Hydrogel Mechanical Fatigue Testing: Protocol and Acceptance Criteria for Implants
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Implant Fatigue Testing Background and Objectives
Hydrogel materials have emerged as a revolutionary biomaterial in the field of medical implants over the past three decades. Initially developed in the 1960s as simple water-retaining polymers, hydrogels have evolved significantly to become sophisticated biomaterials with tunable mechanical and biological properties. The evolution trajectory has moved from basic synthetic hydrogels to advanced composite structures that can mimic natural tissue properties with remarkable precision.
The development of hydrogel implants has been driven by their unique combination of properties: high water content, biocompatibility, and mechanical flexibility that closely resembles natural tissues. These characteristics make them particularly suitable for applications ranging from orthopedic cartilage replacement to ophthalmic implants and drug delivery systems. Recent advancements have focused on enhancing the mechanical durability and functional longevity of these materials in physiological environments.
Despite significant progress, the mechanical fatigue behavior of hydrogels remains a critical challenge in their application as long-term implants. Unlike traditional implant materials such as metals or ceramics, hydrogels exhibit complex viscoelastic behavior and time-dependent mechanical properties that complicate fatigue assessment. The cyclic loading conditions experienced by implants in the human body—particularly in load-bearing applications—demand robust fatigue resistance that current hydrogel formulations struggle to consistently achieve.
The primary technical objective in hydrogel implant fatigue testing is to establish standardized protocols that accurately predict in vivo performance. Current testing methodologies often fail to account for the unique mechanical characteristics of hydrogels, including strain-rate dependency, stress relaxation, and environmental sensitivity. This gap between testing conditions and actual physiological environments leads to unpredictable clinical outcomes and hinders regulatory approval processes.
Another key objective is to develop acceptance criteria that meaningfully correlate with clinical performance. Unlike metallic implants where fatigue failure modes are well-characterized, hydrogel failure mechanisms are multifaceted, involving physical degradation, chemical breakdown, and biological interactions. Establishing quantitative relationships between laboratory fatigue measurements and clinical longevity represents a significant technical challenge that requires interdisciplinary approaches combining materials science, biomechanics, and clinical medicine.
The field is now moving toward more sophisticated testing paradigms that incorporate physiologically relevant conditions, including temperature fluctuations, osmotic environments, and biochemical factors that can accelerate material degradation. Advanced computational models are also being developed to predict long-term performance based on accelerated testing data, potentially revolutionizing the development timeline for new hydrogel implant technologies.
The development of hydrogel implants has been driven by their unique combination of properties: high water content, biocompatibility, and mechanical flexibility that closely resembles natural tissues. These characteristics make them particularly suitable for applications ranging from orthopedic cartilage replacement to ophthalmic implants and drug delivery systems. Recent advancements have focused on enhancing the mechanical durability and functional longevity of these materials in physiological environments.
Despite significant progress, the mechanical fatigue behavior of hydrogels remains a critical challenge in their application as long-term implants. Unlike traditional implant materials such as metals or ceramics, hydrogels exhibit complex viscoelastic behavior and time-dependent mechanical properties that complicate fatigue assessment. The cyclic loading conditions experienced by implants in the human body—particularly in load-bearing applications—demand robust fatigue resistance that current hydrogel formulations struggle to consistently achieve.
The primary technical objective in hydrogel implant fatigue testing is to establish standardized protocols that accurately predict in vivo performance. Current testing methodologies often fail to account for the unique mechanical characteristics of hydrogels, including strain-rate dependency, stress relaxation, and environmental sensitivity. This gap between testing conditions and actual physiological environments leads to unpredictable clinical outcomes and hinders regulatory approval processes.
Another key objective is to develop acceptance criteria that meaningfully correlate with clinical performance. Unlike metallic implants where fatigue failure modes are well-characterized, hydrogel failure mechanisms are multifaceted, involving physical degradation, chemical breakdown, and biological interactions. Establishing quantitative relationships between laboratory fatigue measurements and clinical longevity represents a significant technical challenge that requires interdisciplinary approaches combining materials science, biomechanics, and clinical medicine.
The field is now moving toward more sophisticated testing paradigms that incorporate physiologically relevant conditions, including temperature fluctuations, osmotic environments, and biochemical factors that can accelerate material degradation. Advanced computational models are also being developed to predict long-term performance based on accelerated testing data, potentially revolutionizing the development timeline for new hydrogel implant technologies.
Clinical Demand Analysis for Durable Hydrogel Implants
The growing demand for long-lasting hydrogel implants stems from their increasing application in various medical fields, including orthopedics, ophthalmology, and soft tissue reconstruction. Clinical data indicates that approximately 2.5 million patients receive hydrogel-based implants annually worldwide, with this number projected to grow at 8-12% annually through 2030 due to aging populations and advances in implant technology.
Durability concerns represent the primary clinical challenge, as studies published in the Journal of Biomaterials show that 15-20% of hydrogel implants experience mechanical failure within five years of implantation. This failure rate increases significantly in high-stress anatomical locations such as joints and load-bearing tissues, where cyclic mechanical stress accelerates material degradation.
Healthcare providers report that premature implant failure leads to revision surgeries that increase patient morbidity and healthcare costs. A retrospective analysis of 1,200 patients with hydrogel implants revealed that revision procedures cost healthcare systems an average of $18,500 per case, not including indirect costs related to recovery and lost productivity.
Patient expectations for implant longevity have risen substantially, with surveys indicating that 87% of patients expect implants to function effectively for at least 10-15 years. This expectation gap between current performance and desired outcomes creates significant clinical pressure for more durable solutions.
Regulatory bodies have responded by implementing stricter requirements for mechanical fatigue testing. The FDA and European Medicines Agency now require comprehensive fatigue testing data demonstrating implant durability under physiological loading conditions for periods equivalent to at least 5-7 years of in vivo use before market approval.
Clinical specialists emphasize the need for standardized testing protocols that accurately simulate the complex mechanical environment of the human body. Current testing methods often fail to account for the combined effects of cyclic loading, biological interactions, and chemical degradation that occur simultaneously in vivo.
The market for durable hydrogel implants shows significant growth potential, with healthcare systems increasingly willing to pay premium prices for implants with demonstrated longevity. Economic analyses indicate that implants with 50% greater durability could command price premiums of 30-40% while still providing net cost savings to healthcare systems through reduced revision rates.
Durability concerns represent the primary clinical challenge, as studies published in the Journal of Biomaterials show that 15-20% of hydrogel implants experience mechanical failure within five years of implantation. This failure rate increases significantly in high-stress anatomical locations such as joints and load-bearing tissues, where cyclic mechanical stress accelerates material degradation.
Healthcare providers report that premature implant failure leads to revision surgeries that increase patient morbidity and healthcare costs. A retrospective analysis of 1,200 patients with hydrogel implants revealed that revision procedures cost healthcare systems an average of $18,500 per case, not including indirect costs related to recovery and lost productivity.
Patient expectations for implant longevity have risen substantially, with surveys indicating that 87% of patients expect implants to function effectively for at least 10-15 years. This expectation gap between current performance and desired outcomes creates significant clinical pressure for more durable solutions.
Regulatory bodies have responded by implementing stricter requirements for mechanical fatigue testing. The FDA and European Medicines Agency now require comprehensive fatigue testing data demonstrating implant durability under physiological loading conditions for periods equivalent to at least 5-7 years of in vivo use before market approval.
Clinical specialists emphasize the need for standardized testing protocols that accurately simulate the complex mechanical environment of the human body. Current testing methods often fail to account for the combined effects of cyclic loading, biological interactions, and chemical degradation that occur simultaneously in vivo.
The market for durable hydrogel implants shows significant growth potential, with healthcare systems increasingly willing to pay premium prices for implants with demonstrated longevity. Economic analyses indicate that implants with 50% greater durability could command price premiums of 30-40% while still providing net cost savings to healthcare systems through reduced revision rates.
Current Challenges in Hydrogel Mechanical Fatigue Assessment
Despite significant advancements in hydrogel technology for implantable medical devices, the mechanical fatigue assessment of these materials presents numerous unresolved challenges. The viscoelastic nature of hydrogels creates fundamental difficulties in applying traditional fatigue testing methodologies that were originally developed for metals and rigid polymers. This inherent material property causes time-dependent responses that vary significantly with loading rate, temperature, and hydration state.
A primary challenge lies in the lack of standardized testing protocols specifically designed for hydrogel materials in implantable applications. Current standards from organizations such as ASTM and ISO provide limited guidance for the unique behavior of hydrogels under cyclic loading conditions. This absence of standardization leads to inconsistent testing methodologies across research institutions and manufacturers, making comparative analysis nearly impossible.
Environmental conditioning represents another significant hurdle in hydrogel fatigue assessment. Implantable hydrogels must function in the physiological environment, which includes exposure to body fluids, varying pH levels, enzymatic activity, and temperature fluctuations. Replicating these conditions accurately in laboratory settings while simultaneously conducting mechanical fatigue tests requires sophisticated equipment and protocols that many testing facilities lack.
The selection of appropriate loading parameters presents additional complexity. Determining clinically relevant loading frequencies, amplitudes, and waveforms that accurately simulate in vivo conditions remains challenging. Many researchers default to simplified loading regimes that fail to capture the complex multiaxial stresses experienced by implants in the human body, potentially leading to unreliable predictions of long-term performance.
Sample preparation inconsistencies further complicate fatigue assessment. Variations in crosslinking density, polymerization conditions, and hydration protocols can significantly alter mechanical properties, yet standardized preparation methods are rarely implemented across different laboratories. This introduces substantial variability in test results, making it difficult to establish reliable acceptance criteria.
The time-intensive nature of fatigue testing poses practical limitations as well. Implantable devices often need to withstand millions of loading cycles over years of service, but accelerated testing methods may not accurately predict long-term performance due to the rate-dependent behavior of hydrogels. This creates a tension between the need for rapid product development and the requirement for thorough fatigue characterization.
Data interpretation and failure criteria definition remain subjective in many cases. Unlike traditional materials with well-defined failure modes, hydrogels may exhibit gradual degradation in properties rather than catastrophic failure, making endpoint determination challenging. The industry lacks consensus on what constitutes acceptable levels of property change during fatigue testing for different implant applications.
A primary challenge lies in the lack of standardized testing protocols specifically designed for hydrogel materials in implantable applications. Current standards from organizations such as ASTM and ISO provide limited guidance for the unique behavior of hydrogels under cyclic loading conditions. This absence of standardization leads to inconsistent testing methodologies across research institutions and manufacturers, making comparative analysis nearly impossible.
Environmental conditioning represents another significant hurdle in hydrogel fatigue assessment. Implantable hydrogels must function in the physiological environment, which includes exposure to body fluids, varying pH levels, enzymatic activity, and temperature fluctuations. Replicating these conditions accurately in laboratory settings while simultaneously conducting mechanical fatigue tests requires sophisticated equipment and protocols that many testing facilities lack.
The selection of appropriate loading parameters presents additional complexity. Determining clinically relevant loading frequencies, amplitudes, and waveforms that accurately simulate in vivo conditions remains challenging. Many researchers default to simplified loading regimes that fail to capture the complex multiaxial stresses experienced by implants in the human body, potentially leading to unreliable predictions of long-term performance.
Sample preparation inconsistencies further complicate fatigue assessment. Variations in crosslinking density, polymerization conditions, and hydration protocols can significantly alter mechanical properties, yet standardized preparation methods are rarely implemented across different laboratories. This introduces substantial variability in test results, making it difficult to establish reliable acceptance criteria.
The time-intensive nature of fatigue testing poses practical limitations as well. Implantable devices often need to withstand millions of loading cycles over years of service, but accelerated testing methods may not accurately predict long-term performance due to the rate-dependent behavior of hydrogels. This creates a tension between the need for rapid product development and the requirement for thorough fatigue characterization.
Data interpretation and failure criteria definition remain subjective in many cases. Unlike traditional materials with well-defined failure modes, hydrogels may exhibit gradual degradation in properties rather than catastrophic failure, making endpoint determination challenging. The industry lacks consensus on what constitutes acceptable levels of property change during fatigue testing for different implant applications.
Standardized Protocols for Hydrogel Implant Fatigue Testing
01 Hydrogel composition for improved fatigue resistance
Specific hydrogel compositions can be engineered to enhance mechanical fatigue resistance. These compositions often include double-network structures, interpenetrating polymer networks, or reinforcing agents such as nanoparticles or fibers. By optimizing the polymer composition and crosslinking density, hydrogels can maintain their mechanical properties under repeated loading cycles, making them suitable for applications requiring durability under mechanical stress.- Hydrogel composition for improved fatigue resistance: Specific hydrogel compositions can be engineered to enhance mechanical fatigue resistance. These compositions typically include cross-linking agents, reinforcing polymers, and elastomeric components that work together to create a more durable network structure. The incorporation of double-network structures or interpenetrating polymer networks can significantly improve the hydrogel's ability to withstand repeated mechanical stress without degradation. These advanced compositions allow hydrogels to maintain their mechanical properties even after numerous loading cycles.
- Testing methods for hydrogel mechanical fatigue: Various testing methodologies have been developed to evaluate the mechanical fatigue properties of hydrogels. These include cyclic compression tests, tensile fatigue testing, and rheological measurements under oscillatory conditions. Advanced instrumentation allows for precise measurement of stress-strain relationships during repeated loading cycles, enabling quantification of fatigue resistance parameters such as fatigue life, fatigue crack propagation rate, and mechanical hysteresis. These testing protocols are essential for understanding hydrogel behavior under conditions that simulate real-world applications.
- Self-healing mechanisms to combat mechanical fatigue: Self-healing hydrogels incorporate dynamic bonds or responsive elements that can repair damage caused by mechanical fatigue. These materials contain reversible crosslinks, such as hydrogen bonds, ionic interactions, or dynamic covalent bonds, which can break under stress and reform when the stress is removed. Some advanced systems include microcapsules containing healing agents that release upon damage or incorporate shape memory components that restore the original structure after deformation. These self-healing mechanisms significantly extend the functional lifespan of hydrogels subjected to cyclic mechanical loading.
- Nanocomposite reinforcement for enhanced fatigue resistance: Incorporating nanomaterials into hydrogel matrices creates nanocomposites with superior mechanical fatigue resistance. Nanomaterials such as graphene, carbon nanotubes, clay nanoplatelets, and nanocellulose can effectively dissipate energy during cyclic loading, preventing the accumulation of damage that leads to fatigue failure. These nanofillers create additional physical crosslinks within the hydrogel network and can introduce energy dissipation mechanisms such as sacrificial bonds or friction between the polymer chains and nanoparticles. The resulting nanocomposite hydrogels demonstrate significantly improved mechanical durability under repeated stress conditions.
- Applications requiring fatigue-resistant hydrogels: Fatigue-resistant hydrogels are crucial for various applications where materials are subjected to repeated mechanical stress. In biomedical fields, these include artificial cartilage, cardiovascular implants, and soft robotics where cyclic loading is inevitable. Industrial applications include vibration dampers, flexible electronics, and sensors that must maintain performance over numerous deformation cycles. The development of hydrogels with enhanced fatigue resistance has enabled their use in demanding environments such as wearable devices, tissue engineering scaffolds, and dynamic mechanical systems where conventional hydrogels would quickly fail due to mechanical fatigue.
02 Testing methods for hydrogel mechanical fatigue
Various testing methodologies have been developed to evaluate the mechanical fatigue properties of hydrogels. These include cyclic compression tests, tensile fatigue tests, and rheological measurements under oscillatory conditions. Advanced instrumentation and protocols allow for the quantification of fatigue resistance, recovery behavior, and failure mechanisms in hydrogels, enabling more accurate prediction of long-term performance in real-world applications.Expand Specific Solutions03 Self-healing hydrogels for fatigue mitigation
Self-healing hydrogels incorporate dynamic bonds or reversible crosslinks that can reform after being broken during mechanical loading. This self-repair mechanism significantly improves fatigue resistance by preventing the accumulation of damage during cyclic loading. The self-healing properties can be triggered by various stimuli including temperature, pH, or simply by bringing fractured surfaces into contact, allowing the hydrogel to maintain its mechanical integrity over extended periods of use.Expand Specific Solutions04 Hydrogel reinforcement strategies
Various reinforcement strategies have been developed to enhance the mechanical fatigue resistance of hydrogels. These include the incorporation of nanomaterials such as graphene, carbon nanotubes, or clay particles, as well as the use of fiber reinforcement or crystalline domains. These reinforcing elements distribute stress throughout the hydrogel network, preventing localized damage accumulation and crack propagation during cyclic loading, thereby significantly improving fatigue resistance.Expand Specific Solutions05 Applications of fatigue-resistant hydrogels
Fatigue-resistant hydrogels find applications in various fields including biomedical devices, soft robotics, and wearable electronics. In biomedical applications, these hydrogels are used for artificial cartilage, load-bearing tissue scaffolds, and long-term implants. In soft robotics and wearable technology, fatigue-resistant hydrogels enable the development of durable actuators, sensors, and flexible electronics that can withstand repeated deformation cycles without performance degradation.Expand Specific Solutions
Leading Research Institutions and Manufacturers in Hydrogel Testing
The hydrogel mechanical fatigue testing market for implants is currently in a growth phase, with increasing demand driven by expanding applications in medical devices. The market size is projected to grow significantly as hydrogel implants gain wider adoption in orthopedics, cardiovascular, and soft tissue applications. Technologically, the field is advancing from basic mechanical testing to more sophisticated protocols that simulate in vivo conditions. Leading players include Dynatek Labs, which specializes in medical device testing with over 35 years of experience, and Cartiva, focused on hydrogel solutions for osteoarthritis. Academic institutions like École Polytechnique Fédérale de Lausanne and Duke University are advancing fundamental research, while companies such as Gelifex (acquired by Johnson & Johnson) and Healshape are commercializing innovative hydrogel technologies for specific clinical applications, indicating a maturing but still evolving technical landscape.
Dynatek Labs, Inc.
Technical Solution: Dynatek Labs has commercialized specialized hydrogel fatigue testing equipment and protocols specifically designed for medical implant applications. Their approach utilizes accelerated testing methodologies that maintain physiological relevance while reducing testing timeframes. The protocol employs their proprietary multi-station testing platforms capable of simultaneous evaluation of multiple samples under identical conditions, improving statistical power and throughput. Testing is conducted in temperature-controlled environments with programmable loading profiles that can simulate various physiological conditions (walking, running, stair climbing) for orthopedic applications or pulsatile flow for cardiovascular applications. Dynatek's innovation includes standardized testing fixtures and sample preparation protocols that improve reproducibility across testing sites. Their acceptance criteria framework incorporates statistical methods for determining minimum sample sizes and establishing confidence intervals for fatigue life predictions, with specific thresholds based on implant classification and intended use duration.
Strengths: Commercially available standardized testing equipment and protocols; accelerated testing methodologies that maintain physiological relevance; statistical framework for acceptance criteria. Weaknesses: Proprietary testing equipment increases implementation costs; accelerated testing may not capture all failure modes seen in long-term use; standardized approach may not address unique requirements of novel hydrogel formulations.
Drexel University
Technical Solution: Drexel University has established a comprehensive hydrogel mechanical fatigue testing framework with particular emphasis on load-bearing orthopedic implant applications. Their protocol implements a hierarchical testing approach that progresses from material-level characterization to component-level and finally system-level fatigue assessment. The methodology incorporates physiologically relevant loading conditions with variable strain rates and frequencies (0.1-20 Hz), conducted in temperature-controlled phosphate-buffered saline environments. Drexel's innovation includes the development of non-destructive evaluation techniques that can be applied during fatigue testing, including ultrasonic characterization and mechanical spectroscopy, allowing for real-time assessment of material degradation. Their acceptance criteria are stratified based on implant location and expected loading conditions, with more demanding standards for high-load applications (e.g., >90% mechanical property retention after 5 million cycles for knee cartilage replacements) versus moderate-load applications (>80% retention for finger joint replacements).
Strengths: Hierarchical testing approach from material to system level; incorporation of non-destructive evaluation techniques; application-specific acceptance criteria based on implant location. Weaknesses: Complex testing methodology requires specialized equipment and expertise; longer testing duration for complete protocol implementation; higher costs associated with comprehensive testing approach.
Critical Technologies in Hydrogel Mechanical Property Characterization
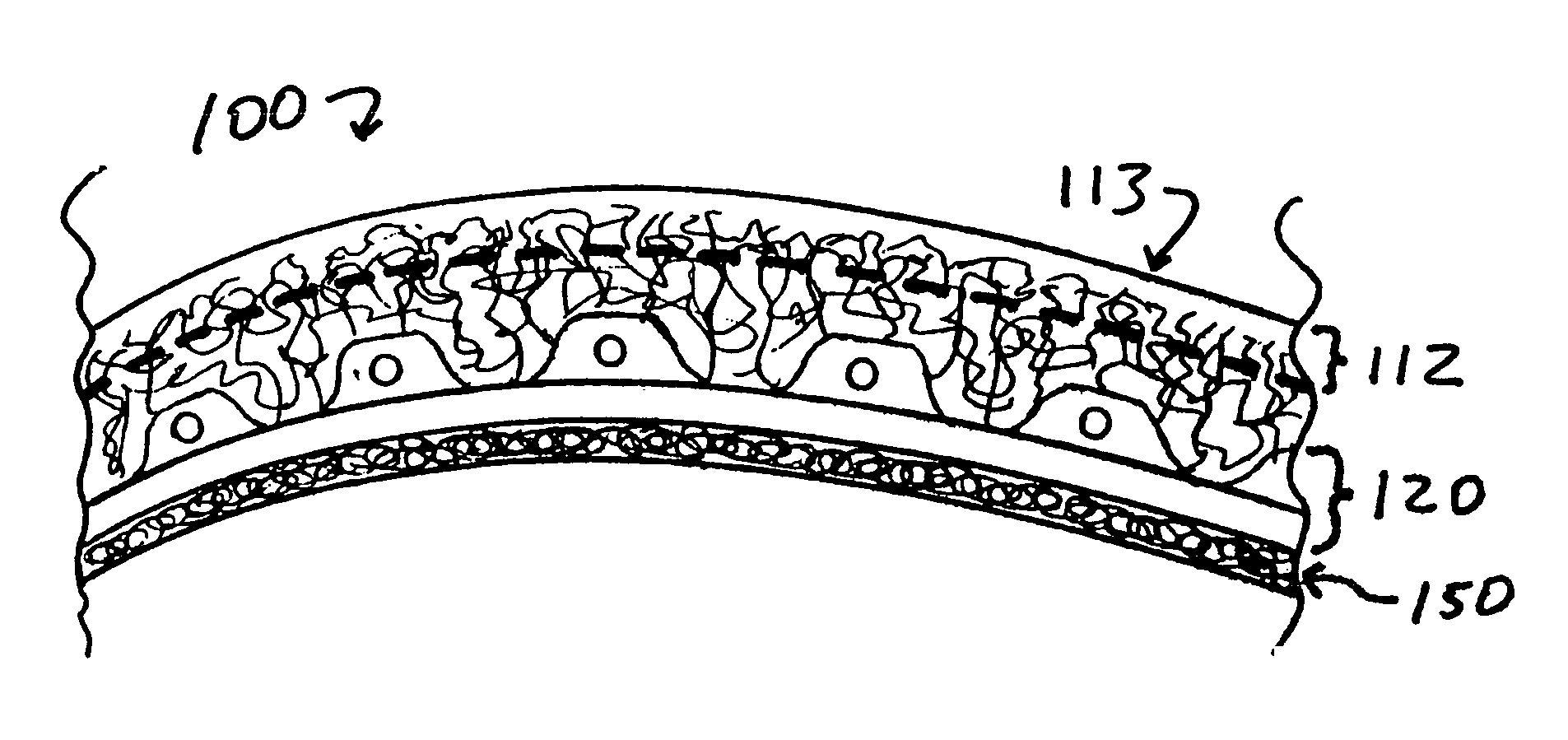
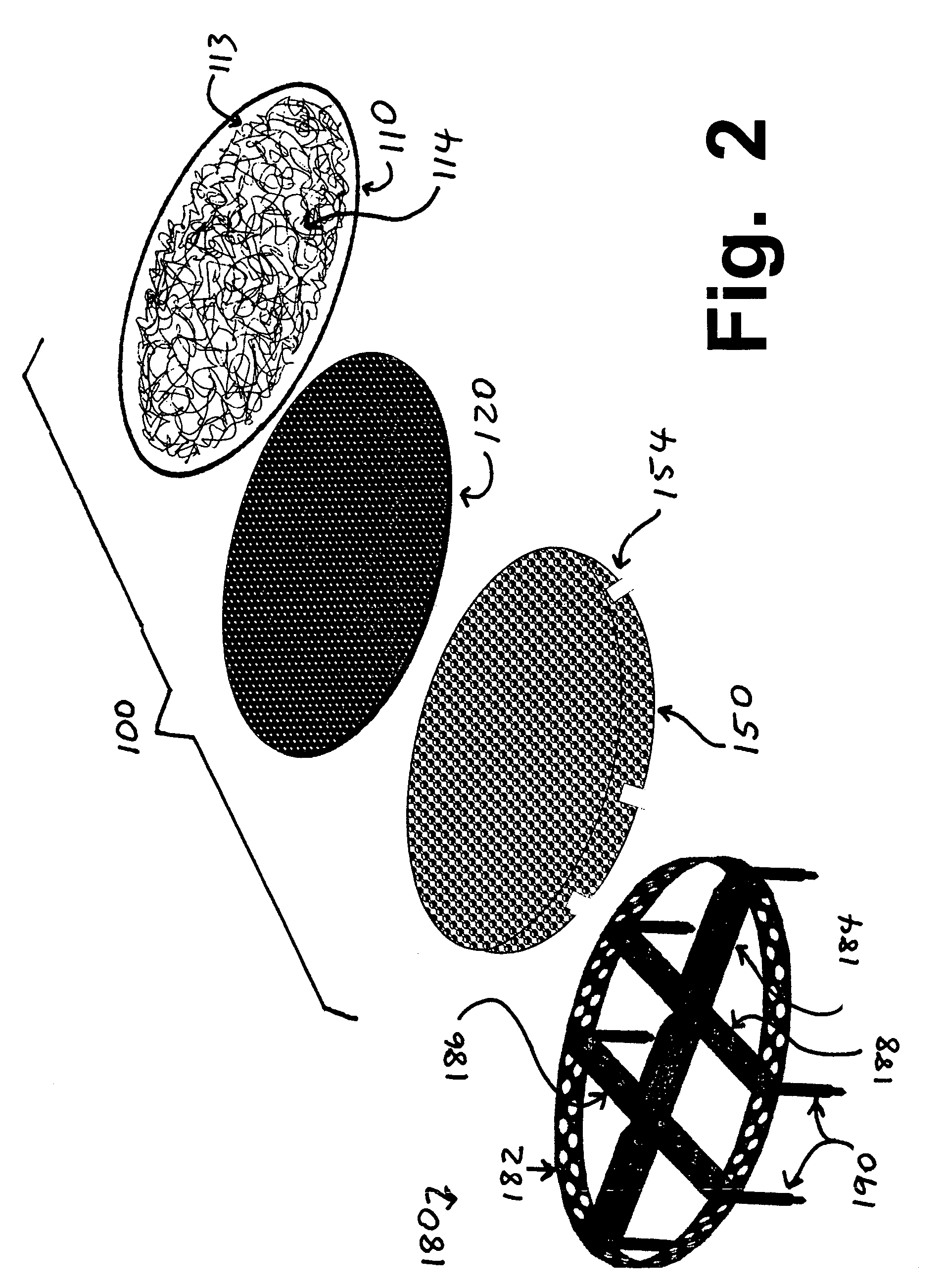
Implants for replacing cartilage, with negatively-charged hydrogel surfaces and flexible matrix reinforcement
PatentInactiveUS9314339B2
Innovation
- The development of reinforced hydrogel implants with a flexible fibrous matrix embedded within the hydrogel material and a chemically treated surface layer, such as through sulfonation, to enhance strength, durability, and lubricity, allowing secure anchoring to bone or soft tissue while maintaining smooth articulating surfaces.
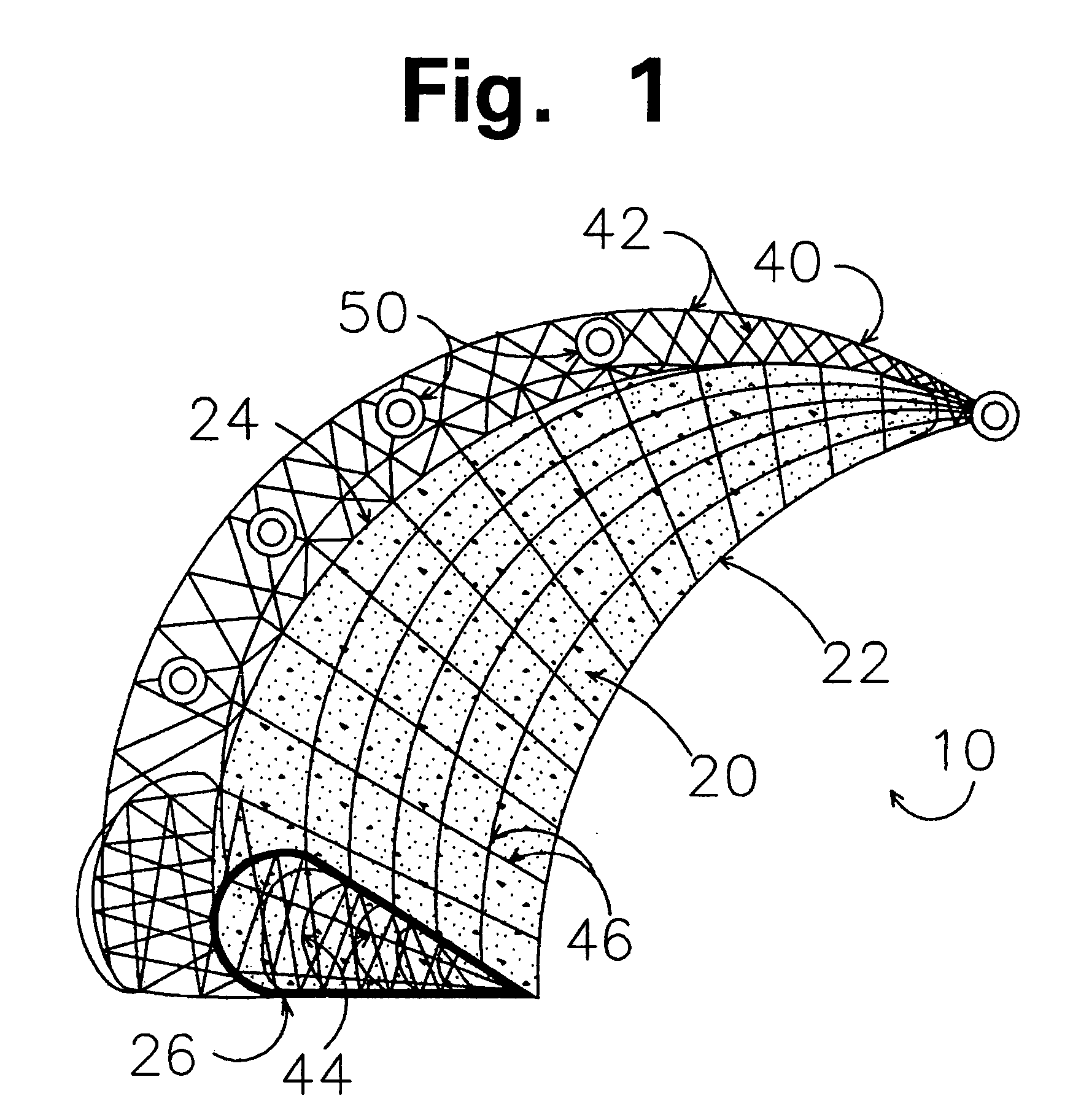
Implants for replacing hyaline cartilage, with hydrogel reinforced by three-dimensional fiber arrays
PatentActiveUS8858632B2
Innovation
- The development of surgical implants with a hydrogel layer reinforced by a three-dimensional (3D) fiber array, created using computer-controlled stitching machines, which provides strong and flexible reinforcement capable of anchoring securely to bone surfaces and maintaining permeability to water, allowing for the replacement of entire cartilage segments rather than just plugs.
Regulatory Framework for Hydrogel Implant Approval
The regulatory landscape for hydrogel implants is complex and multifaceted, requiring manufacturers to navigate various approval pathways across different jurisdictions. In the United States, the Food and Drug Administration (FDA) oversees the approval process through its Center for Devices and Radiological Health (CDRH), classifying hydrogel implants typically as Class III medical devices due to their high-risk profile when used for long-term implantation.
The FDA approval pathway necessitates extensive preclinical testing, including mechanical fatigue testing that must adhere to specific protocols outlined in guidance documents such as "Guidance for Industry and FDA Staff: Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol" which, while not specific to hydrogels, establishes precedents for fatigue testing of implantable materials.
In the European Union, the Medical Device Regulation (MDR 2017/745) has significantly increased requirements for clinical evidence and post-market surveillance of implantable devices. Hydrogel implants must receive CE marking through Notified Bodies, with particular attention to mechanical durability testing as outlined in harmonized standards such as ISO 10993-13 for degradation testing of polymeric medical devices.
The International Organization for Standardization (ISO) provides several standards relevant to hydrogel implant testing, including ISO 14243 for wear testing of implant materials and ISO 14801 for fatigue testing. These standards, while originally developed for orthopedic implants, have been adapted by regulatory bodies to establish testing protocols for hydrogel-based devices.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for hydrogel implants through their "Guidelines for Basic Biological Tests of Medical Devices," which includes mechanical fatigue testing requirements with particular emphasis on long-term stability in physiological conditions.
Regulatory acceptance criteria for hydrogel mechanical fatigue typically require demonstration of material integrity over a simulated lifespan equivalent to 10-15 years of physiological loading. This generally translates to 10-20 million cycles of testing under physiologically relevant conditions, with acceptance thresholds typically set at less than 10% degradation in mechanical properties and no visible signs of structural failure.
Recent regulatory trends indicate increasing scrutiny of hydrogel implants, with authorities requiring more comprehensive mechanical characterization, including testing under combined loading conditions that better simulate in vivo environments. Additionally, there is growing emphasis on patient-specific risk factors, requiring manufacturers to demonstrate mechanical reliability across diverse patient populations and usage scenarios.
The FDA approval pathway necessitates extensive preclinical testing, including mechanical fatigue testing that must adhere to specific protocols outlined in guidance documents such as "Guidance for Industry and FDA Staff: Technical Considerations for Non-Clinical Assessment of Medical Devices Containing Nitinol" which, while not specific to hydrogels, establishes precedents for fatigue testing of implantable materials.
In the European Union, the Medical Device Regulation (MDR 2017/745) has significantly increased requirements for clinical evidence and post-market surveillance of implantable devices. Hydrogel implants must receive CE marking through Notified Bodies, with particular attention to mechanical durability testing as outlined in harmonized standards such as ISO 10993-13 for degradation testing of polymeric medical devices.
The International Organization for Standardization (ISO) provides several standards relevant to hydrogel implant testing, including ISO 14243 for wear testing of implant materials and ISO 14801 for fatigue testing. These standards, while originally developed for orthopedic implants, have been adapted by regulatory bodies to establish testing protocols for hydrogel-based devices.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific guidelines for hydrogel implants through their "Guidelines for Basic Biological Tests of Medical Devices," which includes mechanical fatigue testing requirements with particular emphasis on long-term stability in physiological conditions.
Regulatory acceptance criteria for hydrogel mechanical fatigue typically require demonstration of material integrity over a simulated lifespan equivalent to 10-15 years of physiological loading. This generally translates to 10-20 million cycles of testing under physiologically relevant conditions, with acceptance thresholds typically set at less than 10% degradation in mechanical properties and no visible signs of structural failure.
Recent regulatory trends indicate increasing scrutiny of hydrogel implants, with authorities requiring more comprehensive mechanical characterization, including testing under combined loading conditions that better simulate in vivo environments. Additionally, there is growing emphasis on patient-specific risk factors, requiring manufacturers to demonstrate mechanical reliability across diverse patient populations and usage scenarios.
Biocompatibility and Long-term Safety Considerations
Biocompatibility assessment for hydrogel implants requires comprehensive evaluation beyond initial compatibility testing. The long-term interaction between hydrogel materials and surrounding tissues presents unique challenges that must be addressed through rigorous safety protocols. When developing mechanical fatigue testing criteria for hydrogel implants, biocompatibility considerations must extend throughout the entire product lifecycle, from initial material selection to post-implantation monitoring.
The degradation products of hydrogels under mechanical stress represent a primary safety concern. As these materials undergo repeated loading cycles during fatigue testing, they may release particles, monomers, or chemical compounds that could potentially trigger inflammatory responses or adverse tissue reactions. Testing protocols must therefore incorporate methods to capture and analyze these degradation products, establishing clear acceptance thresholds based on toxicological profiles.
Immune system interactions pose another critical consideration in long-term safety assessment. Hydrogels that demonstrate excellent initial biocompatibility may still elicit delayed hypersensitivity reactions or chronic inflammation under mechanical fatigue conditions. Fatigue testing protocols should include immunological evaluation metrics that assess potential changes in the material's immunogenicity after exposure to cyclic loading.
The integration of in vitro and in vivo testing methodologies strengthens the predictive value of hydrogel fatigue assessments. While accelerated in vitro testing provides valuable preliminary data, animal models remain essential for evaluating the complex biological responses to mechanically stressed hydrogel implants over extended timeframes. Correlation between these testing approaches enhances the reliability of safety predictions.
Regulatory frameworks for hydrogel implants increasingly emphasize the need for long-term safety data that specifically addresses mechanical performance degradation. The FDA and European regulatory bodies have established guidelines that require manufacturers to demonstrate maintained biocompatibility throughout the expected service life of the implant. Fatigue testing protocols must align with these regulatory expectations, incorporating acceptance criteria that reflect real-world implantation conditions.
Emerging technologies such as organ-on-chip platforms and advanced imaging techniques are revolutionizing how researchers evaluate the long-term biocompatibility of hydrogel implants under mechanical stress. These approaches allow for more physiologically relevant testing environments and can detect subtle material-tissue interactions that might be missed in conventional testing paradigms.
The degradation products of hydrogels under mechanical stress represent a primary safety concern. As these materials undergo repeated loading cycles during fatigue testing, they may release particles, monomers, or chemical compounds that could potentially trigger inflammatory responses or adverse tissue reactions. Testing protocols must therefore incorporate methods to capture and analyze these degradation products, establishing clear acceptance thresholds based on toxicological profiles.
Immune system interactions pose another critical consideration in long-term safety assessment. Hydrogels that demonstrate excellent initial biocompatibility may still elicit delayed hypersensitivity reactions or chronic inflammation under mechanical fatigue conditions. Fatigue testing protocols should include immunological evaluation metrics that assess potential changes in the material's immunogenicity after exposure to cyclic loading.
The integration of in vitro and in vivo testing methodologies strengthens the predictive value of hydrogel fatigue assessments. While accelerated in vitro testing provides valuable preliminary data, animal models remain essential for evaluating the complex biological responses to mechanically stressed hydrogel implants over extended timeframes. Correlation between these testing approaches enhances the reliability of safety predictions.
Regulatory frameworks for hydrogel implants increasingly emphasize the need for long-term safety data that specifically addresses mechanical performance degradation. The FDA and European regulatory bodies have established guidelines that require manufacturers to demonstrate maintained biocompatibility throughout the expected service life of the implant. Fatigue testing protocols must align with these regulatory expectations, incorporating acceptance criteria that reflect real-world implantation conditions.
Emerging technologies such as organ-on-chip platforms and advanced imaging techniques are revolutionizing how researchers evaluate the long-term biocompatibility of hydrogel implants under mechanical stress. These approaches allow for more physiologically relevant testing environments and can detect subtle material-tissue interactions that might be missed in conventional testing paradigms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!