Hydrogel Degradation Studies: Accelerated Protocol and Predictive Modeling for Lifetime Estimation
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Degradation Background and Research Objectives
Hydrogels have emerged as versatile biomaterials with applications spanning from tissue engineering and drug delivery to wound healing and biosensors. These three-dimensional networks of hydrophilic polymers can absorb and retain significant amounts of water while maintaining their structural integrity. The evolution of hydrogel technology dates back to the 1960s, with pioneering work by Wichterle and Lim on poly(2-hydroxyethyl methacrylate) hydrogels for contact lenses. Since then, the field has expanded exponentially, incorporating natural polymers like collagen, alginate, and hyaluronic acid, as well as synthetic polymers such as poly(ethylene glycol) and poly(vinyl alcohol).
The degradation behavior of hydrogels represents a critical aspect of their performance in biomedical applications. Degradation can occur through various mechanisms including hydrolysis, enzymatic degradation, dissolution, and physical disintegration. The rate and manner of degradation significantly impact the release kinetics of encapsulated therapeutics, the mechanical properties over time, and the overall functional lifespan of hydrogel-based devices and implants.
Current approaches to studying hydrogel degradation typically involve real-time monitoring under physiological conditions, which can be prohibitively time-consuming for long-lasting formulations. This creates a substantial gap between rapid innovation cycles and the ability to accurately predict long-term performance, potentially hindering the translation of promising hydrogel technologies to clinical applications.
The technical landscape is evolving toward more sophisticated degradation assessment methodologies. Recent advances in material characterization techniques, computational modeling, and high-throughput screening have opened new avenues for accelerated testing protocols. However, establishing reliable correlations between accelerated testing conditions and real-world performance remains challenging due to the complex interplay of factors influencing degradation kinetics.
The primary objective of this research is to develop and validate an accelerated protocol for hydrogel degradation studies that can reliably predict long-term behavior. This entails identifying appropriate stress factors (temperature, pH, mechanical stress, enzymatic concentration) that can accelerate degradation while maintaining mechanistic relevance to physiological conditions.
Furthermore, we aim to establish predictive mathematical models that can translate accelerated testing data into accurate lifetime estimations under normal use conditions. These models should account for the non-linear nature of degradation processes and incorporate multiple degradation pathways. The ultimate goal is to create a standardized framework that enables rapid assessment of hydrogel stability, facilitating faster development cycles while ensuring long-term performance reliability for critical applications.
The degradation behavior of hydrogels represents a critical aspect of their performance in biomedical applications. Degradation can occur through various mechanisms including hydrolysis, enzymatic degradation, dissolution, and physical disintegration. The rate and manner of degradation significantly impact the release kinetics of encapsulated therapeutics, the mechanical properties over time, and the overall functional lifespan of hydrogel-based devices and implants.
Current approaches to studying hydrogel degradation typically involve real-time monitoring under physiological conditions, which can be prohibitively time-consuming for long-lasting formulations. This creates a substantial gap between rapid innovation cycles and the ability to accurately predict long-term performance, potentially hindering the translation of promising hydrogel technologies to clinical applications.
The technical landscape is evolving toward more sophisticated degradation assessment methodologies. Recent advances in material characterization techniques, computational modeling, and high-throughput screening have opened new avenues for accelerated testing protocols. However, establishing reliable correlations between accelerated testing conditions and real-world performance remains challenging due to the complex interplay of factors influencing degradation kinetics.
The primary objective of this research is to develop and validate an accelerated protocol for hydrogel degradation studies that can reliably predict long-term behavior. This entails identifying appropriate stress factors (temperature, pH, mechanical stress, enzymatic concentration) that can accelerate degradation while maintaining mechanistic relevance to physiological conditions.
Furthermore, we aim to establish predictive mathematical models that can translate accelerated testing data into accurate lifetime estimations under normal use conditions. These models should account for the non-linear nature of degradation processes and incorporate multiple degradation pathways. The ultimate goal is to create a standardized framework that enables rapid assessment of hydrogel stability, facilitating faster development cycles while ensuring long-term performance reliability for critical applications.
Market Applications and Demand for Hydrogel Lifetime Prediction
The hydrogel market has experienced significant growth in recent years, with applications spanning medical devices, drug delivery systems, tissue engineering, wound care, and biosensors. The global hydrogel market was valued at $15.3 billion in 2021, with projections indicating growth to reach $26.8 billion by 2028, representing a CAGR of 6.7%. This growth trajectory underscores the increasing demand for reliable lifetime prediction methodologies for hydrogel-based products.
In the medical device sector, hydrogels are extensively used in contact lenses, where precise degradation profiles directly impact product safety and performance. The contact lens market alone represents over $8.5 billion globally, with hydrogel-based lenses comprising approximately 70% of this market. Manufacturers require accurate lifetime prediction models to optimize product development cycles and ensure regulatory compliance.
The wound care segment demonstrates particularly strong demand for hydrogel lifetime prediction, as these materials must maintain structural integrity and therapeutic efficacy throughout the healing process. The advanced wound care market reached $9.4 billion in 2022, with hydrogel dressings showing the fastest growth rate among all dressing types at 8.2% annually. Healthcare providers increasingly demand evidence-based shelf-life data to support procurement decisions.
Drug delivery applications represent another critical market driver, with hydrogel-based controlled release systems requiring precise degradation kinetics to ensure therapeutic efficacy. The controlled release drug delivery market exceeded $36 billion in 2022, with hydrogel-based systems accounting for approximately 18% of this value. Pharmaceutical companies are willing to invest substantially in accelerated testing protocols that can accurately predict in vivo performance.
Tissue engineering applications present perhaps the most stringent requirements for degradation prediction, as scaffold materials must degrade at rates matching tissue regeneration. The global tissue engineering market reached $12.8 billion in 2022, with hydrogel scaffolds representing a rapidly growing segment. Research institutions and biotech companies are actively seeking predictive models that can reduce development timelines from years to months.
Consumer product applications, including agriculture, personal care, and food packaging, represent emerging markets with substantial growth potential. The agricultural hydrogel market alone reached $3.2 billion in 2022, with water retention applications driving demand in drought-prone regions. Manufacturers in these sectors prioritize cost-effective testing protocols that can predict performance across diverse environmental conditions.
Regulatory requirements further intensify market demand for reliable lifetime prediction methodologies. The FDA and equivalent international bodies increasingly require comprehensive stability data for hydrogel-based medical products, creating significant market pull for standardized accelerated testing protocols and validated predictive models.
In the medical device sector, hydrogels are extensively used in contact lenses, where precise degradation profiles directly impact product safety and performance. The contact lens market alone represents over $8.5 billion globally, with hydrogel-based lenses comprising approximately 70% of this market. Manufacturers require accurate lifetime prediction models to optimize product development cycles and ensure regulatory compliance.
The wound care segment demonstrates particularly strong demand for hydrogel lifetime prediction, as these materials must maintain structural integrity and therapeutic efficacy throughout the healing process. The advanced wound care market reached $9.4 billion in 2022, with hydrogel dressings showing the fastest growth rate among all dressing types at 8.2% annually. Healthcare providers increasingly demand evidence-based shelf-life data to support procurement decisions.
Drug delivery applications represent another critical market driver, with hydrogel-based controlled release systems requiring precise degradation kinetics to ensure therapeutic efficacy. The controlled release drug delivery market exceeded $36 billion in 2022, with hydrogel-based systems accounting for approximately 18% of this value. Pharmaceutical companies are willing to invest substantially in accelerated testing protocols that can accurately predict in vivo performance.
Tissue engineering applications present perhaps the most stringent requirements for degradation prediction, as scaffold materials must degrade at rates matching tissue regeneration. The global tissue engineering market reached $12.8 billion in 2022, with hydrogel scaffolds representing a rapidly growing segment. Research institutions and biotech companies are actively seeking predictive models that can reduce development timelines from years to months.
Consumer product applications, including agriculture, personal care, and food packaging, represent emerging markets with substantial growth potential. The agricultural hydrogel market alone reached $3.2 billion in 2022, with water retention applications driving demand in drought-prone regions. Manufacturers in these sectors prioritize cost-effective testing protocols that can predict performance across diverse environmental conditions.
Regulatory requirements further intensify market demand for reliable lifetime prediction methodologies. The FDA and equivalent international bodies increasingly require comprehensive stability data for hydrogel-based medical products, creating significant market pull for standardized accelerated testing protocols and validated predictive models.
Current Challenges in Hydrogel Degradation Assessment
The assessment of hydrogel degradation rates presents significant challenges that impede accurate lifetime prediction in various applications. Traditional degradation studies often require extensive timeframes, sometimes spanning months or years, which is impractical for rapid product development cycles. This temporal disconnect between testing and market demands creates a substantial bottleneck in hydrogel technology advancement.
A fundamental challenge lies in the complex degradation mechanisms that hydrogels undergo. These materials may experience multiple simultaneous degradation pathways including hydrolysis, enzymatic degradation, oxidative processes, and mechanical breakdown. The interplay between these mechanisms varies significantly depending on the specific chemical composition of the hydrogel and its environmental conditions, making standardized testing protocols difficult to establish.
Environmental factors introduce another layer of complexity. Temperature, pH, ionic strength, mechanical stress, and biological factors (such as enzyme concentrations) all significantly influence degradation kinetics. Current testing methodologies struggle to accurately simulate the combination of these factors as they would occur in real-world applications, leading to discrepancies between laboratory predictions and actual performance.
The heterogeneous nature of degradation presents additional challenges. Degradation often occurs non-uniformly throughout hydrogel structures, with surface erosion and bulk degradation occurring at different rates. This spatial variability complicates the development of mathematical models that can accurately predict overall material failure.
Accelerated testing protocols, while necessary for timely development, introduce their own set of challenges. The assumption that increased temperature or concentration of degradative agents will proportionally accelerate degradation without altering the fundamental mechanisms is often flawed. Many hydrogels exhibit threshold effects or mechanism shifts under extreme conditions that do not correlate with normal-use degradation patterns.
Current analytical techniques also present limitations. While techniques such as mass loss measurement, mechanical testing, and spectroscopic analysis provide valuable data, they often fail to capture the multidimensional nature of degradation. Real-time, non-destructive monitoring methods remain limited, forcing researchers to rely on discrete sampling points that may miss critical degradation events.
The translation of laboratory data to predictive models represents perhaps the most significant challenge. Existing models typically rely on simplified assumptions that fail to account for the complex, non-linear nature of hydrogel degradation. The development of comprehensive models that incorporate multiple degradation mechanisms, environmental variables, and material property changes over time requires sophisticated computational approaches that are still evolving in this field.
A fundamental challenge lies in the complex degradation mechanisms that hydrogels undergo. These materials may experience multiple simultaneous degradation pathways including hydrolysis, enzymatic degradation, oxidative processes, and mechanical breakdown. The interplay between these mechanisms varies significantly depending on the specific chemical composition of the hydrogel and its environmental conditions, making standardized testing protocols difficult to establish.
Environmental factors introduce another layer of complexity. Temperature, pH, ionic strength, mechanical stress, and biological factors (such as enzyme concentrations) all significantly influence degradation kinetics. Current testing methodologies struggle to accurately simulate the combination of these factors as they would occur in real-world applications, leading to discrepancies between laboratory predictions and actual performance.
The heterogeneous nature of degradation presents additional challenges. Degradation often occurs non-uniformly throughout hydrogel structures, with surface erosion and bulk degradation occurring at different rates. This spatial variability complicates the development of mathematical models that can accurately predict overall material failure.
Accelerated testing protocols, while necessary for timely development, introduce their own set of challenges. The assumption that increased temperature or concentration of degradative agents will proportionally accelerate degradation without altering the fundamental mechanisms is often flawed. Many hydrogels exhibit threshold effects or mechanism shifts under extreme conditions that do not correlate with normal-use degradation patterns.
Current analytical techniques also present limitations. While techniques such as mass loss measurement, mechanical testing, and spectroscopic analysis provide valuable data, they often fail to capture the multidimensional nature of degradation. Real-time, non-destructive monitoring methods remain limited, forcing researchers to rely on discrete sampling points that may miss critical degradation events.
The translation of laboratory data to predictive models represents perhaps the most significant challenge. Existing models typically rely on simplified assumptions that fail to account for the complex, non-linear nature of hydrogel degradation. The development of comprehensive models that incorporate multiple degradation mechanisms, environmental variables, and material property changes over time requires sophisticated computational approaches that are still evolving in this field.
Accelerated Testing Protocols for Hydrogel Stability Evaluation
01 Polymer composition affecting degradation rate
The degradation rate of hydrogels can be controlled by modifying the polymer composition. Different polymers exhibit varying degradation profiles, with natural polymers like hyaluronic acid typically degrading faster than synthetic polymers. The molecular weight, crosslinking density, and chemical structure of the polymers significantly influence how quickly the hydrogel breaks down in biological environments. By carefully selecting polymer components, researchers can design hydrogels with predetermined degradation timeframes suitable for specific applications.- Polymer composition affecting hydrogel degradation rate: The degradation rate of hydrogels can be controlled by modifying the polymer composition. By selecting specific polymers or combining different polymers, the degradation profile can be tailored for various applications. Factors such as molecular weight, crosslinking density, and hydrophilicity/hydrophobicity balance of the polymers significantly influence the degradation kinetics of hydrogels. These composition modifications enable the development of hydrogels with predictable and controllable degradation rates for medical and pharmaceutical applications.
- Enzymatic degradation mechanisms for hydrogels: Hydrogels can be designed to degrade in response to specific enzymes present in biological environments. By incorporating enzyme-sensitive peptide sequences or linkages into the hydrogel network, the degradation rate can be controlled based on the concentration and activity of target enzymes. This approach allows for site-specific degradation in the body, which is particularly useful for drug delivery systems and tissue engineering applications where controlled release or scaffold resorption is desired.
- pH-responsive hydrogel degradation systems: Hydrogels can be formulated to exhibit pH-dependent degradation rates, allowing for targeted degradation in specific physiological environments. By incorporating pH-sensitive bonds or polymers into the hydrogel structure, degradation can be accelerated or decelerated based on the surrounding pH conditions. This property is particularly valuable for oral drug delivery systems that need to withstand acidic stomach conditions but degrade in the neutral or slightly alkaline environment of the intestines, or for targeted delivery to specific tissues with known pH characteristics.
- Photodegradable hydrogel systems: Photodegradable hydrogels incorporate light-sensitive components that enable controlled degradation upon exposure to specific wavelengths of light. This approach allows for precise spatial and temporal control over the degradation process. By adjusting the light intensity, exposure time, and wavelength, the degradation rate can be finely tuned. These systems are particularly useful for applications requiring on-demand degradation, such as controlled release of therapeutics or dynamic cell culture platforms where matrix properties need to be altered during the experiment.
- Hydrogel degradation rate modifiers and additives: Various additives and modifiers can be incorporated into hydrogel formulations to control their degradation rates. These include catalysts that accelerate hydrolysis, stabilizers that slow degradation, and buffering agents that maintain optimal pH for desired degradation kinetics. Additionally, nanoparticles, ions, and small molecules can be used to modify the local environment within the hydrogel network, thereby influencing degradation behavior. By carefully selecting and dosing these additives, hydrogel degradation can be precisely controlled for specific applications in drug delivery, tissue engineering, and biomedical devices.
02 Enzymatic degradation mechanisms
Hydrogels can be designed to degrade in response to specific enzymes present in biological environments. These enzyme-sensitive hydrogels incorporate peptide sequences or chemical bonds that are cleaved by targeted enzymes, allowing for site-specific degradation. This approach enables precise control over the degradation rate by engineering the hydrogel to respond to enzymes that are upregulated in certain disease states or tissue environments, making them particularly valuable for drug delivery and tissue engineering applications.Expand Specific Solutions03 pH-responsive degradation control
Hydrogels can be formulated to exhibit pH-dependent degradation rates, allowing them to respond to specific physiological environments. These smart hydrogels contain chemical bonds that are stable at certain pH values but become labile at others. For example, hydrogels designed with acid-labile linkages will degrade more rapidly in acidic environments like tumor tissues or the stomach, while remaining stable at neutral pH. This property enables targeted delivery of therapeutics to specific sites in the body based on local pH conditions.Expand Specific Solutions04 Crosslinking density and methods
The degradation rate of hydrogels is significantly influenced by crosslinking density and the methods used to create crosslinks. Higher crosslinking density typically results in slower degradation rates due to increased mechanical stability and reduced water penetration. Various crosslinking methods, including physical crosslinking, chemical crosslinking, and photo-crosslinking, produce different degradation profiles. By adjusting the crosslinker concentration, type, and crosslinking conditions, researchers can fine-tune the degradation timeline to match specific application requirements.Expand Specific Solutions05 Environmental factors affecting degradation
External environmental factors significantly impact hydrogel degradation rates. Temperature, mechanical stress, and the presence of specific ions or biological molecules can accelerate or decelerate degradation. Hydrogels exposed to higher temperatures typically degrade faster due to increased molecular mobility and reaction rates. Mechanical forces can physically break down the hydrogel network, while certain ions may interact with the polymer chains to either stabilize or destabilize the structure. Understanding these environmental influences is crucial for predicting hydrogel performance in real-world applications.Expand Specific Solutions
Leading Research Groups and Companies in Hydrogel Technology
Hydrogel degradation studies are currently in a growth phase, with the market expanding due to increasing applications in biomedical, pharmaceutical, and environmental sectors. The global hydrogel market is projected to reach significant scale as research intensifies on accelerated testing protocols and predictive modeling for lifetime estimation. Technologically, the field shows moderate maturity with established players like Baxter International and Nektar Therapeutics leading commercial applications, while academic institutions such as MIT, Harvard, and Beihang University drive fundamental research innovations. Research collaborations between industry leaders like Toyota and academic powerhouses are accelerating the development of standardized degradation protocols, with organizations like Agency for Science, Technology & Research bridging the gap between theoretical models and practical applications.
Baxter International, Inc.
Technical Solution: Baxter International has developed a comprehensive hydrogel degradation assessment platform tailored for medical devices and drug delivery systems. Their approach combines accelerated aging protocols with sophisticated mathematical modeling to predict long-term stability and performance of hydrogel-based products. Baxter's methodology incorporates multiple degradation pathways including hydrolysis, oxidation, and enzymatic breakdown, with particular attention to physiologically relevant conditions. Their predictive models utilize a combination of empirical data from accelerated testing and theoretical degradation kinetics to generate accurate lifetime estimations across various product categories. The company has implemented standardized protocols that allow for comparative assessment across different hydrogel formulations, enabling rational design decisions during product development. Their approach has been validated through correlation studies between accelerated testing results and real-world clinical performance data from their extensive product portfolio.
Strengths: Robust validation through extensive clinical correlations; comprehensive consideration of multiple degradation mechanisms; well-established regulatory acceptance of their testing protocols. Weaknesses: Primarily focused on medical applications; proprietary aspects limit broader scientific adoption; models may be less applicable to non-medical hydrogel applications.
Nektar Therapeutics
Technical Solution: Nektar Therapeutics has developed proprietary hydrogel degradation testing protocols specifically designed for their advanced drug delivery systems. Their approach focuses on predicting the release kinetics of therapeutic agents from hydrogel matrices under various physiological conditions. The company employs accelerated stability testing chambers that can simulate multiple environmental stressors simultaneously, including temperature cycling, mechanical stress, and enzymatic exposure. Their predictive modeling framework incorporates both empirical data and mechanistic understanding of polymer degradation pathways to generate accurate lifetime estimations for their pharmaceutical products. Nektar has implemented a tiered testing approach that begins with rapid screening methods followed by more detailed characterization of promising formulations, allowing for efficient development timelines while maintaining predictive accuracy for regulatory submissions.
Strengths: Highly specialized for pharmaceutical applications; excellent correlation between accelerated testing and real-world performance; integrated with drug release modeling. Weaknesses: Models primarily focused on pharmaceutical applications; proprietary nature limits broader scientific adoption; less emphasis on mechanical property prediction compared to structural integrity.
Key Scientific Advances in Degradation Mechanism Understanding
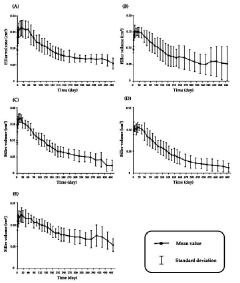
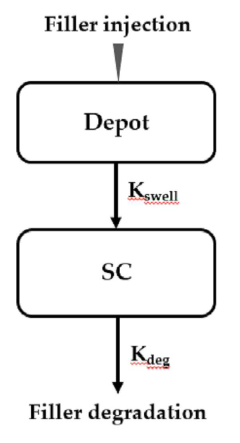
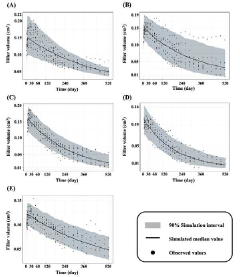
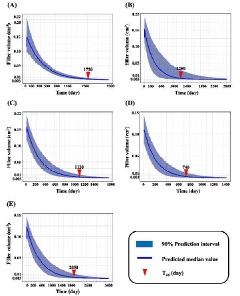
Method for predicting the in vivo degradation time of hyaluronic acid hydrogel
PatentActiveKR1020220117021A
Innovation
- A mathematical model using Equations 1 and 2 to determine the edema and degradation rates (K swell and K deg) of hyaluronic acid hydrogels, allowing for the prediction of complete decomposition time (T cd ) through a two-compartment model.
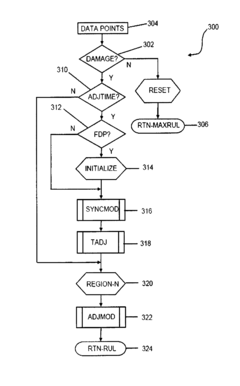
General-purpose adaptive reasoning processor and fault-to-failure progression modeling of a multiplicity of regions of degradation for producing remaining useful life estimations
PatentInactiveUS20070255511A1
Innovation
- A system and method that uses a sensor to monitor a device's defining signature as it accumulates damage, mapping this signature onto a predictive curve to estimate remaining useful lifetime, with a reasoner modifying the curve based on sensed data to provide a personalized prediction and revision of the device's state of health.
Regulatory Considerations for Hydrogel Lifetime Validation
The regulatory landscape for hydrogel lifetime validation presents a complex framework that manufacturers must navigate to ensure compliance and market approval. Regulatory bodies such as the FDA in the United States, the EMA in Europe, and the PMDA in Japan have established specific guidelines for biomaterials with degradation profiles, particularly those intended for medical applications.
For hydrogel-based products, regulatory considerations typically focus on demonstrating the reliability of lifetime predictions through validated testing protocols. The FDA's guidance on "Use of International Standard ISO 10993" specifically addresses the need for degradation studies that accurately reflect in vivo conditions, with particular emphasis on the correlation between accelerated testing and real-time performance.
Regulatory frameworks generally require manufacturers to provide comprehensive documentation of testing methodologies, including justification for acceleration factors used in predictive models. The ISO 10993-13 standard specifically addresses the identification and quantification of degradation products from polymeric medical devices, providing a foundation for hydrogel degradation validation protocols.
Recent regulatory trends indicate increasing scrutiny of mathematical models used for lifetime predictions. Authorities now expect robust statistical validation of these models, including sensitivity analyses and uncertainty quantification. The FDA's 2020 guidance on "Computer Software Assurance for Production and Quality System Software" has implications for computational models used in predicting hydrogel degradation.
Risk classification of the hydrogel product significantly impacts regulatory requirements for lifetime validation. Class III medical devices incorporating hydrogels typically require more extensive degradation studies and validation data compared to lower-risk applications. This includes demonstration of the biological consequences of degradation products over the entire predicted lifetime.
Post-market surveillance requirements are increasingly becoming part of regulatory frameworks for biomaterials with degradation profiles. Manufacturers must implement systems to monitor real-world degradation patterns and compare them against predictive models, potentially leading to refinements in testing protocols.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), are working to standardize requirements for degradation testing across regulatory jurisdictions. However, significant regional variations persist, necessitating tailored approaches to regulatory submissions in different markets.
Regulatory bodies are increasingly emphasizing the need for physiologically relevant testing conditions that account for mechanical stresses, enzymatic activity, and pH variations that may accelerate degradation in vivo. This trend aligns with the broader regulatory movement toward real-world evidence and patient-centered outcomes in medical device evaluation.
For hydrogel-based products, regulatory considerations typically focus on demonstrating the reliability of lifetime predictions through validated testing protocols. The FDA's guidance on "Use of International Standard ISO 10993" specifically addresses the need for degradation studies that accurately reflect in vivo conditions, with particular emphasis on the correlation between accelerated testing and real-time performance.
Regulatory frameworks generally require manufacturers to provide comprehensive documentation of testing methodologies, including justification for acceleration factors used in predictive models. The ISO 10993-13 standard specifically addresses the identification and quantification of degradation products from polymeric medical devices, providing a foundation for hydrogel degradation validation protocols.
Recent regulatory trends indicate increasing scrutiny of mathematical models used for lifetime predictions. Authorities now expect robust statistical validation of these models, including sensitivity analyses and uncertainty quantification. The FDA's 2020 guidance on "Computer Software Assurance for Production and Quality System Software" has implications for computational models used in predicting hydrogel degradation.
Risk classification of the hydrogel product significantly impacts regulatory requirements for lifetime validation. Class III medical devices incorporating hydrogels typically require more extensive degradation studies and validation data compared to lower-risk applications. This includes demonstration of the biological consequences of degradation products over the entire predicted lifetime.
Post-market surveillance requirements are increasingly becoming part of regulatory frameworks for biomaterials with degradation profiles. Manufacturers must implement systems to monitor real-world degradation patterns and compare them against predictive models, potentially leading to refinements in testing protocols.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP), are working to standardize requirements for degradation testing across regulatory jurisdictions. However, significant regional variations persist, necessitating tailored approaches to regulatory submissions in different markets.
Regulatory bodies are increasingly emphasizing the need for physiologically relevant testing conditions that account for mechanical stresses, enzymatic activity, and pH variations that may accelerate degradation in vivo. This trend aligns with the broader regulatory movement toward real-world evidence and patient-centered outcomes in medical device evaluation.
Environmental Impact of Hydrogel Degradation Products
The environmental fate of hydrogel degradation products represents a critical consideration in the development and application of these versatile materials. As hydrogels degrade, they release various compounds into surrounding ecosystems, potentially causing significant environmental impacts that must be thoroughly assessed and mitigated.
Primary degradation products from synthetic hydrogels often include monomers, oligomers, plasticizers, and additives that may persist in the environment. Acrylamide-based hydrogels, for instance, can release acrylamide monomers during degradation, which are known to exhibit neurotoxic and carcinogenic properties. Similarly, polyethylene glycol (PEG) derivatives may break down into shorter-chain molecules with altered environmental mobility and bioavailability.
Natural hydrogels generally produce less concerning degradation products, with most materials yielding biocompatible compounds. Alginate-based hydrogels typically degrade into non-toxic saccharide units, while collagen-based materials break down into amino acids and peptide fragments. However, crosslinking agents used in these natural systems may introduce potentially harmful elements into the environment.
Aquatic ecosystems appear particularly vulnerable to hydrogel degradation impacts. Studies have demonstrated that certain hydrogel degradation products can affect aquatic organisms at multiple trophic levels. For example, research has shown that some synthetic hydrogel fragments can adsorb onto microalgae, potentially disrupting photosynthetic processes and serving as vectors for bioaccumulation up the food chain.
Soil environments also experience significant effects from hydrogel degradation. While some degradation products enhance soil structure and water retention, others may alter microbial community composition or inhibit plant growth. Recent investigations have revealed that certain hydrogel fragments can influence nutrient cycling by affecting the activity of soil enzymes involved in carbon and nitrogen transformations.
Accelerated degradation protocols must therefore incorporate environmental impact assessments to provide comprehensive lifetime estimations. This requires analyzing not only the degradation kinetics but also the ecotoxicological profiles of resulting products. Predictive models should integrate parameters such as bioaccumulation potential, persistence, and toxicity thresholds to accurately forecast environmental risks.
Emerging research suggests potential strategies for developing environmentally benign hydrogels with controlled degradation profiles. These include incorporating enzymatically cleavable linkages that yield non-toxic degradation products, designing systems with predictable degradation pathways, and utilizing green chemistry principles to minimize environmental footprint throughout the material lifecycle.
Primary degradation products from synthetic hydrogels often include monomers, oligomers, plasticizers, and additives that may persist in the environment. Acrylamide-based hydrogels, for instance, can release acrylamide monomers during degradation, which are known to exhibit neurotoxic and carcinogenic properties. Similarly, polyethylene glycol (PEG) derivatives may break down into shorter-chain molecules with altered environmental mobility and bioavailability.
Natural hydrogels generally produce less concerning degradation products, with most materials yielding biocompatible compounds. Alginate-based hydrogels typically degrade into non-toxic saccharide units, while collagen-based materials break down into amino acids and peptide fragments. However, crosslinking agents used in these natural systems may introduce potentially harmful elements into the environment.
Aquatic ecosystems appear particularly vulnerable to hydrogel degradation impacts. Studies have demonstrated that certain hydrogel degradation products can affect aquatic organisms at multiple trophic levels. For example, research has shown that some synthetic hydrogel fragments can adsorb onto microalgae, potentially disrupting photosynthetic processes and serving as vectors for bioaccumulation up the food chain.
Soil environments also experience significant effects from hydrogel degradation. While some degradation products enhance soil structure and water retention, others may alter microbial community composition or inhibit plant growth. Recent investigations have revealed that certain hydrogel fragments can influence nutrient cycling by affecting the activity of soil enzymes involved in carbon and nitrogen transformations.
Accelerated degradation protocols must therefore incorporate environmental impact assessments to provide comprehensive lifetime estimations. This requires analyzing not only the degradation kinetics but also the ecotoxicological profiles of resulting products. Predictive models should integrate parameters such as bioaccumulation potential, persistence, and toxicity thresholds to accurately forecast environmental risks.
Emerging research suggests potential strategies for developing environmentally benign hydrogels with controlled degradation profiles. These include incorporating enzymatically cleavable linkages that yield non-toxic degradation products, designing systems with predictable degradation pathways, and utilizing green chemistry principles to minimize environmental footprint throughout the material lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!