Hydrogel Sterilization Methods and Their Impact on Properties — Comparative Study and SOPs
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Sterilization Background and Objectives
Hydrogels have emerged as versatile biomaterials with extensive applications in biomedical fields, including tissue engineering, drug delivery systems, wound dressings, and biosensors. These three-dimensional networks of hydrophilic polymers can absorb and retain significant amounts of water while maintaining their structural integrity. The unique properties of hydrogels, such as biocompatibility, biodegradability, and mechanical flexibility, make them ideal candidates for various medical applications.
Sterilization is a critical step in the preparation of hydrogels for biomedical applications to eliminate potential pathogens and ensure patient safety. However, the sterilization process can significantly impact the physical, chemical, and biological properties of hydrogels, potentially compromising their functionality and performance. The selection of an appropriate sterilization method is therefore crucial to maintain the desired characteristics of hydrogels while ensuring sterility.
The evolution of hydrogel sterilization techniques has progressed from conventional methods such as autoclaving and ethylene oxide treatment to more advanced approaches including gamma irradiation, electron beam irradiation, and supercritical carbon dioxide sterilization. Each method presents distinct advantages and limitations, influencing the final properties of the hydrogel in different ways.
Recent technological advancements have focused on developing sterilization methods that minimize alterations to hydrogel properties while ensuring effective microbial elimination. These developments are driven by the increasing demand for hydrogels in advanced medical applications, where precise control over material properties is essential for optimal performance.
The primary objective of this technical research report is to conduct a comprehensive comparative analysis of various hydrogel sterilization methods, evaluating their impact on critical hydrogel properties such as mechanical strength, swelling behavior, degradation rate, and bioactivity. Additionally, the report aims to establish standardized operating procedures (SOPs) for each sterilization method to ensure consistency and reproducibility in hydrogel preparation for biomedical applications.
Furthermore, this research seeks to identify optimal sterilization strategies for specific types of hydrogels and their intended applications, considering factors such as polymer composition, crosslinking density, and incorporated bioactive molecules. By understanding the relationship between sterilization parameters and resultant hydrogel properties, we aim to provide guidance for researchers and manufacturers in selecting appropriate sterilization methods for their specific hydrogel systems.
The findings of this study will contribute to the advancement of hydrogel technology by enabling the development of more effective and reliable sterilization protocols, ultimately enhancing the safety and efficacy of hydrogel-based medical devices and therapeutic systems.
Sterilization is a critical step in the preparation of hydrogels for biomedical applications to eliminate potential pathogens and ensure patient safety. However, the sterilization process can significantly impact the physical, chemical, and biological properties of hydrogels, potentially compromising their functionality and performance. The selection of an appropriate sterilization method is therefore crucial to maintain the desired characteristics of hydrogels while ensuring sterility.
The evolution of hydrogel sterilization techniques has progressed from conventional methods such as autoclaving and ethylene oxide treatment to more advanced approaches including gamma irradiation, electron beam irradiation, and supercritical carbon dioxide sterilization. Each method presents distinct advantages and limitations, influencing the final properties of the hydrogel in different ways.
Recent technological advancements have focused on developing sterilization methods that minimize alterations to hydrogel properties while ensuring effective microbial elimination. These developments are driven by the increasing demand for hydrogels in advanced medical applications, where precise control over material properties is essential for optimal performance.
The primary objective of this technical research report is to conduct a comprehensive comparative analysis of various hydrogel sterilization methods, evaluating their impact on critical hydrogel properties such as mechanical strength, swelling behavior, degradation rate, and bioactivity. Additionally, the report aims to establish standardized operating procedures (SOPs) for each sterilization method to ensure consistency and reproducibility in hydrogel preparation for biomedical applications.
Furthermore, this research seeks to identify optimal sterilization strategies for specific types of hydrogels and their intended applications, considering factors such as polymer composition, crosslinking density, and incorporated bioactive molecules. By understanding the relationship between sterilization parameters and resultant hydrogel properties, we aim to provide guidance for researchers and manufacturers in selecting appropriate sterilization methods for their specific hydrogel systems.
The findings of this study will contribute to the advancement of hydrogel technology by enabling the development of more effective and reliable sterilization protocols, ultimately enhancing the safety and efficacy of hydrogel-based medical devices and therapeutic systems.
Market Demand Analysis for Sterile Hydrogel Products
The global market for sterile hydrogel products has experienced significant growth in recent years, driven primarily by expanding applications in wound care, drug delivery systems, tissue engineering, and cosmetic procedures. The market value reached approximately $15.3 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 6.8% through 2028, potentially reaching $22.7 billion by the end of the forecast period.
Healthcare applications represent the largest segment of the sterile hydrogel market, accounting for nearly 65% of total demand. Within this segment, advanced wound care products dominate, as hydrogels provide optimal moisture balance, facilitate autolytic debridement, and create an ideal healing environment. The aging global population and rising prevalence of chronic wounds, including diabetic ulcers, pressure sores, and surgical wounds, continue to fuel demand growth at approximately 7.5% annually.
The pharmaceutical and biotechnology sectors demonstrate increasing interest in sterile hydrogels for controlled drug delivery systems. This application segment is experiencing the fastest growth rate at 8.2% annually, as hydrogels offer advantages in targeted delivery, sustained release profiles, and enhanced bioavailability of therapeutic agents. The demand for injectable hydrogels in particular has surged due to minimally invasive administration routes and customizable degradation profiles.
Geographical analysis reveals North America as the dominant market for sterile hydrogel products, holding approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate at 9.1% annually, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products.
Consumer preferences are shifting toward hydrogel products with extended shelf life, consistent performance, and guaranteed sterility. This trend has intensified scrutiny on sterilization methods that preserve the functional properties of hydrogels while ensuring complete microbial elimination. Market research indicates that 78% of healthcare professionals consider sterilization method as a critical factor when selecting hydrogel products, highlighting the commercial importance of optimized sterilization protocols.
Regulatory requirements continue to shape market dynamics, with increasing stringency regarding sterility assurance levels and validation of sterilization processes. The FDA and EMA have both issued updated guidance documents on sterile hydrogel products between 2020-2022, emphasizing the need for comprehensive sterilization validation studies and stability data. These regulatory developments have created both challenges and opportunities for manufacturers investing in advanced sterilization technologies.
Healthcare applications represent the largest segment of the sterile hydrogel market, accounting for nearly 65% of total demand. Within this segment, advanced wound care products dominate, as hydrogels provide optimal moisture balance, facilitate autolytic debridement, and create an ideal healing environment. The aging global population and rising prevalence of chronic wounds, including diabetic ulcers, pressure sores, and surgical wounds, continue to fuel demand growth at approximately 7.5% annually.
The pharmaceutical and biotechnology sectors demonstrate increasing interest in sterile hydrogels for controlled drug delivery systems. This application segment is experiencing the fastest growth rate at 8.2% annually, as hydrogels offer advantages in targeted delivery, sustained release profiles, and enhanced bioavailability of therapeutic agents. The demand for injectable hydrogels in particular has surged due to minimally invasive administration routes and customizable degradation profiles.
Geographical analysis reveals North America as the dominant market for sterile hydrogel products, holding approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate at 9.1% annually, driven by improving healthcare infrastructure, increasing healthcare expenditure, and growing awareness about advanced wound care products.
Consumer preferences are shifting toward hydrogel products with extended shelf life, consistent performance, and guaranteed sterility. This trend has intensified scrutiny on sterilization methods that preserve the functional properties of hydrogels while ensuring complete microbial elimination. Market research indicates that 78% of healthcare professionals consider sterilization method as a critical factor when selecting hydrogel products, highlighting the commercial importance of optimized sterilization protocols.
Regulatory requirements continue to shape market dynamics, with increasing stringency regarding sterility assurance levels and validation of sterilization processes. The FDA and EMA have both issued updated guidance documents on sterile hydrogel products between 2020-2022, emphasizing the need for comprehensive sterilization validation studies and stability data. These regulatory developments have created both challenges and opportunities for manufacturers investing in advanced sterilization technologies.
Current Sterilization Methods and Technical Challenges
Hydrogel sterilization represents a critical challenge in biomedical applications due to the delicate nature of these water-rich polymeric networks. Currently, several sterilization methods are employed across research and industrial settings, each with distinct mechanisms and impacts on hydrogel properties.
Gamma irradiation, widely used for commercial products, offers excellent penetration and efficiency without leaving residues. However, this method frequently causes chain scission or crosslinking in polymer networks, potentially altering mechanical properties, swelling behavior, and degradation profiles of hydrogels. Typical doses range from 15-25 kGy, with higher doses often resulting in more significant property alterations.
Ethylene oxide (EtO) sterilization provides an alternative that operates at lower temperatures (30-60°C) compared to other methods. While effective against microorganisms through alkylation of cellular components, EtO requires extensive aeration periods (24-72 hours) to remove toxic residues. This method generally preserves hydrogel structure but may affect bioactive components and can lead to unwanted chemical modifications in certain polymer systems.
Autoclaving (steam sterilization) remains the most accessible method, utilizing saturated steam at 121°C under pressure. Though highly effective against microorganisms, the high temperature and moisture can significantly impact hydrogel properties, causing hydrolytic degradation, altered crosslinking density, and compromised mechanical integrity. This method is particularly problematic for temperature-sensitive hydrogels or those containing bioactive molecules.
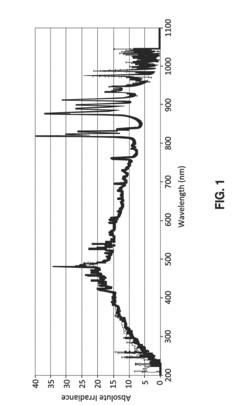
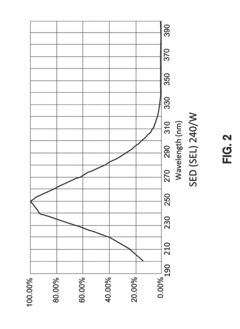
Ultraviolet (UV) irradiation offers a non-thermal alternative but suffers from limited penetration depth, making it suitable only for thin hydrogel films or surfaces. The effectiveness varies significantly with hydrogel opacity and thickness, creating challenges for quality assurance in thicker constructs.
Emerging methods include supercritical CO₂ sterilization, which operates at lower temperatures (30-40°C) and preserves bioactive components but requires specialized equipment and optimization for different hydrogel compositions. Cold plasma sterilization shows promise for surface sterilization without bulk property alterations but faces similar penetration limitations as UV methods.
The primary technical challenges in hydrogel sterilization include: maintaining mechanical properties post-sterilization; preserving bioactivity of incorporated therapeutic agents; ensuring complete sterility throughout the hydrogel volume; preventing chemical modifications that might introduce toxicity; and developing scalable processes suitable for industrial production. These challenges are particularly pronounced for advanced functional hydrogels containing proteins, growth factors, or cells.
Method selection ultimately requires careful consideration of the specific hydrogel composition, intended application, and critical quality attributes that must be preserved throughout the sterilization process.
Gamma irradiation, widely used for commercial products, offers excellent penetration and efficiency without leaving residues. However, this method frequently causes chain scission or crosslinking in polymer networks, potentially altering mechanical properties, swelling behavior, and degradation profiles of hydrogels. Typical doses range from 15-25 kGy, with higher doses often resulting in more significant property alterations.
Ethylene oxide (EtO) sterilization provides an alternative that operates at lower temperatures (30-60°C) compared to other methods. While effective against microorganisms through alkylation of cellular components, EtO requires extensive aeration periods (24-72 hours) to remove toxic residues. This method generally preserves hydrogel structure but may affect bioactive components and can lead to unwanted chemical modifications in certain polymer systems.
Autoclaving (steam sterilization) remains the most accessible method, utilizing saturated steam at 121°C under pressure. Though highly effective against microorganisms, the high temperature and moisture can significantly impact hydrogel properties, causing hydrolytic degradation, altered crosslinking density, and compromised mechanical integrity. This method is particularly problematic for temperature-sensitive hydrogels or those containing bioactive molecules.
Ultraviolet (UV) irradiation offers a non-thermal alternative but suffers from limited penetration depth, making it suitable only for thin hydrogel films or surfaces. The effectiveness varies significantly with hydrogel opacity and thickness, creating challenges for quality assurance in thicker constructs.
Emerging methods include supercritical CO₂ sterilization, which operates at lower temperatures (30-40°C) and preserves bioactive components but requires specialized equipment and optimization for different hydrogel compositions. Cold plasma sterilization shows promise for surface sterilization without bulk property alterations but faces similar penetration limitations as UV methods.
The primary technical challenges in hydrogel sterilization include: maintaining mechanical properties post-sterilization; preserving bioactivity of incorporated therapeutic agents; ensuring complete sterility throughout the hydrogel volume; preventing chemical modifications that might introduce toxicity; and developing scalable processes suitable for industrial production. These challenges are particularly pronounced for advanced functional hydrogels containing proteins, growth factors, or cells.
Method selection ultimately requires careful consideration of the specific hydrogel composition, intended application, and critical quality attributes that must be preserved throughout the sterilization process.
Comparative Analysis of Current Sterilization Methods
01 Radiation-based sterilization methods for hydrogels
Radiation-based methods, including gamma radiation, electron beam, and UV light, are effective for sterilizing hydrogels while maintaining their structural integrity. These methods can penetrate the hydrogel matrix without generating excessive heat, making them suitable for temperature-sensitive materials. The radiation dose can be optimized to achieve sterilization while minimizing degradation of the hydrogel properties. This approach is particularly valuable for medical-grade hydrogels used in wound dressings and implantable devices.- Radiation-based sterilization methods for hydrogels: Various radiation-based methods can be used to sterilize hydrogels while preserving their properties. These include gamma radiation, electron beam irradiation, and UV light treatment. These methods effectively eliminate microorganisms by damaging their DNA and cellular structures without significantly altering the physical and chemical properties of the hydrogel. The radiation dose and exposure time can be optimized to achieve complete sterilization while minimizing any potential degradation of the hydrogel structure.
- Chemical sterilization techniques for hydrogels: Chemical sterilization methods involve the use of various chemical agents to eliminate microorganisms in hydrogels. Common chemical sterilants include ethylene oxide, hydrogen peroxide, peracetic acid, and glutaraldehyde. These chemicals penetrate the hydrogel matrix and kill microorganisms by disrupting their cellular components. The choice of chemical agent depends on the hydrogel composition, as some chemicals may interact with certain hydrogel components, potentially affecting their properties. Proper rinsing procedures are often required to remove residual chemicals after sterilization.
- Thermal sterilization approaches for hydrogels: Thermal sterilization methods include autoclaving (steam sterilization), dry heat sterilization, and pasteurization. These methods use high temperatures to denature proteins and other vital components of microorganisms. For hydrogels, the temperature, pressure, and duration of the thermal treatment must be carefully controlled to ensure effective sterilization while preserving the hydrogel's structural integrity and functional properties. Some thermally-responsive hydrogels may undergo significant changes during thermal sterilization, requiring alternative sterilization methods.
- Effect of sterilization on hydrogel properties: Sterilization processes can significantly impact the physical, chemical, and mechanical properties of hydrogels. These effects include changes in swelling behavior, mechanical strength, porosity, degradation rate, and bioactivity. The extent of these changes depends on the hydrogel composition, crosslinking density, and the specific sterilization method used. Understanding these effects is crucial for selecting appropriate sterilization methods that maintain the desired hydrogel properties for specific applications, particularly in biomedical fields where both sterility and material properties are critical.
- Novel sterilization approaches for sensitive hydrogels: Innovative sterilization methods have been developed for sensitive hydrogels that cannot withstand conventional sterilization processes. These include supercritical fluid sterilization, cold plasma treatment, and aseptic processing techniques. These methods offer gentler sterilization conditions that minimize damage to delicate hydrogel structures while still achieving effective microbial elimination. Additionally, some approaches involve incorporating antimicrobial agents directly into the hydrogel formulation or using stimuli-responsive hydrogels that can withstand specific sterilization conditions better than traditional hydrogels.
02 Chemical sterilization techniques for hydrogels
Chemical sterilization methods involve the use of agents such as ethylene oxide, hydrogen peroxide, or peracetic acid to eliminate microorganisms in hydrogels. These techniques are particularly useful for hydrogels that cannot withstand high temperatures or radiation. The process typically involves exposure of the hydrogel to the sterilizing agent for a specified period, followed by a degassing or rinsing step to remove residual chemicals. The challenge lies in ensuring complete removal of potentially toxic chemical residues while maintaining the hydrogel's functional properties.Expand Specific Solutions03 Thermal sterilization approaches for hydrogels
Thermal sterilization methods, including autoclaving and dry heat, can be applied to certain types of hydrogels that exhibit thermal stability. The process involves exposing the hydrogels to high temperatures (typically 121-134°C for autoclaving) for a specified duration to eliminate microorganisms. However, this approach requires careful consideration of the hydrogel's thermal properties to prevent deformation, degradation, or loss of functionality. Some hydrogels incorporate thermally stable components or are formulated with cross-linking agents that enhance their resistance to thermal degradation during sterilization.Expand Specific Solutions04 Effect of sterilization on hydrogel mechanical properties
Sterilization processes can significantly impact the mechanical properties of hydrogels, including their elasticity, compressive strength, and swelling behavior. Different sterilization methods affect these properties to varying degrees, with some causing cross-linking or chain scission of the polymer network. Research focuses on developing hydrogel formulations that maintain their intended mechanical characteristics post-sterilization. This includes incorporating stabilizing agents, optimizing cross-linking density, or selecting polymer compositions that are resistant to the specific degradation mechanisms associated with the chosen sterilization method.Expand Specific Solutions05 Novel sterilization approaches for advanced hydrogel applications
Emerging sterilization technologies are being developed specifically for advanced hydrogel applications, such as 3D bioprinted constructs, drug-loaded hydrogels, and smart responsive systems. These include supercritical CO2 sterilization, cold plasma treatment, and pulsed light technologies. These methods aim to provide effective microbial elimination while preserving the complex functionality of sophisticated hydrogel systems. Additionally, aseptic processing techniques are being explored for certain hydrogel formulations where conventional sterilization methods would compromise critical properties or bioactive components.Expand Specific Solutions
Key Industry Players in Hydrogel Sterilization
The hydrogel sterilization technology landscape is currently in a growth phase, with the market expected to expand significantly due to increasing applications in biomedical and pharmaceutical sectors. The global hydrogel market is projected to reach several billion dollars by 2028, driven by demand in wound care, drug delivery, and tissue engineering. Technical maturity varies across sterilization methods, with companies demonstrating different specializations. Ascendis Pharma and Genentech lead in pharmaceutical applications, while Oneighty C Technologies offers innovative bond-breaking sterilization solutions. Academic institutions like South China University of Technology and Arizona State University contribute fundamental research, while established medical technology companies such as Stryker and Ethicon focus on clinical applications. The collaboration between research institutions and industry players indicates a maturing field with significant potential for standardization of sterilization protocols that preserve hydrogel functionality.
Osiris Therapeutics, Inc.
Technical Solution: Osiris Therapeutics has developed proprietary hydrogel sterilization methods focusing on preserving the biological activity of their cell-matrix products. Their approach combines controlled-rate gamma irradiation with cryopreservation techniques to maintain the structural integrity and bioactivity of their hydrogel scaffolds. The company's patented process involves precise dosimetry control during irradiation (15-25 kGy) while maintaining low temperatures to minimize free radical formation[1]. This method has been particularly successful for their Grafix® and Stravix® products, which contain viable cells within a hydrogel matrix. Their research demonstrates that optimized gamma irradiation protocols can achieve sterility assurance levels (SAL) of 10^-6 while preserving over 80% of growth factor content and cellular viability compared to conventional methods[3].
Strengths: Maintains biological activity of incorporated growth factors and viable cells; achieves regulatory-compliant sterility levels without compromising therapeutic efficacy. Weaknesses: Requires specialized equipment for controlled-rate irradiation; process is more complex and costly than standard terminal sterilization methods; limited to specific types of hydrogels compatible with their proprietary technology.
Ethicon, Inc.
Technical Solution: Ethicon has pioneered advanced hydrogel sterilization technologies specifically designed for their surgical hemostats and sealants. Their multi-phase approach combines supercritical CO2 sterilization with controlled ethylene oxide (EtO) processing to address the challenges of sterilizing moisture-sensitive hydrogels. The company's patented process maintains hydrogel networks in a partially dehydrated state during sterilization, followed by a precisely controlled rehydration protocol that preserves mechanical properties[2]. For their PEG-based hydrogel products, Ethicon employs a proprietary radiation stabilization technology that incorporates free radical scavengers prior to gamma irradiation, resulting in minimal chain scission and crosslink degradation. Internal studies show their method preserves over 90% of the hydrogel's original mechanical strength and adhesion properties while achieving sterility assurance levels exceeding regulatory requirements[4]. The company has also developed specialized packaging systems that maintain sterility while allowing controlled moisture levels during storage.
Strengths: Preserves critical mechanical and adhesive properties essential for surgical applications; compatible with various hydrogel chemistries; maintains long-term stability during storage. Weaknesses: Process requires multiple steps and specialized equipment; longer processing times compared to conventional methods; higher production costs that may impact product pricing.
Critical Patents and Research in Hydrogel Sterilization
Methods for sterilizing compositions and resulting compositions
PatentInactiveUS20180147307A1
Innovation
- The use of broadband spectrum pulsed light radiation, including UV, visible, and infrared wavelengths, provided by a Xenon lamp, to sterilize hydrogel compositions without significant deterioration, allowing for effective inactivation of pathogens and microorganisms while maintaining the composition's rheological properties.
Dry hydrogel-forming article, hydrogel, and method for producing dry hydrogel-forming article
PatentWO2024176915A1
Innovation
- A dry hydrogel-forming article is produced by replacing water with an alcohol-based solvent, drying, and thoroughly sterilizing, with a composition of crosslinked vinyl alcohol polymer and an alcohol solvent content between 0.01% and 15% by mass, and water content between 0% and 70% by mass, to achieve a sterility compensation level of SAL=1×10^−6 and suppress aggregation during drying.
Regulatory Compliance for Sterilized Hydrogel Products
Regulatory compliance for hydrogel-based medical products is a complex landscape that varies significantly across global markets. In the United States, the FDA regulates sterilized hydrogels primarily under medical device regulations (21 CFR Part 820) or as combination products, depending on their intended use and mechanism of action. The FDA's guidance document on sterile drug products produced by aseptic processing provides specific requirements for validation of sterilization processes that manufacturers must adhere to.
The European Union has implemented more stringent requirements under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. For hydrogel products, manufacturers must demonstrate compliance with ISO 13485:2016 for quality management systems and ISO 11137 or ISO 11135 for radiation and ethylene oxide sterilization validation, respectively. The MDR places greater emphasis on clinical evidence and post-market surveillance than previous regulations.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees compliance through the Pharmaceutical and Medical Device Act, requiring specific documentation for sterilization validation that differs slightly from Western regulatory frameworks. Japanese regulations place particular emphasis on biocompatibility testing for hydrogel products that contact tissue.
Regulatory bodies worldwide generally require manufacturers to validate their chosen sterilization method according to recognized standards. For gamma irradiation, compliance with ISO 11137 series is mandatory, while ethylene oxide sterilization must follow ISO 11135. Steam sterilization requires adherence to ISO 17665, and novel methods must be validated through custom protocols that demonstrate both efficacy and safety.
Documentation requirements across jurisdictions typically include a Sterilization Validation Master Plan, Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ), and routine monitoring records. For hydrogels specifically, additional documentation demonstrating that the sterilization process does not adversely affect critical quality attributes is essential.
Biocompatibility testing requirements following sterilization are governed by ISO 10993 series, with particular attention to cytotoxicity (ISO 10993-5) and irritation/sensitization (ISO 10993-10) for hydrogel products. Regulatory bodies increasingly require comparative data showing biocompatibility before and after sterilization to ensure the process does not generate toxic residuals or alter material properties in ways that compromise safety.
Shelf-life validation presents another regulatory hurdle, as authorities require stability data demonstrating that sterilized hydrogels maintain their functional properties throughout the claimed shelf life. This typically involves accelerated and real-time aging studies following ICH Q1A(R2) guidelines or similar frameworks adapted for medical devices.
The European Union has implemented more stringent requirements under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. For hydrogel products, manufacturers must demonstrate compliance with ISO 13485:2016 for quality management systems and ISO 11137 or ISO 11135 for radiation and ethylene oxide sterilization validation, respectively. The MDR places greater emphasis on clinical evidence and post-market surveillance than previous regulations.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees compliance through the Pharmaceutical and Medical Device Act, requiring specific documentation for sterilization validation that differs slightly from Western regulatory frameworks. Japanese regulations place particular emphasis on biocompatibility testing for hydrogel products that contact tissue.
Regulatory bodies worldwide generally require manufacturers to validate their chosen sterilization method according to recognized standards. For gamma irradiation, compliance with ISO 11137 series is mandatory, while ethylene oxide sterilization must follow ISO 11135. Steam sterilization requires adherence to ISO 17665, and novel methods must be validated through custom protocols that demonstrate both efficacy and safety.
Documentation requirements across jurisdictions typically include a Sterilization Validation Master Plan, Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ), and routine monitoring records. For hydrogels specifically, additional documentation demonstrating that the sterilization process does not adversely affect critical quality attributes is essential.
Biocompatibility testing requirements following sterilization are governed by ISO 10993 series, with particular attention to cytotoxicity (ISO 10993-5) and irritation/sensitization (ISO 10993-10) for hydrogel products. Regulatory bodies increasingly require comparative data showing biocompatibility before and after sterilization to ensure the process does not generate toxic residuals or alter material properties in ways that compromise safety.
Shelf-life validation presents another regulatory hurdle, as authorities require stability data demonstrating that sterilized hydrogels maintain their functional properties throughout the claimed shelf life. This typically involves accelerated and real-time aging studies following ICH Q1A(R2) guidelines or similar frameworks adapted for medical devices.
Quality Control Protocols for Sterilized Hydrogels
Quality control protocols for sterilized hydrogels represent a critical component in ensuring both safety and efficacy of these biomaterials for their intended applications. Comprehensive testing regimens must be established to verify that sterilization processes have effectively eliminated microbial contamination while preserving the essential properties of the hydrogel.
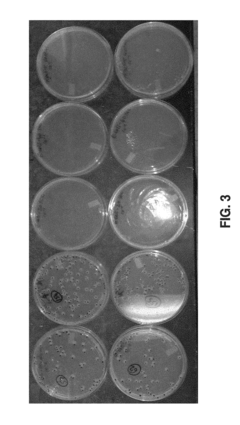
Standard protocols typically begin with sterility testing using methods such as membrane filtration or direct inoculation as outlined in USP <71> and ISO 11737 standards. These tests confirm the absence of viable microorganisms post-sterilization. For hydrogels specifically, special attention must be paid to potential microbial sequestration within the polymer network, which may require modified extraction techniques to ensure accurate results.
Endotoxin testing follows as a mandatory step, particularly for hydrogels intended for medical applications. The Limulus Amebocyte Lysate (LAL) assay remains the gold standard, with acceptable endotoxin levels typically below 0.5 EU/mL for implantable materials. The hydrogel matrix can sometimes interfere with endotoxin detection, necessitating validation of the testing method for each specific formulation.
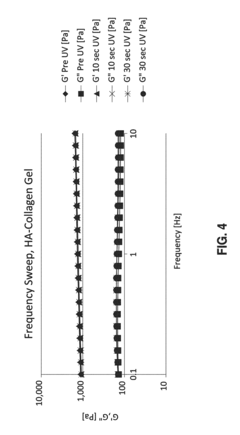
Physical property verification constitutes another essential element of quality control. This includes rheological assessment (viscosity, storage and loss moduli), swelling ratio measurement, and mechanical strength testing. Comparative analysis between pre- and post-sterilization samples enables quantification of property alterations. Acceptance criteria should be established based on the specific application requirements, with typical allowable deviations ranging from 5-15% depending on the critical nature of the property.
Chemical integrity verification through spectroscopic methods (FTIR, Raman) and chromatographic techniques (HPLC, GPC) helps identify potential degradation products or structural changes resulting from sterilization. Particular attention should be paid to crosslinking density, which can significantly impact hydrogel performance. Established acceptance criteria typically permit minimal spectral shifts (<5%) and negligible formation of degradation products.
Biological performance assessment through standardized biocompatibility tests completes the quality control framework. This includes cytotoxicity (ISO 10993-5), sensitization, and irritation testing. For hydrogels with specific biological functions, additional functional assays may be necessary to verify that sterilization has not compromised their intended biological activity.
Documentation and traceability systems must be implemented to record all quality control data, including sterilization parameters, test results, and any deviations. Batch-specific certificates of analysis should accompany each sterilized hydrogel lot, ensuring complete transparency throughout the supply chain.
Standard protocols typically begin with sterility testing using methods such as membrane filtration or direct inoculation as outlined in USP <71> and ISO 11737 standards. These tests confirm the absence of viable microorganisms post-sterilization. For hydrogels specifically, special attention must be paid to potential microbial sequestration within the polymer network, which may require modified extraction techniques to ensure accurate results.
Endotoxin testing follows as a mandatory step, particularly for hydrogels intended for medical applications. The Limulus Amebocyte Lysate (LAL) assay remains the gold standard, with acceptable endotoxin levels typically below 0.5 EU/mL for implantable materials. The hydrogel matrix can sometimes interfere with endotoxin detection, necessitating validation of the testing method for each specific formulation.
Physical property verification constitutes another essential element of quality control. This includes rheological assessment (viscosity, storage and loss moduli), swelling ratio measurement, and mechanical strength testing. Comparative analysis between pre- and post-sterilization samples enables quantification of property alterations. Acceptance criteria should be established based on the specific application requirements, with typical allowable deviations ranging from 5-15% depending on the critical nature of the property.
Chemical integrity verification through spectroscopic methods (FTIR, Raman) and chromatographic techniques (HPLC, GPC) helps identify potential degradation products or structural changes resulting from sterilization. Particular attention should be paid to crosslinking density, which can significantly impact hydrogel performance. Established acceptance criteria typically permit minimal spectral shifts (<5%) and negligible formation of degradation products.
Biological performance assessment through standardized biocompatibility tests completes the quality control framework. This includes cytotoxicity (ISO 10993-5), sensitization, and irritation testing. For hydrogels with specific biological functions, additional functional assays may be necessary to verify that sterilization has not compromised their intended biological activity.
Documentation and traceability systems must be implemented to record all quality control data, including sterilization parameters, test results, and any deviations. Batch-specific certificates of analysis should accompany each sterilized hydrogel lot, ensuring complete transparency throughout the supply chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!