
Hokkaido University
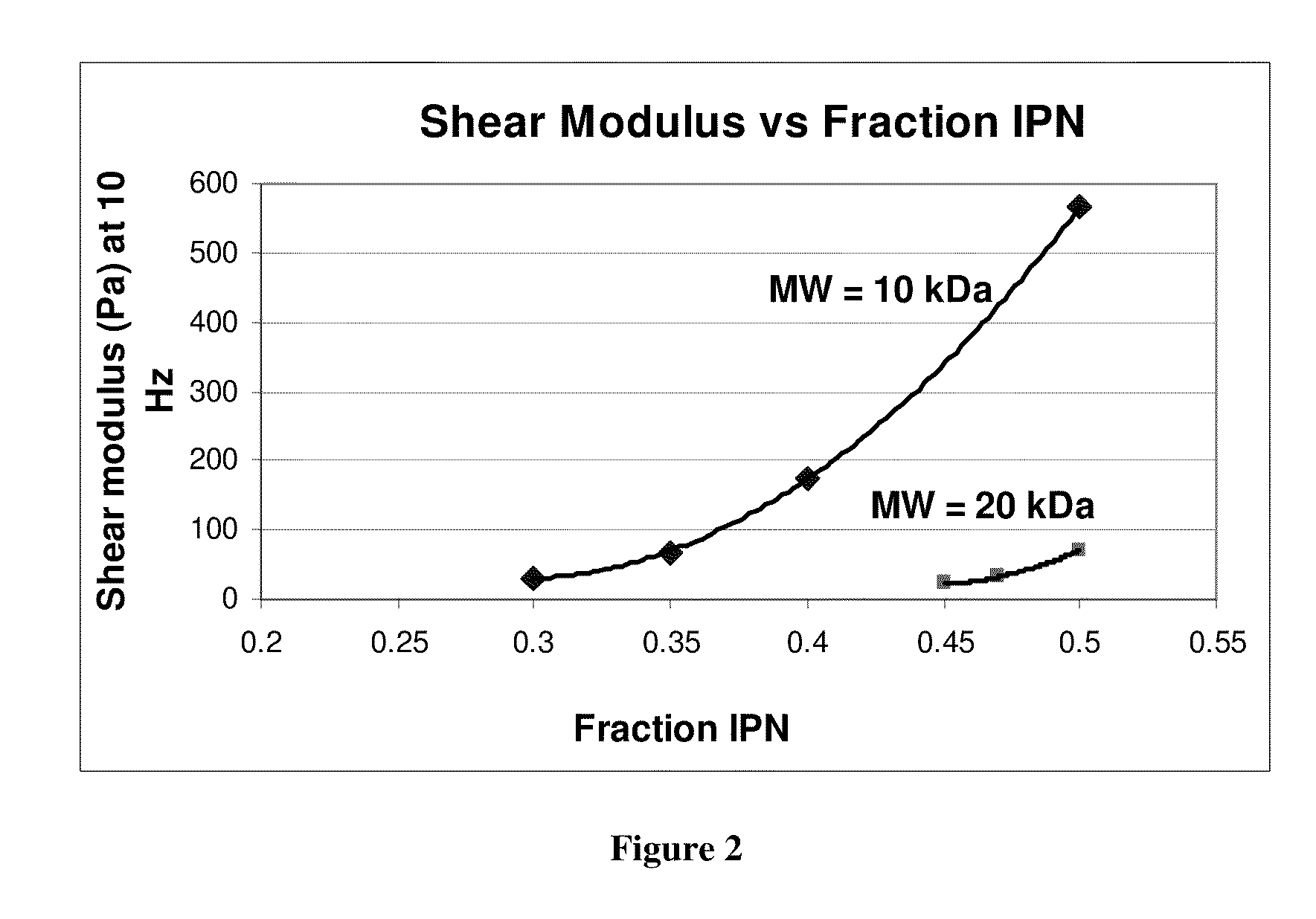
Technical Solution: Hokkaido University has developed innovative double-network (DN) hydrogels specifically designed for soft tissue repair applications. Their technology utilizes interpenetrating polymer networks with contrasting properties - a rigid, densely crosslinked first network combined with a ductile, sparsely crosslinked second network - creating materials with exceptional mechanical strength and fracture resistance[1]. These DN hydrogels achieve cartilage-like mechanical properties while maintaining high water content and biocompatibility. Hokkaido researchers have pioneered techniques to incorporate cell-adhesive domains and growth factors within these networks to enhance tissue integration[3]. Their animal studies have demonstrated superior performance in cartilage repair applications, with evidence of chondrogenic differentiation of mesenchymal stem cells and reduced inflammatory responses[5]. Recent innovations include the development of self-healing variants that can be delivered minimally invasively and reform robust networks in situ. Their translational approach includes optimization of sterilization methods compatible with the complex network structure and development of storage protocols to maintain shelf stability.
Strengths: Exceptional mechanical properties surpassing conventional hydrogels; innovative network architecture providing both strength and biocompatibility; extensive validation in cartilage repair applications. Weaknesses: Complex manufacturing process potentially limiting scale-up; relatively new technology with limited long-term clinical data; primarily focused on load-bearing applications with fewer solutions for soft tissue repair.
Key Patents and Scientific Literature
Decellularized tissue/nanofiber/hydrogel hybrid material for optimized tissue regeneration
PatentWO2016133887A1
Innovation
- A hybrid material comprising decellularized tissue encased and stabilized in a PEG hydrogel matrix with nanofibers, where the PEG hydrogel is formed via oxime ligation reactions, providing tunable mechanical properties and controlled degradation for enhanced wound healing.
Hydrogels for vocal cord and soft tissue augmentation and repair
PatentInactiveUS20100055184A1
Innovation
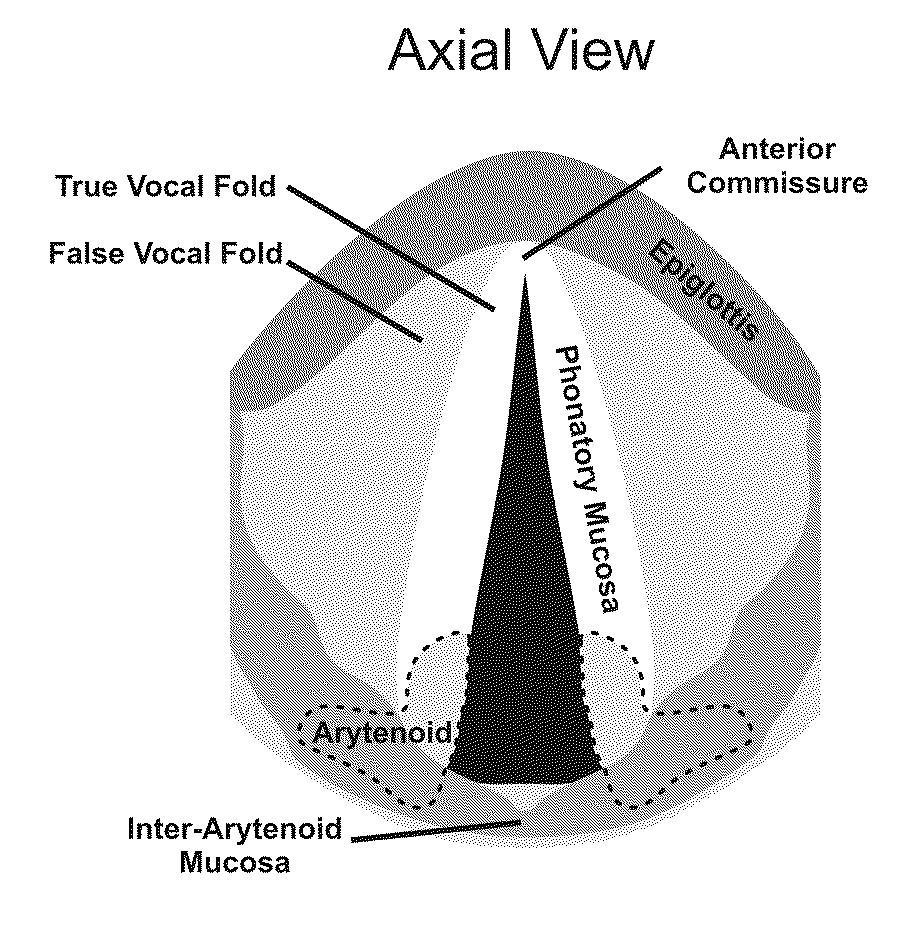
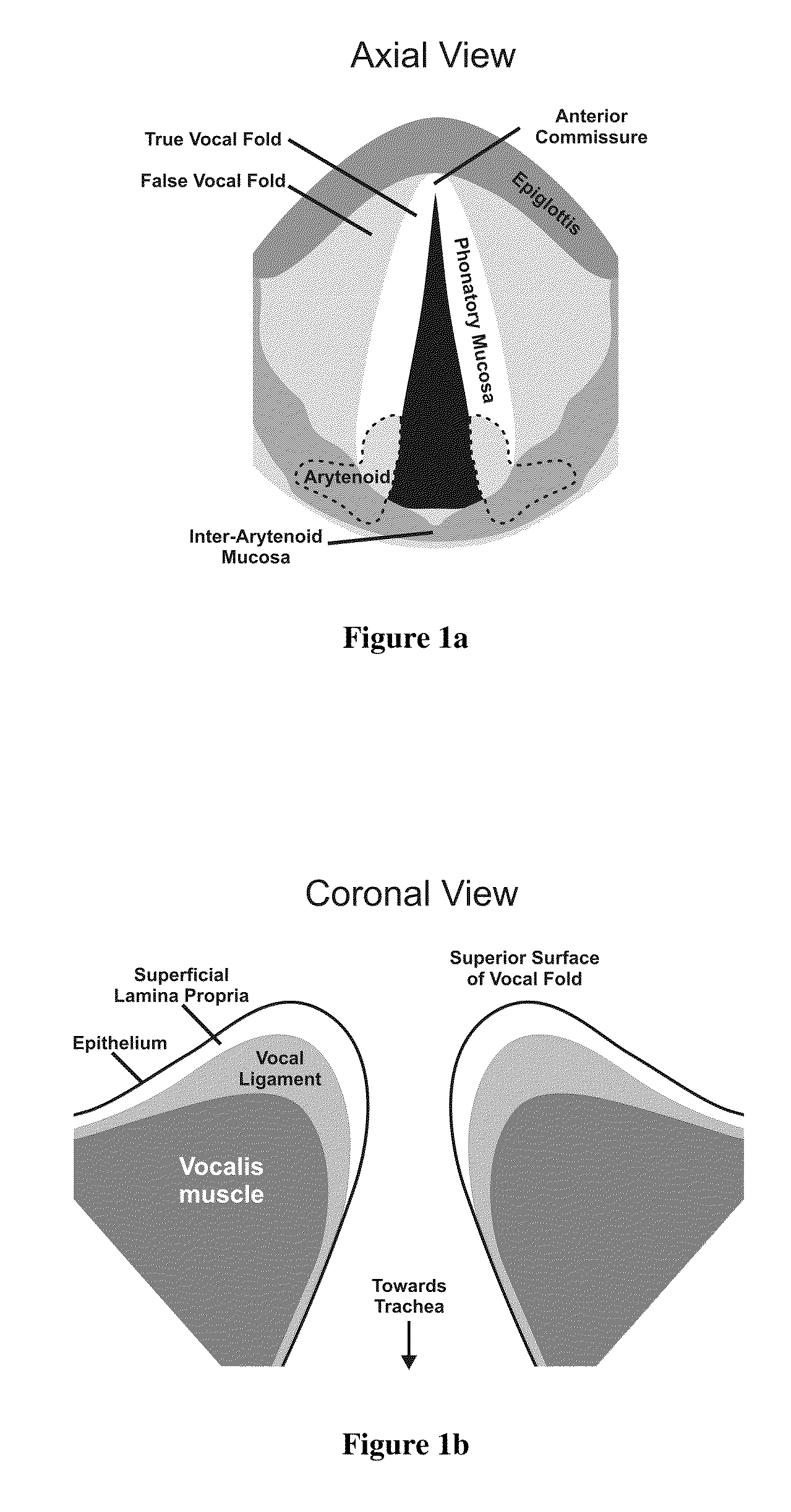
- The use of hydrogels with an elastic shear modulus of approximately 25 Pa, specifically semi-interpenetrating networks, is injected into the subepithelial phonatory mucosal region to restore phonatory mucosal pliability and normal vocal cord vibration, potentially combined with biologically active agents or stem cells for enhanced repair.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations represent critical aspects in the development and application of hydrogels for soft tissue repair. The interaction between implanted hydrogels and host tissues determines not only the efficacy of the repair but also the potential for adverse reactions and long-term outcomes.
Hydrogels must undergo rigorous biocompatibility testing according to ISO 10993 standards, which evaluate cytotoxicity, sensitization, irritation, and systemic toxicity. Recent studies indicate that natural polymer-based hydrogels such as collagen, hyaluronic acid, and alginate generally exhibit superior biocompatibility compared to synthetic alternatives, though advances in synthetic polymer chemistry have significantly improved the biocompatibility profiles of materials like polyethylene glycol (PEG) and poly(vinyl alcohol) (PVA).
Immunogenicity remains a significant concern, particularly with animal-derived hydrogel components. Research demonstrates that decellularization techniques can effectively reduce immunogenic potential while preserving the beneficial extracellular matrix architecture. The inflammatory response to hydrogel implantation typically follows a predictable pattern: acute inflammation (0-7 days), chronic inflammation (1-3 weeks), and resolution/remodeling phase. Hydrogels designed with controlled degradation profiles that match tissue regeneration rates show optimal outcomes in animal models.
Degradation byproducts present another critical safety consideration. Hydrolytic and enzymatic degradation mechanisms must be thoroughly characterized to ensure that breakdown products are non-toxic and can be metabolized or excreted through normal physiological pathways. Studies have demonstrated that some synthetic hydrogel degradation products may accumulate in organs, necessitating long-term safety monitoring.
The mechanical properties of hydrogels must be carefully engineered to match target tissue characteristics. Mismatched mechanical properties can lead to stress shielding or excessive loading, potentially compromising repair integrity. Advanced hydrogel systems incorporating gradient mechanical properties have shown promising results in addressing this challenge, particularly at tissue interfaces.
Sterilization methods significantly impact hydrogel safety and functionality. While gamma irradiation offers efficient sterilization, it may alter crosslinking density and mechanical properties. Ethylene oxide sterilization presents fewer structural alterations but requires extensive aeration to remove residual toxic compounds. Terminal filtration remains viable only for certain hydrogel precursors prior to gelation.
Regulatory pathways for hydrogel-based products vary significantly based on their classification (device, drug, biologic, or combination product). The FDA's regulatory approach typically requires comprehensive preclinical safety data, including biocompatibility testing, degradation studies, and toxicological assessments before advancing to first-in-human trials.
Hydrogels must undergo rigorous biocompatibility testing according to ISO 10993 standards, which evaluate cytotoxicity, sensitization, irritation, and systemic toxicity. Recent studies indicate that natural polymer-based hydrogels such as collagen, hyaluronic acid, and alginate generally exhibit superior biocompatibility compared to synthetic alternatives, though advances in synthetic polymer chemistry have significantly improved the biocompatibility profiles of materials like polyethylene glycol (PEG) and poly(vinyl alcohol) (PVA).
Immunogenicity remains a significant concern, particularly with animal-derived hydrogel components. Research demonstrates that decellularization techniques can effectively reduce immunogenic potential while preserving the beneficial extracellular matrix architecture. The inflammatory response to hydrogel implantation typically follows a predictable pattern: acute inflammation (0-7 days), chronic inflammation (1-3 weeks), and resolution/remodeling phase. Hydrogels designed with controlled degradation profiles that match tissue regeneration rates show optimal outcomes in animal models.
Degradation byproducts present another critical safety consideration. Hydrolytic and enzymatic degradation mechanisms must be thoroughly characterized to ensure that breakdown products are non-toxic and can be metabolized or excreted through normal physiological pathways. Studies have demonstrated that some synthetic hydrogel degradation products may accumulate in organs, necessitating long-term safety monitoring.
The mechanical properties of hydrogels must be carefully engineered to match target tissue characteristics. Mismatched mechanical properties can lead to stress shielding or excessive loading, potentially compromising repair integrity. Advanced hydrogel systems incorporating gradient mechanical properties have shown promising results in addressing this challenge, particularly at tissue interfaces.
Sterilization methods significantly impact hydrogel safety and functionality. While gamma irradiation offers efficient sterilization, it may alter crosslinking density and mechanical properties. Ethylene oxide sterilization presents fewer structural alterations but requires extensive aeration to remove residual toxic compounds. Terminal filtration remains viable only for certain hydrogel precursors prior to gelation.
Regulatory pathways for hydrogel-based products vary significantly based on their classification (device, drug, biologic, or combination product). The FDA's regulatory approach typically requires comprehensive preclinical safety data, including biocompatibility testing, degradation studies, and toxicological assessments before advancing to first-in-human trials.
Regulatory Pathway for Clinical Translation
The regulatory pathway for hydrogel-based medical devices for soft tissue repair involves navigating complex approval processes across different global jurisdictions. In the United States, the FDA typically classifies these products as Class II or III medical devices, depending on their intended use and risk profile. Hydrogels used for wound healing or superficial tissue repair generally fall under Class II, requiring a 510(k) premarket notification demonstrating substantial equivalence to a legally marketed device. However, hydrogels designed for internal tissue repair, especially those incorporating bioactive components or intended for long-term implantation, may be classified as Class III devices requiring the more rigorous Premarket Approval (PMA) pathway.
The European regulatory framework under the Medical Device Regulation (MDR) similarly categorizes hydrogels based on risk classification. Most tissue repair hydrogels fall under Class IIb or III, requiring conformity assessment by a Notified Body and comprehensive clinical evaluation reports. The MDR's increased emphasis on clinical evidence and post-market surveillance presents additional challenges for manufacturers seeking CE marking.
Clinical translation requires strategic planning of preclinical studies to address specific regulatory requirements. This includes biocompatibility testing according to ISO 10993 standards, which evaluates cytotoxicity, sensitization, irritation, and systemic toxicity. For implantable hydrogels, additional tests for genotoxicity, carcinogenicity, and long-term implantation effects are mandatory.
Manufacturers must develop a comprehensive regulatory strategy early in product development. This includes determining the appropriate regulatory pathway, identifying predicate devices for comparison, and planning clinical trials that will generate sufficient safety and efficacy data. For novel hydrogels without clear predicates, early consultation with regulatory bodies through pre-submission meetings is advisable to establish appropriate testing protocols and data requirements.
Quality management systems compliant with ISO 13485 are essential throughout development and manufacturing. Documentation of design controls, risk management processes (ISO 14971), and manufacturing validation are critical components of regulatory submissions. For hydrogels incorporating biological components or drugs, additional regulatory considerations apply, potentially involving combination product regulations requiring coordination between different regulatory divisions.
Post-market surveillance plans must be robust, particularly under the MDR in Europe, which requires manufacturers to actively collect and analyze real-world performance data. This includes adverse event reporting systems, periodic safety update reports, and post-approval studies to monitor long-term outcomes and identify potential safety signals not detected during clinical trials.
The European regulatory framework under the Medical Device Regulation (MDR) similarly categorizes hydrogels based on risk classification. Most tissue repair hydrogels fall under Class IIb or III, requiring conformity assessment by a Notified Body and comprehensive clinical evaluation reports. The MDR's increased emphasis on clinical evidence and post-market surveillance presents additional challenges for manufacturers seeking CE marking.
Clinical translation requires strategic planning of preclinical studies to address specific regulatory requirements. This includes biocompatibility testing according to ISO 10993 standards, which evaluates cytotoxicity, sensitization, irritation, and systemic toxicity. For implantable hydrogels, additional tests for genotoxicity, carcinogenicity, and long-term implantation effects are mandatory.
Manufacturers must develop a comprehensive regulatory strategy early in product development. This includes determining the appropriate regulatory pathway, identifying predicate devices for comparison, and planning clinical trials that will generate sufficient safety and efficacy data. For novel hydrogels without clear predicates, early consultation with regulatory bodies through pre-submission meetings is advisable to establish appropriate testing protocols and data requirements.
Quality management systems compliant with ISO 13485 are essential throughout development and manufacturing. Documentation of design controls, risk management processes (ISO 14971), and manufacturing validation are critical components of regulatory submissions. For hydrogels incorporating biological components or drugs, additional regulatory considerations apply, potentially involving combination product regulations requiring coordination between different regulatory divisions.
Post-market surveillance plans must be robust, particularly under the MDR in Europe, which requires manufacturers to actively collect and analyze real-world performance data. This includes adverse event reporting systems, periodic safety update reports, and post-approval studies to monitor long-term outcomes and identify potential safety signals not detected during clinical trials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!