Hydrogel 3D Printing: Ink Formulation, Crosslinking and Print Fidelity Metrics
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel 3D Printing Background and Objectives
Hydrogel 3D printing has emerged as a transformative technology at the intersection of materials science, bioengineering, and additive manufacturing. Since its inception in the early 2000s, this field has evolved from rudimentary extrusion-based approaches to sophisticated multi-material, high-resolution fabrication techniques capable of producing complex functional structures with biomimetic properties.
The evolution of hydrogel 3D printing has been driven by parallel advancements in biomaterials, printing hardware, and computational design. Initially limited to simple alginate and polyethylene glycol (PEG) systems, the material palette has expanded dramatically to include natural polymers like collagen, gelatin, and hyaluronic acid, as well as synthetic alternatives with tunable mechanical and biological properties.
A significant technological shift occurred around 2015-2017 with the development of more advanced crosslinking mechanisms that enabled improved structural integrity while maintaining cell viability. This period marked the transition from primarily research-focused applications to early commercial and clinical translation efforts, particularly in tissue engineering and regenerative medicine.
Current technological trajectories indicate continued refinement in three critical areas: ink formulation complexity, crosslinking precision, and print fidelity assessment. The ability to precisely control material properties at multiple scales remains a central challenge and opportunity in the field.
The primary objectives of hydrogel 3D printing technology development are multifaceted. First, to establish standardized approaches for ink formulation that balance printability, mechanical performance, and biological functionality. Second, to develop crosslinking strategies that enable spatial and temporal control over material properties during and after printing. Third, to define quantitative metrics for print fidelity that can serve as industry benchmarks.
Long-term technical goals include achieving sub-cellular printing resolution while maintaining high throughput, developing fully automated quality control systems, and creating predictive models that can accurately forecast printed construct performance based on ink formulation parameters and printing conditions.
The convergence of hydrogel 3D printing with complementary technologies such as artificial intelligence for design optimization, in situ monitoring systems, and advanced bioreactor integration represents the next frontier. These developments aim to address the persistent challenges of scalability, reproducibility, and functional performance that currently limit broader industrial and clinical adoption.
The evolution of hydrogel 3D printing has been driven by parallel advancements in biomaterials, printing hardware, and computational design. Initially limited to simple alginate and polyethylene glycol (PEG) systems, the material palette has expanded dramatically to include natural polymers like collagen, gelatin, and hyaluronic acid, as well as synthetic alternatives with tunable mechanical and biological properties.
A significant technological shift occurred around 2015-2017 with the development of more advanced crosslinking mechanisms that enabled improved structural integrity while maintaining cell viability. This period marked the transition from primarily research-focused applications to early commercial and clinical translation efforts, particularly in tissue engineering and regenerative medicine.
Current technological trajectories indicate continued refinement in three critical areas: ink formulation complexity, crosslinking precision, and print fidelity assessment. The ability to precisely control material properties at multiple scales remains a central challenge and opportunity in the field.
The primary objectives of hydrogel 3D printing technology development are multifaceted. First, to establish standardized approaches for ink formulation that balance printability, mechanical performance, and biological functionality. Second, to develop crosslinking strategies that enable spatial and temporal control over material properties during and after printing. Third, to define quantitative metrics for print fidelity that can serve as industry benchmarks.
Long-term technical goals include achieving sub-cellular printing resolution while maintaining high throughput, developing fully automated quality control systems, and creating predictive models that can accurately forecast printed construct performance based on ink formulation parameters and printing conditions.
The convergence of hydrogel 3D printing with complementary technologies such as artificial intelligence for design optimization, in situ monitoring systems, and advanced bioreactor integration represents the next frontier. These developments aim to address the persistent challenges of scalability, reproducibility, and functional performance that currently limit broader industrial and clinical adoption.
Market Analysis for Bioprinted Hydrogel Applications
The global bioprinted hydrogel market is experiencing significant growth, driven by increasing applications in tissue engineering, regenerative medicine, and drug discovery. Currently valued at approximately $1.2 billion, the market is projected to reach $3.5 billion by 2028, representing a compound annual growth rate (CAGR) of 19.7% during the forecast period.
Healthcare applications dominate the market landscape, accounting for over 60% of the total market share. Within this segment, tissue engineering represents the largest application area, followed by drug screening and personalized medicine. The pharmaceutical industry has emerged as a major adopter of bioprinted hydrogel technologies, utilizing these platforms for drug testing and development to reduce animal testing and accelerate the drug discovery process.
Regionally, North America leads the market with approximately 40% share, attributed to substantial research funding, presence of major biotechnology companies, and advanced healthcare infrastructure. Europe follows closely at 30%, with significant contributions from countries like Germany, the UK, and Switzerland. The Asia-Pacific region is witnessing the fastest growth rate of 22.3% annually, primarily driven by increasing healthcare expenditure in China, Japan, and South Korea.
Consumer demand is increasingly focused on personalized medicine applications, with patient-specific implants and tissues representing a high-growth segment. The market for organ-on-chip models utilizing bioprinted hydrogels is expected to grow at 25% annually, reflecting the increasing need for more physiologically relevant drug testing platforms.
Key market restraints include high production costs, regulatory challenges, and technical limitations in achieving complex tissue structures. The average cost of bioprinted hydrogel constructs remains significantly higher than traditional biomaterials, limiting widespread clinical adoption. Additionally, regulatory pathways for bioprinted products remain complex and time-consuming, with approval processes varying significantly across different regions.
Investment in the sector has shown remarkable growth, with venture capital funding increasing by 35% year-over-year. Strategic partnerships between academic institutions and industry players have become increasingly common, accelerating technology transfer and commercialization efforts. Major pharmaceutical companies have allocated an average of 15% of their R&D budgets toward bioprinting technologies, signaling strong industry confidence in the long-term potential of this field.
Healthcare applications dominate the market landscape, accounting for over 60% of the total market share. Within this segment, tissue engineering represents the largest application area, followed by drug screening and personalized medicine. The pharmaceutical industry has emerged as a major adopter of bioprinted hydrogel technologies, utilizing these platforms for drug testing and development to reduce animal testing and accelerate the drug discovery process.
Regionally, North America leads the market with approximately 40% share, attributed to substantial research funding, presence of major biotechnology companies, and advanced healthcare infrastructure. Europe follows closely at 30%, with significant contributions from countries like Germany, the UK, and Switzerland. The Asia-Pacific region is witnessing the fastest growth rate of 22.3% annually, primarily driven by increasing healthcare expenditure in China, Japan, and South Korea.
Consumer demand is increasingly focused on personalized medicine applications, with patient-specific implants and tissues representing a high-growth segment. The market for organ-on-chip models utilizing bioprinted hydrogels is expected to grow at 25% annually, reflecting the increasing need for more physiologically relevant drug testing platforms.
Key market restraints include high production costs, regulatory challenges, and technical limitations in achieving complex tissue structures. The average cost of bioprinted hydrogel constructs remains significantly higher than traditional biomaterials, limiting widespread clinical adoption. Additionally, regulatory pathways for bioprinted products remain complex and time-consuming, with approval processes varying significantly across different regions.
Investment in the sector has shown remarkable growth, with venture capital funding increasing by 35% year-over-year. Strategic partnerships between academic institutions and industry players have become increasingly common, accelerating technology transfer and commercialization efforts. Major pharmaceutical companies have allocated an average of 15% of their R&D budgets toward bioprinting technologies, signaling strong industry confidence in the long-term potential of this field.
Technical Challenges in Hydrogel Ink Development
The development of hydrogel inks for 3D bioprinting faces several significant technical challenges that limit their widespread application. One primary obstacle is achieving optimal rheological properties that enable both printability and structural integrity. Hydrogel inks must possess shear-thinning behavior to flow through printing nozzles while maintaining sufficient viscosity to retain their shape post-extrusion. This delicate balance is difficult to achieve, as formulations that are too fluid collapse under their own weight, while overly viscous materials require excessive pressure that can damage encapsulated cells.
Biocompatibility presents another major challenge, particularly for biomedical applications. The ink formulation must support cell viability throughout the printing process and subsequent culture period. Chemical crosslinkers, photoinitiators, and other additives essential for gelation often exhibit cytotoxicity at concentrations required for effective crosslinking. Researchers must carefully optimize these components to minimize cellular damage while maintaining structural properties.
Crosslinking kinetics pose a significant technical hurdle in hydrogel development. The crosslinking reaction must occur rapidly enough to stabilize the printed structure but slowly enough to allow proper layer adhesion during the printing process. Current approaches include photocrosslinking, ionic crosslinking, and enzymatic methods, each with distinct limitations. Photocrosslinking offers precise spatial control but may cause cellular damage from UV exposure. Ionic crosslinking is biocompatible but often results in mechanically weaker constructs. Enzymatic methods provide excellent biocompatibility but typically proceed too slowly for efficient printing.
The mechanical properties of crosslinked hydrogels frequently fall short of target tissue requirements. Most hydrogel formulations yield structures with elastic moduli in the 1-100 kPa range, suitable for soft tissues but inadequate for cartilage or bone applications. Enhancing mechanical strength typically requires higher polymer concentrations or crosslinking densities, which adversely affect cell viability and nutrient diffusion.
Degradation control represents another significant challenge. Ideal hydrogel constructs should degrade at rates matching tissue regeneration, but achieving predictable degradation profiles remains difficult. Environmental factors such as pH, temperature, and enzymatic activity in vivo can dramatically alter degradation kinetics compared to laboratory conditions.
Finally, batch-to-batch consistency in natural polymer-based hydrogels presents a persistent challenge. Natural materials like gelatin, alginate, and hyaluronic acid exhibit inherent variability in molecular weight, purity, and functional group distribution, leading to inconsistent printing outcomes despite identical processing parameters. This variability complicates standardization efforts and regulatory approval pathways for clinical applications.
Biocompatibility presents another major challenge, particularly for biomedical applications. The ink formulation must support cell viability throughout the printing process and subsequent culture period. Chemical crosslinkers, photoinitiators, and other additives essential for gelation often exhibit cytotoxicity at concentrations required for effective crosslinking. Researchers must carefully optimize these components to minimize cellular damage while maintaining structural properties.
Crosslinking kinetics pose a significant technical hurdle in hydrogel development. The crosslinking reaction must occur rapidly enough to stabilize the printed structure but slowly enough to allow proper layer adhesion during the printing process. Current approaches include photocrosslinking, ionic crosslinking, and enzymatic methods, each with distinct limitations. Photocrosslinking offers precise spatial control but may cause cellular damage from UV exposure. Ionic crosslinking is biocompatible but often results in mechanically weaker constructs. Enzymatic methods provide excellent biocompatibility but typically proceed too slowly for efficient printing.
The mechanical properties of crosslinked hydrogels frequently fall short of target tissue requirements. Most hydrogel formulations yield structures with elastic moduli in the 1-100 kPa range, suitable for soft tissues but inadequate for cartilage or bone applications. Enhancing mechanical strength typically requires higher polymer concentrations or crosslinking densities, which adversely affect cell viability and nutrient diffusion.
Degradation control represents another significant challenge. Ideal hydrogel constructs should degrade at rates matching tissue regeneration, but achieving predictable degradation profiles remains difficult. Environmental factors such as pH, temperature, and enzymatic activity in vivo can dramatically alter degradation kinetics compared to laboratory conditions.
Finally, batch-to-batch consistency in natural polymer-based hydrogels presents a persistent challenge. Natural materials like gelatin, alginate, and hyaluronic acid exhibit inherent variability in molecular weight, purity, and functional group distribution, leading to inconsistent printing outcomes despite identical processing parameters. This variability complicates standardization efforts and regulatory approval pathways for clinical applications.
Current Hydrogel Ink Formulation Approaches
01 Dimensional accuracy and shape fidelity metrics
Dimensional accuracy and shape fidelity are critical metrics for evaluating hydrogel 3D printing quality. These metrics assess how closely the printed structure matches the intended design dimensions and geometry. Measurement techniques include comparing the printed object's dimensions with the original CAD model, analyzing cross-sectional profiles, and evaluating feature resolution. Advanced imaging techniques such as micro-CT scanning and confocal microscopy are employed to quantify these parameters with high precision.- Dimensional accuracy and shape fidelity metrics: Dimensional accuracy and shape fidelity are critical metrics for evaluating hydrogel 3D printing quality. These metrics measure how closely the printed structure matches the intended design dimensions and geometry. Methods for quantifying these include comparing the printed object's dimensions with the original CAD model, measuring feature resolution, and assessing structural integrity. Advanced imaging techniques such as micro-CT scanning and confocal microscopy are employed to evaluate these parameters with high precision.
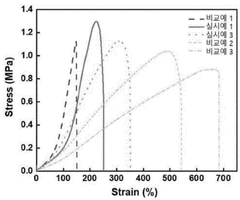
- Mechanical property assessment for printed hydrogels: Mechanical properties serve as important metrics for hydrogel print fidelity, including compression strength, tensile strength, elastic modulus, and shear resistance. These properties determine the functionality and durability of the printed structures. Testing methods include rheological analysis, compression testing, and dynamic mechanical analysis. The consistency of mechanical properties throughout the printed structure is particularly important for biomedical applications where specific mechanical behaviors are required for tissue engineering or drug delivery systems.
- Biocompatibility and cell viability metrics: For biomedical applications, metrics related to biocompatibility and cell viability are essential for evaluating hydrogel print fidelity. These include cell adhesion, proliferation rates, and cell distribution within the printed structure. Methods for assessment include live/dead staining, metabolic activity assays, and immunohistochemistry. The preservation of biological functionality during and after the printing process is crucial for applications such as tissue engineering and regenerative medicine, where the printed hydrogel serves as a scaffold for cellular growth and tissue formation.
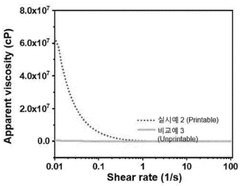
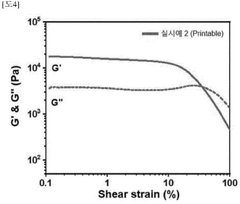
- Printability and process parameter metrics: Printability metrics focus on the behavior of hydrogels during the printing process, including extrusion consistency, flow properties, and gelation kinetics. These metrics help optimize printing parameters such as extrusion pressure, printing speed, and nozzle diameter. Rheological properties like viscosity, yield stress, and shear-thinning behavior are particularly important for predicting how hydrogels will perform during printing. Advanced monitoring systems can track these parameters in real-time to ensure consistent print quality and identify potential issues before they affect the final structure.
- Post-printing stability and degradation metrics: Long-term stability and controlled degradation are important metrics for hydrogel print fidelity, especially for applications requiring specific functional lifespans. These metrics include swelling ratio, degradation rate, and structural integrity over time under physiological conditions. Assessment methods include weight loss measurements, mechanical testing at various time points, and imaging of structural changes. For biomedical applications, the correlation between degradation rate and tissue regeneration is particularly important, as the hydrogel scaffold should ideally degrade at a rate that matches new tissue formation.
02 Mechanical property assessment for print quality
Mechanical properties serve as important metrics for hydrogel print fidelity. These include measurements of compressive strength, tensile strength, elastic modulus, and viscoelastic behavior of the printed structures. Mechanical testing protocols specifically adapted for hydrogel constructs help evaluate how well the printing process maintains the intended mechanical characteristics. The correlation between printing parameters and resulting mechanical properties provides valuable insights into print quality and functional performance of the hydrogel structures.Expand Specific Solutions03 Structural integrity and porosity characterization
Structural integrity and porosity are essential metrics for hydrogel 3D printing fidelity. These metrics evaluate the internal architecture, pore size distribution, interconnectivity, and overall structural cohesion of printed hydrogel constructs. Methods for assessment include mercury intrusion porosimetry, fluid flow tests, and various imaging techniques. The ability to consistently achieve desired porosity profiles directly impacts the functionality of printed hydrogels, particularly for tissue engineering and drug delivery applications.Expand Specific Solutions04 Surface quality and resolution metrics
Surface quality and resolution metrics quantify the precision of hydrogel 3D printing at the microscale level. These metrics include surface roughness measurements, edge definition analysis, and minimum feature size capabilities. Advanced characterization techniques such as scanning electron microscopy, atomic force microscopy, and optical profilometry are employed to evaluate surface topography and texture. The ability to reproduce fine details and maintain smooth surfaces significantly impacts both the aesthetic and functional aspects of printed hydrogel structures.Expand Specific Solutions05 Biocompatibility and functional performance metrics
Biocompatibility and functional performance metrics assess how well 3D printed hydrogels maintain their intended biological and chemical properties. These metrics include cell viability within printed constructs, protein retention, drug release kinetics, and degradation profiles. Specialized assays evaluate the preservation of bioactive components throughout the printing process. For biomedical applications, the ability to maintain these functional properties while achieving good print fidelity is crucial for creating effective tissue models, drug delivery systems, and implantable devices.Expand Specific Solutions
Leading Companies and Research Institutions
Hydrogel 3D printing technology is currently in a growth phase, with the market expected to expand significantly due to increasing applications in biomedical engineering, tissue engineering, and drug delivery systems. The global market size for hydrogel 3D printing is projected to reach substantial value as research advances toward commercialization. Technologically, the field is progressing from experimental to early commercial applications, with varying levels of maturity across different sectors. Leading academic institutions like Northwestern University, National University of Singapore, and ETH Zurich are driving fundamental research in ink formulation and crosslinking mechanisms, while companies including 3D Systems, HP Development, and IBM are developing commercial applications with improved print fidelity metrics. Collaboration between research institutions and industry players is accelerating technological maturation, particularly in biomedical applications where ALDAVER and Lung Biotechnology are pioneering tissue-mimicking constructs.
The Regents of the University of California
Technical Solution: The University of California has developed advanced hydrogel bioinks with tunable mechanical properties for 3D bioprinting applications. Their formulation incorporates a dual-crosslinking mechanism combining ionic and photocrosslinking to enhance structural integrity. The system utilizes methacrylated gelatin (GelMA) and alginate, allowing for initial ionic gelation with calcium ions followed by UV photopolymerization to create stable constructs. This approach enables printing of complex tissue-like structures with high shape fidelity and cell viability (>90%). Their research has established quantitative metrics for print fidelity assessment, including shape retention, structural stability under physiological conditions, and swelling behavior characterization. The university has also pioneered the development of rheological characterization methods specifically tailored for hydrogel bioinks, correlating printability with viscoelastic properties such as yield stress, shear-thinning behavior, and recovery kinetics.
Strengths: Superior control over mechanical properties through dual-crosslinking mechanisms; excellent cell viability in printed constructs; comprehensive print fidelity metrics system. Weaknesses: UV crosslinking may limit cell types that can be incorporated; potential challenges in scaling up production; relatively slow printing process compared to non-biological printing methods.
Northwestern University
Technical Solution: Northwestern University has pioneered a revolutionary approach to hydrogel 3D printing through their development of "FRESH" (Freeform Reversible Embedding of Suspended Hydrogels) technology. This method utilizes a support bath of gelatin microparticles that acts as a temporary support during printing, allowing for the creation of complex anatomical structures with unprecedented resolution. Their bioink formulations incorporate naturally derived polymers like collagen, fibrin, and hyaluronic acid, modified with functional groups to enable controlled crosslinking. Northwestern researchers have developed proprietary crosslinking mechanisms that combine enzymatic, ionic, and photoinitiated processes to achieve precise control over mechanical properties. Their print fidelity assessment framework includes quantitative metrics for structural accuracy, mechanical integrity, and functional performance of printed constructs. The university has also developed advanced rheological characterization techniques that correlate ink viscoelasticity with printability parameters, establishing predictive models for optimal printing conditions.
Strengths: FRESH technology enables printing of complex anatomical structures with exceptional resolution; multi-modal crosslinking provides precise control over mechanical properties; comprehensive print fidelity assessment framework. Weaknesses: Support bath removal can be challenging for certain geometries; relatively slow printing process; potential batch-to-batch variability in naturally derived polymer components.
Key Crosslinking Mechanisms and Print Fidelity Assessment
Hydrogel composition for 3D printing and method for fabricating artificial blood vessel using same
PatentWO2025089835A1
Innovation
- A hydrogel composition for 3D printing is developed, comprising a granular hydrogel and a matrix hydrogel solution. The matrix solution includes an acrylamide compound, a crosslinking agent, an initiator, and a solvent with at least 2 hydroxy groups, which increases viscosity and allows for the creation of artificial blood vessels with similar texture and physical properties to natural vessels.
Hydrogel compositions for direct-write printing applications
PatentActiveUS20170267883A1
Innovation
- A crosslinkable composition comprising a water-soluble ABA type polyether triblock copolymer, a water-soluble crosslinking agent with methylenethiol groups, and a photoinitiator capable of abstracting hydrogen from a thiol group under UV light, allowing for photo-crosslinking and forming covalently crosslinked structures suitable for direct-write printing.
Regulatory Framework for Bioprinted Materials
The regulatory landscape for bioprinted hydrogel materials represents a complex and evolving framework that spans multiple jurisdictions and oversight bodies. Currently, the FDA's Center for Biologics Evaluation and Research (CBER) and Center for Devices and Radiological Health (CDRH) share primary responsibility for regulating bioprinted materials in the United States, with classification typically depending on the intended use and mechanism of action of the final product.
For hydrogel-based bioprinted constructs, regulatory considerations must address both the biomaterial components and the cellular elements when present. The FDA has established a tiered risk-based approach, where materials intended for structural support face different requirements than those designed for therapeutic delivery or tissue replacement. Notably, the 21st Century Cures Act of 2016 created accelerated approval pathways for regenerative medicine advanced therapies (RMATs), potentially benefiting certain hydrogel bioprinting applications.
In the European Union, the regulatory framework centers around the Medical Device Regulation (MDR) and the Advanced Therapy Medicinal Products (ATMP) Regulation. Hydrogel bioprinted products may fall under either framework depending on their primary mode of action, with combination products requiring consultation with the European Medicines Agency (EMA) to determine the appropriate regulatory pathway.
Quality control metrics for bioprinted hydrogels present particular challenges for regulators. Current standards focus on mechanical properties, degradation profiles, biocompatibility, and sterility. However, the industry lacks standardized protocols specifically for assessing print fidelity and structural integrity of hydrogel constructs. Organizations such as ASTM International and the International Organization for Standardization (ISO) are working to develop consensus standards for bioprinted materials.
Regulatory compliance for hydrogel ink formulations requires thorough documentation of all components, including crosslinking agents, photoinitiators, and any bioactive molecules. Manufacturers must demonstrate both safety and consistency in production. The FDA's Chemistry, Manufacturing, and Controls (CMC) guidelines provide a framework for this documentation, though they were not specifically designed for bioprinting applications.
Looking forward, regulatory bodies are increasingly adopting collaborative approaches with industry stakeholders to develop appropriate frameworks for emerging bioprinting technologies. The FDA's Technical Considerations for Additive Manufactured Medical Devices guidance document, while not specific to hydrogels, provides valuable insights into the agency's thinking on 3D printed medical products and may inform future regulatory developments for hydrogel bioprinting.
For hydrogel-based bioprinted constructs, regulatory considerations must address both the biomaterial components and the cellular elements when present. The FDA has established a tiered risk-based approach, where materials intended for structural support face different requirements than those designed for therapeutic delivery or tissue replacement. Notably, the 21st Century Cures Act of 2016 created accelerated approval pathways for regenerative medicine advanced therapies (RMATs), potentially benefiting certain hydrogel bioprinting applications.
In the European Union, the regulatory framework centers around the Medical Device Regulation (MDR) and the Advanced Therapy Medicinal Products (ATMP) Regulation. Hydrogel bioprinted products may fall under either framework depending on their primary mode of action, with combination products requiring consultation with the European Medicines Agency (EMA) to determine the appropriate regulatory pathway.
Quality control metrics for bioprinted hydrogels present particular challenges for regulators. Current standards focus on mechanical properties, degradation profiles, biocompatibility, and sterility. However, the industry lacks standardized protocols specifically for assessing print fidelity and structural integrity of hydrogel constructs. Organizations such as ASTM International and the International Organization for Standardization (ISO) are working to develop consensus standards for bioprinted materials.
Regulatory compliance for hydrogel ink formulations requires thorough documentation of all components, including crosslinking agents, photoinitiators, and any bioactive molecules. Manufacturers must demonstrate both safety and consistency in production. The FDA's Chemistry, Manufacturing, and Controls (CMC) guidelines provide a framework for this documentation, though they were not specifically designed for bioprinting applications.
Looking forward, regulatory bodies are increasingly adopting collaborative approaches with industry stakeholders to develop appropriate frameworks for emerging bioprinting technologies. The FDA's Technical Considerations for Additive Manufactured Medical Devices guidance document, while not specific to hydrogels, provides valuable insights into the agency's thinking on 3D printed medical products and may inform future regulatory developments for hydrogel bioprinting.
Sustainability Aspects of Hydrogel Manufacturing
The sustainability of hydrogel manufacturing processes represents a critical consideration in the advancement of 3D bioprinting technologies. Current hydrogel production methods often involve energy-intensive processes and environmentally concerning chemicals, necessitating a comprehensive sustainability assessment framework.
Raw material sourcing presents the first sustainability challenge in hydrogel manufacturing. Many conventional hydrogels utilize petroleum-derived polymers, while more sustainable alternatives incorporate naturally derived materials such as alginate, cellulose, and chitosan. These bio-based alternatives typically demonstrate lower environmental footprints across their lifecycle, though challenges remain in ensuring consistent quality and performance characteristics.
Water consumption constitutes another significant sustainability factor, as hydrogel synthesis and processing typically require substantial volumes. Advanced manufacturing techniques have begun implementing closed-loop water systems that recapture and purify process water, reducing overall consumption by up to 60% compared to traditional methods. Such water conservation approaches prove particularly valuable in regions facing water scarcity.
Energy efficiency in crosslinking processes represents a key area for sustainability improvement. Physical crosslinking methods generally demonstrate lower energy requirements compared to chemical approaches, while photo-initiated crosslinking systems offer precision with moderate energy demands. Recent innovations in enzyme-mediated crosslinking show promise for ambient temperature processing, potentially reducing energy consumption by 30-45% compared to thermal methods.
Waste generation during hydrogel manufacturing primarily stems from unused precursors, failed prints, and support materials. Emerging circular economy approaches focus on developing recyclable hydrogel systems where uncrosslinked materials can be recovered and reprocessed. Additionally, biodegradable support structures minimize landfill contributions while maintaining print quality.
Toxicity considerations extend beyond environmental impacts to include workplace safety and biocompatibility. The transition toward green chemistry principles has accelerated the development of non-toxic crosslinking agents and solvent-free processing methods. These approaches not only reduce environmental hazards but also enhance the safety profile of the resulting hydrogel products.
Life cycle assessment (LCA) methodologies specifically adapted for hydrogel manufacturing processes provide quantitative metrics for sustainability evaluation. Recent studies indicate that bio-based hydrogel systems typically demonstrate 40-70% lower carbon footprints compared to synthetic alternatives, though these advantages may be partially offset by land use considerations and agricultural inputs required for biomass production.
Raw material sourcing presents the first sustainability challenge in hydrogel manufacturing. Many conventional hydrogels utilize petroleum-derived polymers, while more sustainable alternatives incorporate naturally derived materials such as alginate, cellulose, and chitosan. These bio-based alternatives typically demonstrate lower environmental footprints across their lifecycle, though challenges remain in ensuring consistent quality and performance characteristics.
Water consumption constitutes another significant sustainability factor, as hydrogel synthesis and processing typically require substantial volumes. Advanced manufacturing techniques have begun implementing closed-loop water systems that recapture and purify process water, reducing overall consumption by up to 60% compared to traditional methods. Such water conservation approaches prove particularly valuable in regions facing water scarcity.
Energy efficiency in crosslinking processes represents a key area for sustainability improvement. Physical crosslinking methods generally demonstrate lower energy requirements compared to chemical approaches, while photo-initiated crosslinking systems offer precision with moderate energy demands. Recent innovations in enzyme-mediated crosslinking show promise for ambient temperature processing, potentially reducing energy consumption by 30-45% compared to thermal methods.
Waste generation during hydrogel manufacturing primarily stems from unused precursors, failed prints, and support materials. Emerging circular economy approaches focus on developing recyclable hydrogel systems where uncrosslinked materials can be recovered and reprocessed. Additionally, biodegradable support structures minimize landfill contributions while maintaining print quality.
Toxicity considerations extend beyond environmental impacts to include workplace safety and biocompatibility. The transition toward green chemistry principles has accelerated the development of non-toxic crosslinking agents and solvent-free processing methods. These approaches not only reduce environmental hazards but also enhance the safety profile of the resulting hydrogel products.
Life cycle assessment (LCA) methodologies specifically adapted for hydrogel manufacturing processes provide quantitative metrics for sustainability evaluation. Recent studies indicate that bio-based hydrogel systems typically demonstrate 40-70% lower carbon footprints compared to synthetic alternatives, though these advantages may be partially offset by land use considerations and agricultural inputs required for biomass production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!