How to Formulate Antimicrobial Hydrogel Dressings — Active Agents, Release Kinetics and Safety Tests
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Antimicrobial Hydrogel Technology Background and Objectives
Antimicrobial hydrogel dressings represent a significant advancement in wound care technology, evolving from traditional passive wound coverings to active therapeutic platforms. The development of these sophisticated biomaterials began in the 1960s with simple hydrogel formulations, progressing through the 1980s and 1990s with the incorporation of antimicrobial agents to address infection concerns in chronic wounds. The last two decades have witnessed exponential growth in this field, driven by increasing antibiotic resistance and the need for more effective wound management solutions.
The technological evolution of antimicrobial hydrogels has been characterized by several key milestones, including the development of sustained-release systems, smart responsive hydrogels that react to wound environment changes, and biocompatible matrices that support tissue regeneration while combating pathogens. Recent innovations have focused on incorporating nanotechnology to enhance antimicrobial efficacy and developing multi-functional hydrogels that simultaneously address multiple aspects of wound healing.
Current research trends indicate a shift toward precision medicine approaches in hydrogel design, with customizable formulations that can be tailored to specific wound types and patient conditions. The integration of advanced manufacturing techniques such as 3D bioprinting has further expanded the potential for creating complex, patient-specific antimicrobial dressings with precisely controlled architecture and release profiles.
The primary technical objectives in this field center around optimizing the balance between antimicrobial efficacy and biocompatibility. This includes developing formulations that maintain potent antimicrobial activity while minimizing cytotoxicity to healthy tissues, ensuring appropriate mechanical properties that match wound tissue characteristics, and creating controlled release systems that maintain therapeutic concentrations of active agents over clinically relevant timeframes.
Additional objectives include improving the stability and shelf-life of these advanced dressings, developing cost-effective manufacturing processes to enable broader clinical adoption, and creating standardized testing protocols to evaluate both antimicrobial performance and safety profiles. There is also growing emphasis on developing sustainable and environmentally friendly formulations that reduce healthcare waste.
The ultimate goal of antimicrobial hydrogel technology development is to create "intelligent" wound dressings that can sense the wound environment, respond appropriately to changing conditions, deliver antimicrobial agents precisely when and where needed, and actively promote tissue regeneration—all while preventing or treating infection with minimal side effects and reduced risk of developing antimicrobial resistance.
The technological evolution of antimicrobial hydrogels has been characterized by several key milestones, including the development of sustained-release systems, smart responsive hydrogels that react to wound environment changes, and biocompatible matrices that support tissue regeneration while combating pathogens. Recent innovations have focused on incorporating nanotechnology to enhance antimicrobial efficacy and developing multi-functional hydrogels that simultaneously address multiple aspects of wound healing.
Current research trends indicate a shift toward precision medicine approaches in hydrogel design, with customizable formulations that can be tailored to specific wound types and patient conditions. The integration of advanced manufacturing techniques such as 3D bioprinting has further expanded the potential for creating complex, patient-specific antimicrobial dressings with precisely controlled architecture and release profiles.
The primary technical objectives in this field center around optimizing the balance between antimicrobial efficacy and biocompatibility. This includes developing formulations that maintain potent antimicrobial activity while minimizing cytotoxicity to healthy tissues, ensuring appropriate mechanical properties that match wound tissue characteristics, and creating controlled release systems that maintain therapeutic concentrations of active agents over clinically relevant timeframes.
Additional objectives include improving the stability and shelf-life of these advanced dressings, developing cost-effective manufacturing processes to enable broader clinical adoption, and creating standardized testing protocols to evaluate both antimicrobial performance and safety profiles. There is also growing emphasis on developing sustainable and environmentally friendly formulations that reduce healthcare waste.
The ultimate goal of antimicrobial hydrogel technology development is to create "intelligent" wound dressings that can sense the wound environment, respond appropriately to changing conditions, deliver antimicrobial agents precisely when and where needed, and actively promote tissue regeneration—all while preventing or treating infection with minimal side effects and reduced risk of developing antimicrobial resistance.
Market Analysis for Advanced Wound Care Solutions
The global advanced wound care market is experiencing robust growth, valued at approximately $10.3 billion in 2022 and projected to reach $15.2 billion by 2027, with a compound annual growth rate (CAGR) of 8.1%. This expansion is primarily driven by the increasing prevalence of chronic wounds, including diabetic foot ulcers, pressure ulcers, and venous leg ulcers, which affect an estimated 6.5 million patients in the United States alone.
Antimicrobial hydrogel dressings represent a rapidly growing segment within this market, currently accounting for about 18% of the advanced wound care solutions. Their popularity stems from the dual functionality of maintaining a moist wound environment while simultaneously combating infection—addressing two critical aspects of wound healing simultaneously.
Demographic trends significantly influence market demand, with the aging global population being a key driver. Individuals over 65 years of age are more susceptible to chronic wounds, and this demographic is expected to comprise 16% of the world population by 2050, up from 9% in 2020. Additionally, the rising incidence of diabetes, affecting approximately 537 million adults worldwide in 2021, further expands the potential user base for advanced wound care products.
Healthcare expenditure patterns also shape market dynamics, with developed regions like North America and Europe currently dominating the market share at 45% and 30% respectively. However, the Asia-Pacific region is emerging as the fastest-growing market with a CAGR of 9.7%, driven by improving healthcare infrastructure and increasing awareness about advanced wound care solutions.
Consumer preferences are shifting toward products that offer reduced dressing change frequency, minimal pain during application and removal, and enhanced patient comfort. This has created a demand for hydrogel dressings with controlled release mechanisms that can maintain antimicrobial efficacy over extended periods, typically 3-7 days.
Reimbursement policies significantly impact market adoption, with favorable coverage in developed markets accelerating uptake. However, in emerging economies, out-of-pocket expenses remain a barrier to widespread adoption, creating a market opportunity for cost-effective solutions that maintain clinical efficacy.
The competitive landscape features both established medical device companies and innovative startups. Major players include Smith & Nephew, Mölnlycke Health Care, and ConvaTec, collectively holding approximately 65% market share, while emerging companies focus on novel antimicrobial agents and delivery systems to differentiate their offerings in this increasingly competitive space.
Antimicrobial hydrogel dressings represent a rapidly growing segment within this market, currently accounting for about 18% of the advanced wound care solutions. Their popularity stems from the dual functionality of maintaining a moist wound environment while simultaneously combating infection—addressing two critical aspects of wound healing simultaneously.
Demographic trends significantly influence market demand, with the aging global population being a key driver. Individuals over 65 years of age are more susceptible to chronic wounds, and this demographic is expected to comprise 16% of the world population by 2050, up from 9% in 2020. Additionally, the rising incidence of diabetes, affecting approximately 537 million adults worldwide in 2021, further expands the potential user base for advanced wound care products.
Healthcare expenditure patterns also shape market dynamics, with developed regions like North America and Europe currently dominating the market share at 45% and 30% respectively. However, the Asia-Pacific region is emerging as the fastest-growing market with a CAGR of 9.7%, driven by improving healthcare infrastructure and increasing awareness about advanced wound care solutions.
Consumer preferences are shifting toward products that offer reduced dressing change frequency, minimal pain during application and removal, and enhanced patient comfort. This has created a demand for hydrogel dressings with controlled release mechanisms that can maintain antimicrobial efficacy over extended periods, typically 3-7 days.
Reimbursement policies significantly impact market adoption, with favorable coverage in developed markets accelerating uptake. However, in emerging economies, out-of-pocket expenses remain a barrier to widespread adoption, creating a market opportunity for cost-effective solutions that maintain clinical efficacy.
The competitive landscape features both established medical device companies and innovative startups. Major players include Smith & Nephew, Mölnlycke Health Care, and ConvaTec, collectively holding approximately 65% market share, while emerging companies focus on novel antimicrobial agents and delivery systems to differentiate their offerings in this increasingly competitive space.
Current Challenges in Antimicrobial Hydrogel Development
Despite significant advancements in antimicrobial hydrogel technology, several critical challenges persist in their development and clinical application. One of the foremost obstacles is achieving optimal antimicrobial efficacy while maintaining biocompatibility. Many potent antimicrobial agents exhibit cytotoxicity to human cells at concentrations required for effective pathogen elimination, creating a narrow therapeutic window that complicates formulation.
The development of bacterial resistance presents another significant challenge. As antimicrobial agents are increasingly deployed in clinical settings, pathogens evolve resistance mechanisms that reduce treatment efficacy. This necessitates continuous innovation in active agent selection and delivery strategies to stay ahead of evolving microbial defense mechanisms.
Controlled release kinetics remain problematic in hydrogel systems. Many formulations exhibit an initial burst release followed by subtherapeutic levels of antimicrobial agents, potentially creating conditions favorable for resistance development. Engineering hydrogels with predictable, sustained release profiles that maintain antimicrobial concentrations within the therapeutic window over extended periods requires sophisticated polymer chemistry and cross-linking strategies.
Stability issues further complicate antimicrobial hydrogel development. Many active agents degrade under storage conditions or lose efficacy when incorporated into hydrogel matrices. Preserving antimicrobial activity throughout the product shelf-life while maintaining the structural integrity of the hydrogel presents significant formulation challenges.
Scalable manufacturing represents another hurdle. Laboratory-scale formulations often encounter difficulties in translation to industrial production, with issues in consistency, sterility, and quality control. The complex nature of hydrogel systems, particularly those incorporating multiple active agents or advanced release mechanisms, complicates large-scale manufacturing processes.
Regulatory pathways for antimicrobial hydrogels are increasingly stringent, requiring extensive safety and efficacy testing. The classification of these products often falls into gray areas between drugs, medical devices, and wound care products, creating regulatory uncertainty that extends development timelines and increases costs.
Cost-effectiveness remains a critical consideration, particularly for widespread clinical adoption. Many advanced antimicrobial hydrogel formulations utilize expensive materials or complex manufacturing processes, limiting their accessibility in resource-constrained healthcare settings where wound infections are often most prevalent.
Finally, the lack of standardized testing protocols for antimicrobial hydrogels creates challenges in comparing efficacy across different formulations and establishing clear performance benchmarks. This hampers both regulatory approval processes and clinical decision-making regarding product selection for specific wound types and patient populations.
The development of bacterial resistance presents another significant challenge. As antimicrobial agents are increasingly deployed in clinical settings, pathogens evolve resistance mechanisms that reduce treatment efficacy. This necessitates continuous innovation in active agent selection and delivery strategies to stay ahead of evolving microbial defense mechanisms.
Controlled release kinetics remain problematic in hydrogel systems. Many formulations exhibit an initial burst release followed by subtherapeutic levels of antimicrobial agents, potentially creating conditions favorable for resistance development. Engineering hydrogels with predictable, sustained release profiles that maintain antimicrobial concentrations within the therapeutic window over extended periods requires sophisticated polymer chemistry and cross-linking strategies.
Stability issues further complicate antimicrobial hydrogel development. Many active agents degrade under storage conditions or lose efficacy when incorporated into hydrogel matrices. Preserving antimicrobial activity throughout the product shelf-life while maintaining the structural integrity of the hydrogel presents significant formulation challenges.
Scalable manufacturing represents another hurdle. Laboratory-scale formulations often encounter difficulties in translation to industrial production, with issues in consistency, sterility, and quality control. The complex nature of hydrogel systems, particularly those incorporating multiple active agents or advanced release mechanisms, complicates large-scale manufacturing processes.
Regulatory pathways for antimicrobial hydrogels are increasingly stringent, requiring extensive safety and efficacy testing. The classification of these products often falls into gray areas between drugs, medical devices, and wound care products, creating regulatory uncertainty that extends development timelines and increases costs.
Cost-effectiveness remains a critical consideration, particularly for widespread clinical adoption. Many advanced antimicrobial hydrogel formulations utilize expensive materials or complex manufacturing processes, limiting their accessibility in resource-constrained healthcare settings where wound infections are often most prevalent.
Finally, the lack of standardized testing protocols for antimicrobial hydrogels creates challenges in comparing efficacy across different formulations and establishing clear performance benchmarks. This hampers both regulatory approval processes and clinical decision-making regarding product selection for specific wound types and patient populations.
Current Antimicrobial Agent Incorporation Strategies
01 Hydrogel composition for controlled antimicrobial release
Hydrogel dressings can be formulated with specific polymeric compositions to control the release kinetics of antimicrobial agents. These compositions typically include hydrophilic polymers that form a three-dimensional network capable of absorbing and retaining water while gradually releasing incorporated antimicrobial agents. The release rate can be modulated by adjusting the crosslinking density, polymer concentration, and the interaction between the antimicrobial agent and the polymer matrix.- Hydrogel composition for controlled antimicrobial release: Hydrogel dressings can be formulated with specific polymeric compositions to control the release kinetics of antimicrobial agents. These compositions typically include hydrophilic polymers that form a three-dimensional network capable of absorbing and retaining water while gradually releasing incorporated antimicrobial compounds. The polymer composition, crosslinking density, and hydrophilic/hydrophobic balance significantly influence the diffusion rate of antimicrobial agents from the hydrogel matrix, allowing for sustained release over extended periods.
- Silver-based antimicrobial systems in hydrogel dressings: Silver-containing hydrogel dressings represent a significant advancement in wound care, offering controlled release of silver ions that provide broad-spectrum antimicrobial activity. These dressings incorporate silver in various forms including nanoparticles, silver salts, or silver complexes within the hydrogel matrix. The release kinetics of silver ions can be modulated by adjusting the silver concentration, the form of silver used, and the hydrogel composition, allowing for sustained antimicrobial activity while minimizing cytotoxicity to surrounding healthy tissues.
- Stimuli-responsive hydrogels for triggered antimicrobial release: Stimuli-responsive hydrogel dressings can release antimicrobial agents in response to specific environmental triggers present in the wound environment. These smart hydrogels can respond to changes in pH, temperature, enzyme activity, or bacterial metabolites to modulate the release kinetics of incorporated antimicrobials. This targeted approach ensures that antimicrobial agents are released primarily when and where they are needed, improving efficacy while reducing the risk of developing antimicrobial resistance and minimizing potential toxicity to healing tissues.
- Natural polymer-based hydrogels for antimicrobial delivery: Hydrogel dressings formulated with natural polymers such as chitosan, alginate, and hyaluronic acid offer unique advantages for controlling antimicrobial release kinetics. These biopolymers possess inherent biocompatibility and biodegradability while some also exhibit intrinsic antimicrobial properties. The release of incorporated antimicrobial agents from these natural polymer matrices can be controlled through various mechanisms including diffusion, polymer degradation, and specific interactions between the antimicrobial compounds and the polymer chains, resulting in sustained release profiles that maintain effective antimicrobial concentrations at the wound site.
- Multi-layer hydrogel systems for sequential antimicrobial release: Multi-layered hydrogel dressing designs enable sophisticated control over antimicrobial release kinetics through the strategic arrangement of different hydrogel layers with varying compositions and properties. These systems can provide sequential or simultaneous release of multiple antimicrobial agents, with each layer engineered to have specific release characteristics. The layered structure allows for initial burst release from outer layers to rapidly eliminate existing infection, followed by sustained release from inner layers to prevent reinfection, creating a comprehensive antimicrobial strategy that adapts to the changing needs of the wound healing process.
02 pH-responsive release mechanisms in antimicrobial hydrogels
pH-responsive hydrogel systems can be designed to release antimicrobial agents in response to changes in the wound environment. These smart hydrogels contain pH-sensitive polymers that undergo conformational changes when the pH of the wound shifts, typically during infection. This targeted release mechanism ensures that antimicrobial agents are delivered precisely when needed, improving efficacy while minimizing unnecessary exposure and potential resistance development.Expand Specific Solutions03 Nanoparticle incorporation for sustained antimicrobial release
Incorporating nanoparticles into hydrogel dressings can significantly modify the release kinetics of antimicrobial agents. Nanoparticles such as silver, zinc oxide, or loaded polymeric nanoparticles can serve as reservoirs for antimicrobial compounds, providing sustained release over extended periods. This approach creates a dual-release system where antimicrobials are released both from the hydrogel matrix and from the embedded nanoparticles, resulting in prolonged antimicrobial activity at the wound site.Expand Specific Solutions04 Enzymatically degradable hydrogels for triggered antimicrobial release
Enzymatically degradable hydrogels can be designed to release antimicrobial agents in response to specific enzymes present in infected wounds. These hydrogels contain linkages that can be cleaved by enzymes such as matrix metalloproteinases or bacterial enzymes, triggering the release of antimicrobial compounds. This mechanism allows for targeted delivery specifically at infection sites, where enzyme levels are elevated, while maintaining minimal release in healthy tissue environments.Expand Specific Solutions05 Multi-layer hydrogel systems for sequential antimicrobial release
Multi-layered hydrogel dressing designs can provide sequential release of different antimicrobial agents. These systems typically consist of distinct layers with varying compositions, crosslinking densities, or degradation rates, allowing for programmed release of multiple therapeutic agents. The outer layers may release fast-acting antimicrobials for immediate effect, while inner layers provide sustained release of secondary agents, creating a comprehensive and prolonged antimicrobial effect suitable for complex wound management.Expand Specific Solutions
Key Industry Players in Wound Care and Biomaterials
The antimicrobial hydrogel dressing market is currently in a growth phase, with increasing demand driven by rising chronic wound prevalence and healthcare-associated infections. The global market size is estimated at $1.2-1.5 billion, expanding at 6-8% CAGR. From a technological maturity perspective, the field shows varied development levels. Academic institutions (Zhejiang University, Cornell University) focus on fundamental research, while established medical device companies (Mölnlycke Health Care, PAUL HARTMANN AG) have commercialized products with controlled release mechanisms. Specialized firms like First Water Ltd. and Archimed LLP are advancing proprietary superabsorbent polymers and novel antimicrobial delivery systems. Pharmaceutical giants (Novartis AG) are exploring synergies between drug delivery and wound care, while research agencies (A*STAR, NSTDA) bridge the gap between academic innovation and industrial application.
Mölnlycke Health Care, AB.
Technical Solution: Mölnlycke has developed advanced antimicrobial hydrogel dressings utilizing their patented Safetac® technology combined with silver sulfate as the active agent. Their formulation incorporates a three-dimensional polymer network that maintains a moist wound environment while allowing controlled release of silver ions. The release kinetics are optimized through a dual-phase delivery system: an initial burst release to rapidly reduce bioburden, followed by a sustained release phase maintaining antimicrobial efficacy for up to 7 days[1]. Their safety testing protocol is comprehensive, including cytotoxicity assessments using ISO 10993 standards, skin sensitization tests, and clinical evaluations measuring wound healing rates and patient comfort. Mölnlycke's hydrogels also incorporate hydrofiber technology that absorbs exudate while maintaining structural integrity, preventing maceration of surrounding tissue[2].
Strengths: Superior sustained release profile maintaining antimicrobial activity for extended periods; excellent biocompatibility with minimal cytotoxicity; strong clinical evidence supporting efficacy. Weaknesses: Higher production costs compared to conventional dressings; potential for silver accumulation in tissues with repeated use; limited efficacy against certain resistant bacterial strains.
Cornell University
Technical Solution: Cornell University researchers have developed an advanced antimicrobial hydrogel platform utilizing a quaternary ammonium methacrylate-based polymer network incorporating silver nanoparticles. Their innovative approach combines contact-killing properties of quaternary ammonium compounds with the release-based activity of silver ions. The hydrogel is synthesized through a controlled radical polymerization process, allowing precise tuning of crosslinking density to optimize mechanical properties and release kinetics. Release studies demonstrate a sustained silver ion elution over 14 days, with approximately 30% released in the first 48 hours followed by zero-order kinetics thereafter[9]. Safety testing employs a comprehensive approach including in vitro cytotoxicity against human dermal fibroblasts, hemolysis assays, and in vivo biocompatibility studies using murine wound models. The research team has also developed specialized rheological testing protocols to ensure optimal viscoelastic properties for wound conformability. Their formulation incorporates additional functional components including N-acetylcysteine as a biofilm disruptor and hyaluronic acid to promote wound healing, creating a multifunctional therapeutic platform[10].
Strengths: Dual antimicrobial mechanism provides enhanced efficacy against resistant organisms; extended release profile offers prolonged protection; additional biofilm-disrupting capabilities. Weaknesses: Complex synthesis process may present scale-up challenges; quaternary ammonium compounds have potential for skin irritation; limited clinical validation compared to commercial products.
Critical Patents and Research in Controlled Release Systems
Antimicrobial hydrogel formulation
PatentActiveUS20180092780A1
Innovation
- A transparent film dressing coated with a medical-grade hydrogel formulation containing a silane quaternary ammonium salt, specifically 3-(trimethoxysilyl) propyldimethyloctadecyl ammonium chloride, providing long-lasting antimicrobial activity without using up active ingredients, and is applied using a knife-over-roll method on a polyurethane film with an optional release liner.
Antimicrobial hydrogel wound dressing
PatentInactiveUS20130052257A1
Innovation
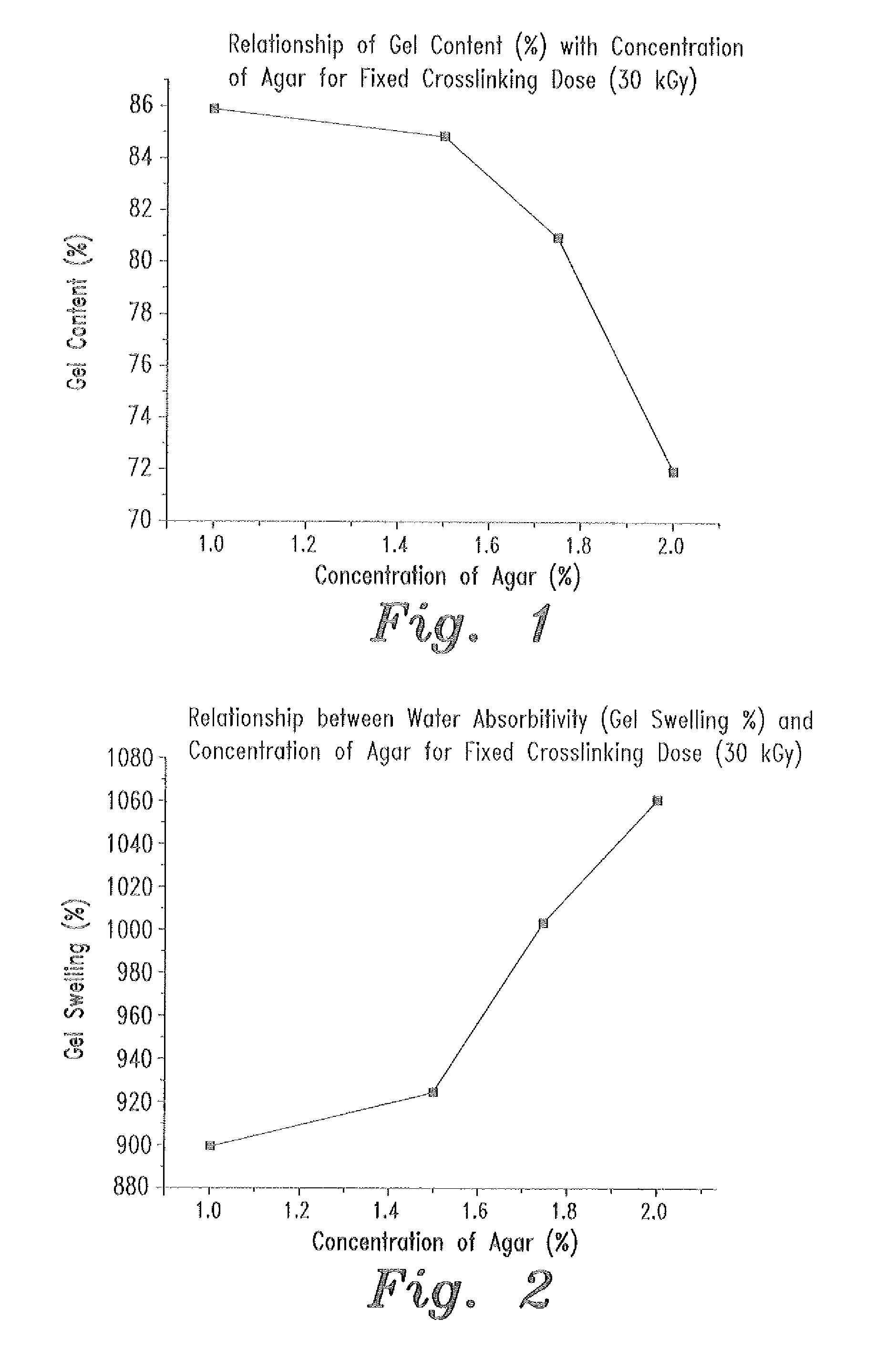
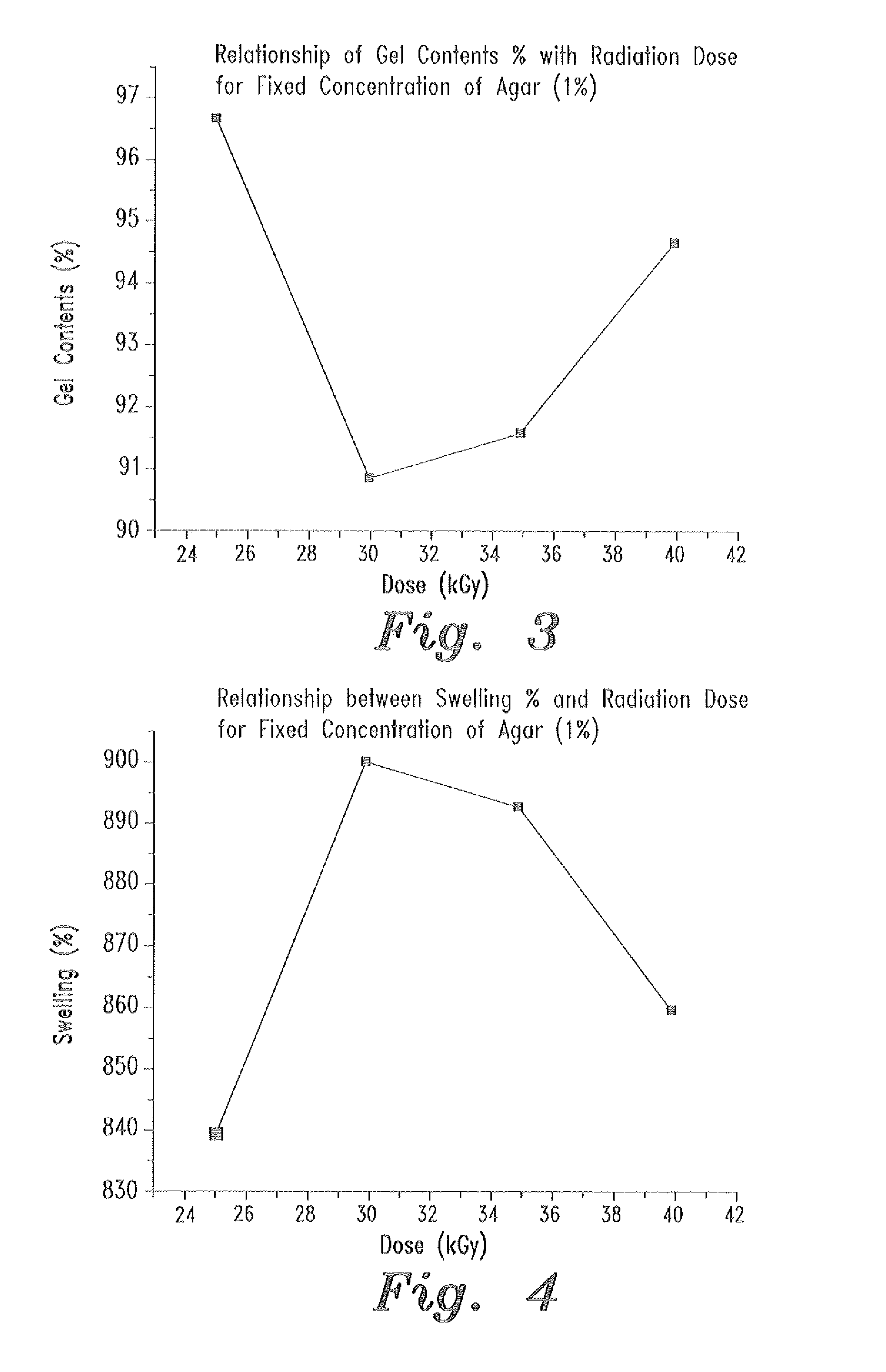
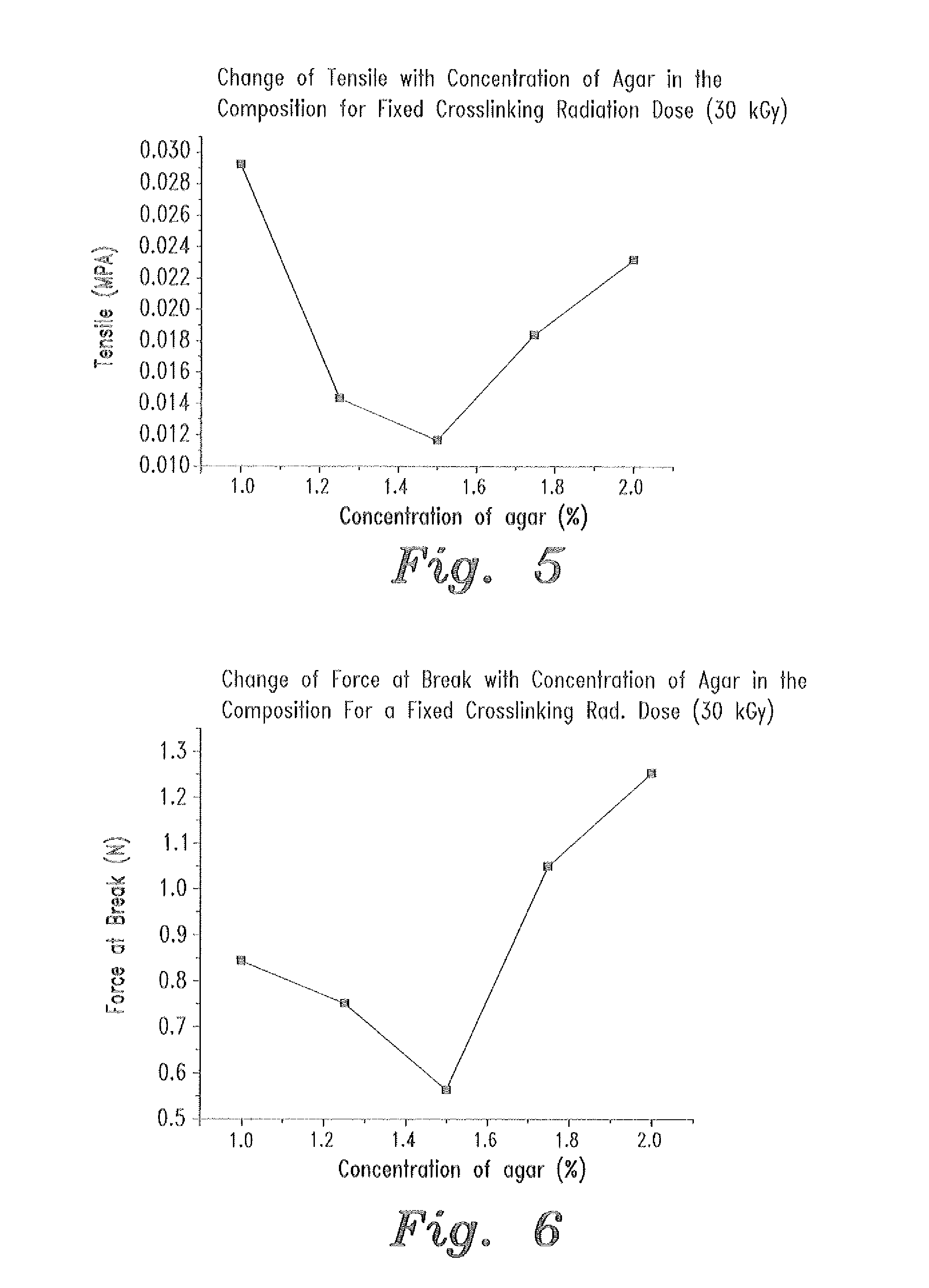
- A swellable polymer gel composed of 7-9% polyvinyl alcohol, 0.1% polyvinyl pyrrolidone, and 1-2% agar, crosslinked by gamma radiation, with 10,000 IU of polymyxin B sulfate and 5 mg of neomycin per gram, providing effective protection against both gram-positive and gram-negative microorganisms and sufficient mechanical strength for use as a wound dressing.
Regulatory Framework for Medical Device Approval
The regulatory landscape for antimicrobial hydrogel dressings is complex and multifaceted, requiring manufacturers to navigate various approval pathways depending on the jurisdiction. In the United States, the Food and Drug Administration (FDA) classifies most hydrogel wound dressings as Class II medical devices, requiring a 510(k) premarket notification unless they contain antimicrobial agents that significantly alter the body's function, which may elevate them to Class III requiring premarket approval (PMA).
The European Union operates under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in May 2021. Under this framework, antimicrobial hydrogel dressings typically fall into Class IIb or III, depending on their intended use and the nature of the antimicrobial agents incorporated. The conformity assessment procedure requires involvement of a Notified Body and implementation of a comprehensive Quality Management System.
For antimicrobial hydrogel dressings specifically, regulatory bodies focus on several critical aspects. The safety and efficacy of the active antimicrobial agents must be thoroughly documented, with particular attention to potential cytotoxicity, sensitization, and systemic absorption. The release kinetics of these agents must be characterized to ensure therapeutic levels are maintained without causing toxicity.
Biocompatibility testing according to ISO 10993 standards is mandatory across most jurisdictions, with specific emphasis on parts 1 (evaluation and testing), 5 (cytotoxicity), 10 (irritation and skin sensitization), and 11 (systemic toxicity). Additionally, antimicrobial efficacy testing must follow standards such as ASTM E2180 or JIS Z 2801 to validate claims against specific pathogens.
Clinical evidence requirements vary by region but generally include data demonstrating the dressing's performance in wound healing while maintaining antimicrobial efficacy. For novel antimicrobial agents or delivery systems, more extensive clinical trials may be necessary to establish safety profiles and efficacy benchmarks.
Post-market surveillance has become increasingly stringent, particularly under the EU MDR, requiring manufacturers to implement proactive monitoring systems and periodic safety update reports. This includes vigilance reporting for adverse events and the ability to trace products throughout the supply chain.
Regulatory strategies for these products should consider the classification implications of antimicrobial claims, as these may trigger more rigorous review processes. Manufacturers must carefully balance innovation in formulation with regulatory compliance to ensure timely market access while maintaining patient safety as the paramount concern.
The European Union operates under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in May 2021. Under this framework, antimicrobial hydrogel dressings typically fall into Class IIb or III, depending on their intended use and the nature of the antimicrobial agents incorporated. The conformity assessment procedure requires involvement of a Notified Body and implementation of a comprehensive Quality Management System.
For antimicrobial hydrogel dressings specifically, regulatory bodies focus on several critical aspects. The safety and efficacy of the active antimicrobial agents must be thoroughly documented, with particular attention to potential cytotoxicity, sensitization, and systemic absorption. The release kinetics of these agents must be characterized to ensure therapeutic levels are maintained without causing toxicity.
Biocompatibility testing according to ISO 10993 standards is mandatory across most jurisdictions, with specific emphasis on parts 1 (evaluation and testing), 5 (cytotoxicity), 10 (irritation and skin sensitization), and 11 (systemic toxicity). Additionally, antimicrobial efficacy testing must follow standards such as ASTM E2180 or JIS Z 2801 to validate claims against specific pathogens.
Clinical evidence requirements vary by region but generally include data demonstrating the dressing's performance in wound healing while maintaining antimicrobial efficacy. For novel antimicrobial agents or delivery systems, more extensive clinical trials may be necessary to establish safety profiles and efficacy benchmarks.
Post-market surveillance has become increasingly stringent, particularly under the EU MDR, requiring manufacturers to implement proactive monitoring systems and periodic safety update reports. This includes vigilance reporting for adverse events and the ability to trace products throughout the supply chain.
Regulatory strategies for these products should consider the classification implications of antimicrobial claims, as these may trigger more rigorous review processes. Manufacturers must carefully balance innovation in formulation with regulatory compliance to ensure timely market access while maintaining patient safety as the paramount concern.
Biocompatibility and Cytotoxicity Assessment Methods
Biocompatibility assessment of antimicrobial hydrogel dressings requires comprehensive testing protocols to ensure patient safety. The evaluation typically begins with in vitro cytotoxicity tests using standardized cell lines such as fibroblasts or keratinocytes, which represent the primary cell types involved in wound healing. These tests measure cell viability, proliferation, and morphological changes when exposed to hydrogel extracts or direct contact with the material.
The ISO 10993 series provides the regulatory framework for biocompatibility testing of medical devices, with ISO 10993-5 specifically addressing cytotoxicity evaluation. Methods include extract tests (where cells are exposed to material extracts), direct contact tests (where the material is placed directly on a cell monolayer), and indirect contact tests (using a barrier between cells and the test material). Quantitative assessment typically employs MTT or XTT assays, which measure mitochondrial activity as an indicator of cell viability.
Hemolysis testing represents another critical aspect of biocompatibility assessment, particularly for dressings that may contact blood. This test evaluates the potential of the hydrogel to cause red blood cell lysis, which could indicate membrane-damaging properties. The standard procedure involves incubating the material with diluted blood and measuring hemoglobin release spectrophotometrically.
For more comprehensive biocompatibility evaluation, in vivo tests are often necessary. These include irritation and sensitization tests, typically performed on animal models such as rabbits or guinea pigs. The Draize test and guinea pig maximization test are commonly employed protocols, though ethical considerations have led to the development of alternative methods. Implantation tests may also be conducted to evaluate tissue response over extended periods.
Advanced biocompatibility assessment incorporates specific tests relevant to antimicrobial hydrogels. These include evaluation of inflammatory responses through quantification of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) and assessment of potential interference with the wound healing process. Cell migration assays and scratch wound healing models provide valuable information about how the antimicrobial components might affect keratinocyte and fibroblast behavior during the healing process.
The emergence of 3D tissue models and organ-on-chip technologies offers promising alternatives to traditional testing methods. These advanced platforms can better mimic the complexity of human tissues and provide more physiologically relevant data on material-tissue interactions. Such models are particularly valuable for antimicrobial hydrogels, as they can simultaneously evaluate antimicrobial efficacy and tissue compatibility in a more representative environment.
The ISO 10993 series provides the regulatory framework for biocompatibility testing of medical devices, with ISO 10993-5 specifically addressing cytotoxicity evaluation. Methods include extract tests (where cells are exposed to material extracts), direct contact tests (where the material is placed directly on a cell monolayer), and indirect contact tests (using a barrier between cells and the test material). Quantitative assessment typically employs MTT or XTT assays, which measure mitochondrial activity as an indicator of cell viability.
Hemolysis testing represents another critical aspect of biocompatibility assessment, particularly for dressings that may contact blood. This test evaluates the potential of the hydrogel to cause red blood cell lysis, which could indicate membrane-damaging properties. The standard procedure involves incubating the material with diluted blood and measuring hemoglobin release spectrophotometrically.
For more comprehensive biocompatibility evaluation, in vivo tests are often necessary. These include irritation and sensitization tests, typically performed on animal models such as rabbits or guinea pigs. The Draize test and guinea pig maximization test are commonly employed protocols, though ethical considerations have led to the development of alternative methods. Implantation tests may also be conducted to evaluate tissue response over extended periods.
Advanced biocompatibility assessment incorporates specific tests relevant to antimicrobial hydrogels. These include evaluation of inflammatory responses through quantification of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6) and assessment of potential interference with the wound healing process. Cell migration assays and scratch wound healing models provide valuable information about how the antimicrobial components might affect keratinocyte and fibroblast behavior during the healing process.
The emergence of 3D tissue models and organ-on-chip technologies offers promising alternatives to traditional testing methods. These advanced platforms can better mimic the complexity of human tissues and provide more physiologically relevant data on material-tissue interactions. Such models are particularly valuable for antimicrobial hydrogels, as they can simultaneously evaluate antimicrobial efficacy and tissue compatibility in a more representative environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!