Hydrogel Crosslinker Selection Guide: Chemical, Physical and Enzymatic Options — Pros & Cons
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Crosslinking Technology Background and Objectives
Hydrogel technology has evolved significantly since its inception in the 1960s, with crosslinking mechanisms representing a critical aspect of hydrogel development. The field has progressed from simple chemically crosslinked networks to sophisticated systems incorporating physical interactions and enzymatic processes. This technological evolution has been driven by increasing demands for biocompatible materials with tunable properties for applications ranging from biomedical devices to tissue engineering and drug delivery systems.
The crosslinking process fundamentally determines hydrogel properties including mechanical strength, degradation profile, swelling behavior, and biocompatibility. Early hydrogel development focused primarily on chemical crosslinking methods using agents such as glutaraldehyde and formaldehyde. However, concerns regarding cytotoxicity prompted exploration of alternative approaches, leading to the development of physical crosslinking methods based on non-covalent interactions and enzymatically-mediated crosslinking systems that mimic natural biological processes.
Recent technological trends indicate a shift toward hybrid crosslinking approaches that combine multiple mechanisms to achieve precise control over hydrogel properties. This convergence of chemical, physical, and enzymatic crosslinking represents a significant advancement in the field, enabling the creation of "smart" hydrogels that respond to specific environmental stimuli such as temperature, pH, or the presence of particular biomolecules.
The global hydrogel market has experienced substantial growth, with projections indicating continued expansion at a CAGR of approximately 6-8% through 2027. This growth is largely attributed to increasing applications in wound care, drug delivery, and tissue engineering, all of which benefit from advances in crosslinking technology. The biomedical sector represents the largest application segment, accounting for over 40% of the market share.
The primary objective of this technical research is to provide a comprehensive analysis of available crosslinking options for hydrogel synthesis, evaluating their respective advantages and limitations. This includes assessment of chemical crosslinkers (e.g., glutaraldehyde, carbodiimides, genipin), physical crosslinking methods (e.g., ionic interactions, hydrogen bonding, crystallization), and enzymatic approaches (e.g., transglutaminase, horseradish peroxidase, tyrosinase).
Additionally, this research aims to establish selection criteria for crosslinking methods based on intended application requirements, including biocompatibility considerations, mechanical property needs, degradation timelines, and processing constraints. The ultimate goal is to develop a decision framework that enables researchers and product developers to systematically identify optimal crosslinking strategies for specific hydrogel applications, thereby accelerating innovation in this rapidly evolving field.
The crosslinking process fundamentally determines hydrogel properties including mechanical strength, degradation profile, swelling behavior, and biocompatibility. Early hydrogel development focused primarily on chemical crosslinking methods using agents such as glutaraldehyde and formaldehyde. However, concerns regarding cytotoxicity prompted exploration of alternative approaches, leading to the development of physical crosslinking methods based on non-covalent interactions and enzymatically-mediated crosslinking systems that mimic natural biological processes.
Recent technological trends indicate a shift toward hybrid crosslinking approaches that combine multiple mechanisms to achieve precise control over hydrogel properties. This convergence of chemical, physical, and enzymatic crosslinking represents a significant advancement in the field, enabling the creation of "smart" hydrogels that respond to specific environmental stimuli such as temperature, pH, or the presence of particular biomolecules.
The global hydrogel market has experienced substantial growth, with projections indicating continued expansion at a CAGR of approximately 6-8% through 2027. This growth is largely attributed to increasing applications in wound care, drug delivery, and tissue engineering, all of which benefit from advances in crosslinking technology. The biomedical sector represents the largest application segment, accounting for over 40% of the market share.
The primary objective of this technical research is to provide a comprehensive analysis of available crosslinking options for hydrogel synthesis, evaluating their respective advantages and limitations. This includes assessment of chemical crosslinkers (e.g., glutaraldehyde, carbodiimides, genipin), physical crosslinking methods (e.g., ionic interactions, hydrogen bonding, crystallization), and enzymatic approaches (e.g., transglutaminase, horseradish peroxidase, tyrosinase).
Additionally, this research aims to establish selection criteria for crosslinking methods based on intended application requirements, including biocompatibility considerations, mechanical property needs, degradation timelines, and processing constraints. The ultimate goal is to develop a decision framework that enables researchers and product developers to systematically identify optimal crosslinking strategies for specific hydrogel applications, thereby accelerating innovation in this rapidly evolving field.
Market Applications and Demand Analysis for Advanced Hydrogels
The global hydrogel market has witnessed substantial growth in recent years, driven primarily by increasing applications in healthcare, agriculture, and consumer products. Advanced hydrogels with tailored crosslinking mechanisms are experiencing particularly strong demand due to their enhanced functionality and performance characteristics. The healthcare sector represents the largest market segment, with applications spanning wound care, drug delivery systems, tissue engineering, and hygiene products.
In the wound care segment, advanced hydrogels are valued for their moisture retention properties, biocompatibility, and ability to promote healing while reducing infection risks. Market analysis indicates that the wound dressing segment alone is projected to grow at a compound annual growth rate of over 6% through 2027, with hydrogels capturing an increasing share of this expansion.
The pharmaceutical industry demonstrates growing interest in hydrogels for controlled drug delivery applications. The ability to fine-tune release profiles through specific crosslinker selection has positioned advanced hydrogels as premium solutions for targeted therapeutic delivery. This segment shows particularly strong demand for enzymatically crosslinked hydrogels due to their biocompatibility and potential for in-situ formation.
Agricultural applications represent an emerging market with significant growth potential. Hydrogels used for soil conditioning and controlled release of fertilizers and pesticides are gaining traction as sustainable solutions for water conservation and crop yield improvement. Market penetration remains relatively low but is expected to accelerate as climate change concerns drive adoption of water-efficient agricultural practices.
Consumer products incorporating hydrogels, particularly in personal care and cosmetics, show steady demand growth. The preference for natural and biocompatible ingredients has increased interest in physically crosslinked hydrogels derived from natural polymers. This trend aligns with broader consumer shifts toward sustainable and environmentally friendly products.
Regional analysis reveals that North America and Europe currently dominate the advanced hydrogel market, primarily due to established healthcare infrastructure and higher adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to demonstrate the highest growth rate, driven by expanding healthcare access, increasing disposable incomes, and growing agricultural technology adoption.
Demand analysis indicates that crosslinker selection is increasingly influenced by application-specific requirements rather than cost considerations alone. End-users are willing to pay premium prices for hydrogels with precisely engineered properties, particularly in high-value applications such as tissue engineering and specialized drug delivery systems. This trend suggests significant market opportunities for advanced crosslinking technologies that can deliver enhanced performance characteristics.
In the wound care segment, advanced hydrogels are valued for their moisture retention properties, biocompatibility, and ability to promote healing while reducing infection risks. Market analysis indicates that the wound dressing segment alone is projected to grow at a compound annual growth rate of over 6% through 2027, with hydrogels capturing an increasing share of this expansion.
The pharmaceutical industry demonstrates growing interest in hydrogels for controlled drug delivery applications. The ability to fine-tune release profiles through specific crosslinker selection has positioned advanced hydrogels as premium solutions for targeted therapeutic delivery. This segment shows particularly strong demand for enzymatically crosslinked hydrogels due to their biocompatibility and potential for in-situ formation.
Agricultural applications represent an emerging market with significant growth potential. Hydrogels used for soil conditioning and controlled release of fertilizers and pesticides are gaining traction as sustainable solutions for water conservation and crop yield improvement. Market penetration remains relatively low but is expected to accelerate as climate change concerns drive adoption of water-efficient agricultural practices.
Consumer products incorporating hydrogels, particularly in personal care and cosmetics, show steady demand growth. The preference for natural and biocompatible ingredients has increased interest in physically crosslinked hydrogels derived from natural polymers. This trend aligns with broader consumer shifts toward sustainable and environmentally friendly products.
Regional analysis reveals that North America and Europe currently dominate the advanced hydrogel market, primarily due to established healthcare infrastructure and higher adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to demonstrate the highest growth rate, driven by expanding healthcare access, increasing disposable incomes, and growing agricultural technology adoption.
Demand analysis indicates that crosslinker selection is increasingly influenced by application-specific requirements rather than cost considerations alone. End-users are willing to pay premium prices for hydrogels with precisely engineered properties, particularly in high-value applications such as tissue engineering and specialized drug delivery systems. This trend suggests significant market opportunities for advanced crosslinking technologies that can deliver enhanced performance characteristics.
Current Crosslinker Technologies and Challenges
The hydrogel crosslinking landscape has evolved significantly over the past decade, with three primary categories now dominating the field: chemical, physical, and enzymatic crosslinking methods. Chemical crosslinkers remain the most widely utilized, with glutaraldehyde and carbodiimides (particularly EDC/NHS chemistry) being industry standards due to their established protocols and commercial availability. However, these traditional approaches face persistent challenges related to cytotoxicity and limited control over crosslinking kinetics.
Physical crosslinking technologies have gained traction as alternatives that avoid potentially harmful chemical residues. Methods such as ionic interactions (e.g., alginate with calcium ions), thermal gelation (as seen in PNIPAAm systems), and photocrosslinking represent significant advancements. Photocrosslinking, especially using UV-activated systems with photoinitiators like Irgacure, offers precise spatial and temporal control but struggles with limited penetration depth in thicker constructs.
Enzymatic crosslinking represents the newest frontier, with transglutaminase and horseradish peroxidase leading innovations in this category. These biologically-derived crosslinkers offer exceptional biocompatibility and specificity but face challenges in scalability and batch-to-batch consistency. The higher cost of enzyme-based approaches has also limited their widespread industrial adoption despite their promising performance in research settings.
A significant technical challenge across all crosslinking approaches is achieving homogeneous network formation. Heterogeneous crosslinking density often results in inconsistent mechanical properties and unpredictable degradation profiles. This issue is particularly pronounced in injectable hydrogels where mixing conditions are suboptimal and reaction kinetics must be carefully balanced against gelation time.
Degradability control represents another major hurdle, especially for biomedical applications. While enzymatically degradable linkages offer promising solutions, engineering precise degradation rates that match tissue regeneration timelines remains difficult. Recent innovations incorporating dual crosslinking mechanisms (e.g., combining physical and chemical approaches) show promise in addressing this challenge.
Scalability and reproducibility issues persist across the field, with many novel crosslinking technologies demonstrating excellent performance in laboratory settings but failing to translate to industrial-scale production. This gap is particularly evident with complex stimuli-responsive systems and bio-orthogonal chemistries that require specialized handling or equipment.
The geographical distribution of crosslinker technology development shows concentration in North America and Europe for novel chemistry approaches, while Asia leads in scaling traditional crosslinkers for mass production. This regional specialization has created interesting collaborative opportunities but also challenges in technology transfer and standardization.
Physical crosslinking technologies have gained traction as alternatives that avoid potentially harmful chemical residues. Methods such as ionic interactions (e.g., alginate with calcium ions), thermal gelation (as seen in PNIPAAm systems), and photocrosslinking represent significant advancements. Photocrosslinking, especially using UV-activated systems with photoinitiators like Irgacure, offers precise spatial and temporal control but struggles with limited penetration depth in thicker constructs.
Enzymatic crosslinking represents the newest frontier, with transglutaminase and horseradish peroxidase leading innovations in this category. These biologically-derived crosslinkers offer exceptional biocompatibility and specificity but face challenges in scalability and batch-to-batch consistency. The higher cost of enzyme-based approaches has also limited their widespread industrial adoption despite their promising performance in research settings.
A significant technical challenge across all crosslinking approaches is achieving homogeneous network formation. Heterogeneous crosslinking density often results in inconsistent mechanical properties and unpredictable degradation profiles. This issue is particularly pronounced in injectable hydrogels where mixing conditions are suboptimal and reaction kinetics must be carefully balanced against gelation time.
Degradability control represents another major hurdle, especially for biomedical applications. While enzymatically degradable linkages offer promising solutions, engineering precise degradation rates that match tissue regeneration timelines remains difficult. Recent innovations incorporating dual crosslinking mechanisms (e.g., combining physical and chemical approaches) show promise in addressing this challenge.
Scalability and reproducibility issues persist across the field, with many novel crosslinking technologies demonstrating excellent performance in laboratory settings but failing to translate to industrial-scale production. This gap is particularly evident with complex stimuli-responsive systems and bio-orthogonal chemistries that require specialized handling or equipment.
The geographical distribution of crosslinker technology development shows concentration in North America and Europe for novel chemistry approaches, while Asia leads in scaling traditional crosslinkers for mass production. This regional specialization has created interesting collaborative opportunities but also challenges in technology transfer and standardization.
Comparative Analysis of Chemical, Physical and Enzymatic Crosslinkers
01 Chemical crosslinkers for hydrogels
Various chemical compounds can be used as crosslinkers to form hydrogel networks with improved stability and mechanical properties. These include glutaraldehyde, formaldehyde, epoxy compounds, and carbodiimides that form covalent bonds between polymer chains. The selection of appropriate chemical crosslinkers significantly impacts the crosslinking efficiency, affecting the final hydrogel properties such as swelling ratio, mechanical strength, and degradation rate.- Chemical crosslinkers for hydrogel formation: Various chemical compounds can be used as crosslinkers to form hydrogels with enhanced efficiency. These include glutaraldehyde, formaldehyde, and other aldehyde-based compounds that form covalent bonds with polymer chains. Additionally, carbodiimides, epoxides, and isocyanates are effective crosslinkers that can react with functional groups on polymer backbones to create stable three-dimensional networks. The selection of appropriate chemical crosslinkers significantly impacts the mechanical properties, swelling behavior, and stability of the resulting hydrogels.
- Physical crosslinking methods for hydrogels: Physical crosslinking methods offer alternatives to chemical crosslinking for hydrogel formation. These include freeze-thaw cycling, ionic interactions, hydrogen bonding, and crystallization. Physical crosslinking can be advantageous as it often avoids the use of potentially toxic crosslinking agents and can be reversible under certain conditions. The efficiency of physical crosslinking depends on factors such as polymer concentration, temperature, pH, and ionic strength of the solution. These methods can produce hydrogels with unique properties suitable for specific biomedical applications.
- Photoinitiated crosslinking systems: Photoinitiated crosslinking systems utilize light energy to trigger the formation of crosslinks in hydrogel precursors. This approach offers spatial and temporal control over the crosslinking process, allowing for precise patterning and in situ gelation. The efficiency of photocrosslinking depends on factors such as photoinitiator type, light intensity, exposure time, and the concentration of reactive groups. Common photoinitiators include Irgacure compounds, riboflavin, and ruthenium complexes. This method is particularly valuable for biomedical applications where minimally invasive delivery is required.
- Enzymatic crosslinking for biocompatible hydrogels: Enzymatic crosslinking utilizes specific enzymes to catalyze the formation of crosslinks between polymer chains, offering a highly biocompatible approach for hydrogel formation. Enzymes such as transglutaminase, horseradish peroxidase, and tyrosinase can effectively crosslink various natural and synthetic polymers under mild physiological conditions. The efficiency of enzymatic crosslinking depends on enzyme concentration, substrate availability, reaction time, and environmental factors such as pH and temperature. This method is particularly valuable for tissue engineering and drug delivery applications where biocompatibility is crucial.
- Optimization strategies for crosslinking efficiency: Various strategies can be employed to optimize crosslinking efficiency in hydrogel systems. These include controlling the ratio of crosslinker to polymer, adjusting reaction conditions such as temperature and pH, incorporating catalysts, and using dual or sequential crosslinking approaches. The molecular weight and architecture of the polymer backbone also significantly impact crosslinking efficiency. Additionally, the use of spacer molecules, click chemistry reactions, and microwave-assisted crosslinking can enhance reaction rates and uniformity. Optimization of these parameters allows for the development of hydrogels with tailored mechanical properties, degradation profiles, and biological responses.
02 Photo-initiated crosslinking methods
Photo-initiated crosslinking uses light-sensitive compounds (photoinitiators) to generate free radicals upon exposure to specific wavelengths of light, typically UV or visible light. This method allows for spatial and temporal control of the crosslinking process, resulting in improved crosslinking efficiency. The technique enables in situ gelation and can be performed under mild conditions, making it suitable for biomedical applications where cells or bioactive molecules are incorporated into the hydrogel.Expand Specific Solutions03 Enzymatic crosslinking approaches
Enzymatic crosslinking utilizes specific enzymes such as transglutaminase, horseradish peroxidase, or tyrosinase to catalyze the formation of crosslinks between polymer chains. This approach offers high specificity and can be performed under physiological conditions, making it particularly suitable for biomedical applications. Enzymatic crosslinking typically results in high crosslinking efficiency while maintaining the biocompatibility of the resulting hydrogels.Expand Specific Solutions04 Physical crosslinking methods
Physical crosslinking methods rely on non-covalent interactions such as hydrogen bonding, ionic interactions, or hydrophobic associations to form hydrogel networks. These methods often involve stimuli-responsive polymers that can undergo sol-gel transitions in response to changes in temperature, pH, or ionic strength. Physical crosslinking typically offers reversible gel formation but may result in lower mechanical stability compared to chemical crosslinking methods.Expand Specific Solutions05 Factors affecting crosslinking efficiency
Several factors influence the efficiency of hydrogel crosslinking, including polymer concentration, functional group density, reaction conditions (temperature, pH, solvent), and crosslinker concentration. Optimization of these parameters is crucial for achieving desired hydrogel properties. Advanced techniques such as dual-crosslinking systems, which combine different crosslinking mechanisms, can be employed to enhance crosslinking efficiency and improve the mechanical properties of hydrogels.Expand Specific Solutions
Leading Companies and Research Institutions in Hydrogel Development
The hydrogel crosslinker selection market is currently in a growth phase, with increasing applications across biomedical, pharmaceutical, and cosmetic industries. The global market size is estimated to exceed $2 billion, driven by rising demand for advanced wound care products and drug delivery systems. Technologically, chemical crosslinkers dominate due to their established methodologies, while enzymatic and physical crosslinking approaches are gaining traction for their biocompatibility. Leading companies like Medtronic (Covidien), BASF, and Beiersdorf are investing heavily in R&D to develop proprietary crosslinking technologies, while academic institutions such as Harvard, Zhejiang University, and EPFL are advancing fundamental research. Emerging players like SupraPolix and VelociGro are introducing innovative crosslinking solutions, particularly in reversible and stimuli-responsive systems.
President & Fellows of Harvard College
Technical Solution: Harvard's research teams have pioneered advanced biocompatible hydrogel crosslinking technologies with particular focus on enzymatic and bio-orthogonal chemistry approaches. Their platform utilizes transglutaminase-mediated protein crosslinking that mimics natural extracellular matrix formation, enabling cell-friendly hydrogel formation under physiological conditions. Harvard researchers have developed click chemistry-based systems using strain-promoted azide-alkyne cycloaddition (SPAAC) that proceed without cytotoxic catalysts. Their dual-network hydrogel technology combines covalent and physical crosslinking mechanisms to create materials with exceptional mechanical properties including self-healing capabilities and high fracture toughness. Recent innovations include light-triggered dynamic crosslinking systems that allow spatiotemporal control over hydrogel properties, enabling 4D bioprinting applications and dynamic tissue engineering constructs.
Strengths: Exceptional biocompatibility for cell encapsulation and tissue engineering; sophisticated control over degradation kinetics; innovative approaches to dynamic materials. Weaknesses: Complex synthesis procedures may limit large-scale production; enzymatic crosslinking systems can be expensive and have stability challenges.
BASF Corp.
Technical Solution: BASF has developed a comprehensive hydrogel crosslinking platform utilizing multiple chemical approaches. Their primary technology focuses on free radical polymerization with acrylic-based crosslinkers that offer tunable mechanical properties. They've pioneered the use of N,N'-methylenebisacrylamide (MBA) and poly(ethylene glycol) diacrylate (PEGDA) systems that allow precise control over crosslinking density. BASF has also developed environmentally responsive hydrogels using stimuli-sensitive crosslinkers that respond to pH, temperature, and ionic strength changes. Their recent innovation includes bio-based crosslinkers derived from renewable resources, reducing environmental impact while maintaining performance characteristics. The company has integrated these technologies into agricultural, cosmetic, and medical applications with controlled release properties.
Strengths: Extensive chemical expertise allows for precise control of mechanical properties; scalable manufacturing capabilities; diverse portfolio of crosslinkers for various applications. Weaknesses: Chemical crosslinkers may present toxicity concerns for certain biomedical applications; some systems require potentially harmful initiators or catalysts.
Key Patents and Scientific Breakthroughs in Crosslinking Technology
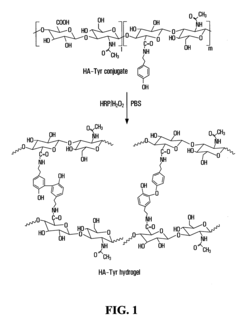
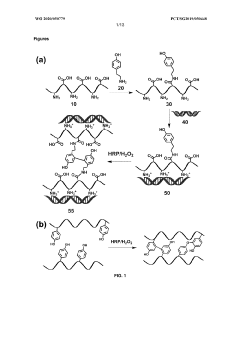
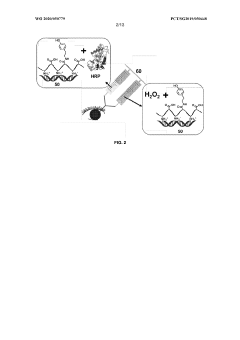
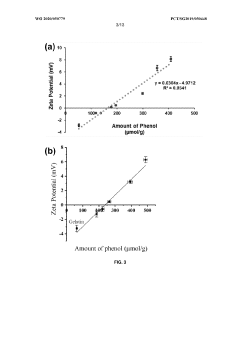
Formation of hydrogel in the presence of peroxidase and low concentration of hydrogen peroxide
PatentInactiveUS8287906B2
Innovation
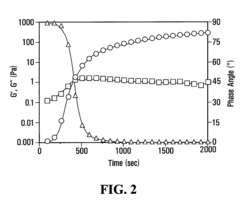
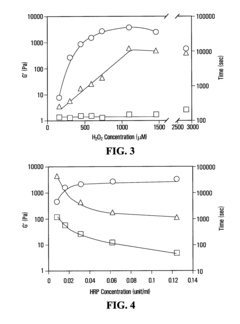
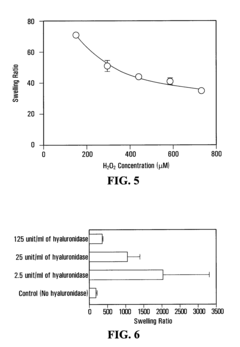
- A method is developed to determine a range for hydrogen peroxide molarity and a threshold for horseradish peroxidase concentration, allowing independent control of gelation rate and crosslinking density in hydrogel formation by varying H2O2 molarity and HRP concentration within specific ranges, using a polymer with crosslinkable phenol groups, such as hyaluronic acid-tyramine conjugates, to form hydrogels with pre-selected storage moduli.
Hydrogels with tunable electrostatic properties
PatentWO2020050779A1
Innovation
- A sustained release composition comprising a crosslinked hydrogel with tunable electrostatic properties, formed from a heterobifunctional polymer and crosslinking agent, facilitates the delivery of active agents like siRNA and cisplatin, providing controlled release and reduced degradation, suitable for treating glaucoma and other conditions.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when selecting hydrogel crosslinkers for biomedical applications. The interaction between crosslinked hydrogels and biological systems determines their suitability for applications ranging from drug delivery to tissue engineering and wound healing.
Chemical crosslinkers such as glutaraldehyde and formaldehyde have historically raised significant biocompatibility concerns due to their cytotoxicity. These agents can leach from incompletely reacted hydrogels, causing local tissue inflammation, cell death, and potential systemic toxicity. In contrast, EDC/NHS chemistry offers improved biocompatibility profiles while maintaining effective crosslinking capabilities, making it increasingly preferred for biomedical applications.
Physical crosslinking methods generally present fewer safety concerns as they avoid potentially toxic chemical agents. Ionic crosslinking using calcium ions with alginate demonstrates excellent biocompatibility, though calcium release kinetics must be carefully controlled to prevent adverse cellular responses. Similarly, temperature-responsive crosslinking systems like PNIPAAm show minimal toxicity but require precise thermal management to prevent thermal damage to surrounding tissues.
Enzymatic crosslinkers represent the gold standard for biocompatibility in many applications. Transglutaminase and horseradish peroxidase facilitate crosslinking under physiological conditions without generating toxic byproducts. These enzymes closely mimic natural biological processes, significantly reducing foreign body responses and improving integration with host tissues.
Regulatory considerations heavily influence crosslinker selection, with FDA approval status varying widely among available options. Photocrosslinking systems using riboflavin have gained regulatory acceptance for corneal applications, while certain chemical crosslinkers face stringent limitations due to safety concerns. The regulatory landscape continues to evolve as new biocompatibility data emerges.
Degradation products present another critical safety consideration. Ideal crosslinkers should degrade into non-toxic metabolites that can be safely eliminated from the body. Hydrolytically degradable crosslinkers like those based on PEG chemistry offer predictable degradation profiles with minimal inflammatory response, whereas persistent crosslinkers may trigger chronic foreign body reactions.
Sterilization compatibility must also be evaluated when selecting crosslinkers. Some crosslinking chemistries may be compromised by standard sterilization techniques such as gamma irradiation or ethylene oxide treatment, potentially altering mechanical properties or generating toxic byproducts. This necessitates careful validation of sterilization protocols for each crosslinker system.
Chemical crosslinkers such as glutaraldehyde and formaldehyde have historically raised significant biocompatibility concerns due to their cytotoxicity. These agents can leach from incompletely reacted hydrogels, causing local tissue inflammation, cell death, and potential systemic toxicity. In contrast, EDC/NHS chemistry offers improved biocompatibility profiles while maintaining effective crosslinking capabilities, making it increasingly preferred for biomedical applications.
Physical crosslinking methods generally present fewer safety concerns as they avoid potentially toxic chemical agents. Ionic crosslinking using calcium ions with alginate demonstrates excellent biocompatibility, though calcium release kinetics must be carefully controlled to prevent adverse cellular responses. Similarly, temperature-responsive crosslinking systems like PNIPAAm show minimal toxicity but require precise thermal management to prevent thermal damage to surrounding tissues.
Enzymatic crosslinkers represent the gold standard for biocompatibility in many applications. Transglutaminase and horseradish peroxidase facilitate crosslinking under physiological conditions without generating toxic byproducts. These enzymes closely mimic natural biological processes, significantly reducing foreign body responses and improving integration with host tissues.
Regulatory considerations heavily influence crosslinker selection, with FDA approval status varying widely among available options. Photocrosslinking systems using riboflavin have gained regulatory acceptance for corneal applications, while certain chemical crosslinkers face stringent limitations due to safety concerns. The regulatory landscape continues to evolve as new biocompatibility data emerges.
Degradation products present another critical safety consideration. Ideal crosslinkers should degrade into non-toxic metabolites that can be safely eliminated from the body. Hydrolytically degradable crosslinkers like those based on PEG chemistry offer predictable degradation profiles with minimal inflammatory response, whereas persistent crosslinkers may trigger chronic foreign body reactions.
Sterilization compatibility must also be evaluated when selecting crosslinkers. Some crosslinking chemistries may be compromised by standard sterilization techniques such as gamma irradiation or ethylene oxide treatment, potentially altering mechanical properties or generating toxic byproducts. This necessitates careful validation of sterilization protocols for each crosslinker system.
Scalability and Manufacturing Challenges
The scalability of hydrogel production represents a critical challenge in transitioning from laboratory-scale synthesis to commercial manufacturing. Chemical crosslinking methods generally offer superior scalability advantages, with glutaraldehyde and EDC/NHS chemistry demonstrating established protocols for large-batch production. However, these methods face significant challenges including reaction homogeneity maintenance across larger volumes and heat dissipation issues during exothermic crosslinking reactions, which can lead to structural inconsistencies in the final hydrogel product.
Physical crosslinking approaches present mixed scalability profiles. Freeze-thaw methods require substantial energy inputs and specialized equipment for temperature cycling, creating significant cost barriers for industrial-scale implementation. Conversely, ionic crosslinking (particularly calcium-alginate systems) demonstrates excellent scalability potential due to straightforward processing requirements and minimal specialized equipment needs, though maintaining consistent crosslink density throughout large batches remains problematic.
Enzymatic crosslinking methods currently face the most significant manufacturing hurdles. The high cost of enzyme production and purification dramatically impacts production economics at scale. Additionally, enzymes often require specific environmental conditions (pH, temperature, ionic strength) that must be precisely maintained throughout the manufacturing process, adding complexity to reactor design and process control systems.
Quality control represents another dimension of the scalability challenge. Chemical crosslinking methods benefit from established analytical techniques for verification, while enzymatic approaches often require more sophisticated and expensive testing protocols. This disparity in quality assurance capabilities can significantly impact regulatory approval timelines and manufacturing costs.
Equipment compatibility also varies significantly across crosslinking approaches. Many existing pharmaceutical and medical device manufacturing facilities are optimized for chemical processes, requiring substantial capital investment to accommodate enzymatic or specialized physical crosslinking methods. This infrastructure limitation often drives initial commercialization strategies toward chemically crosslinked hydrogel systems despite potential performance advantages of alternative approaches.
Sterilization compatibility further complicates manufacturing scale-up. Chemical crosslinkers typically produce materials compatible with standard sterilization techniques (ethylene oxide, gamma irradiation), while physically and enzymatically crosslinked hydrogels may require more specialized approaches that add complexity to the manufacturing process and supply chain management.
Physical crosslinking approaches present mixed scalability profiles. Freeze-thaw methods require substantial energy inputs and specialized equipment for temperature cycling, creating significant cost barriers for industrial-scale implementation. Conversely, ionic crosslinking (particularly calcium-alginate systems) demonstrates excellent scalability potential due to straightforward processing requirements and minimal specialized equipment needs, though maintaining consistent crosslink density throughout large batches remains problematic.
Enzymatic crosslinking methods currently face the most significant manufacturing hurdles. The high cost of enzyme production and purification dramatically impacts production economics at scale. Additionally, enzymes often require specific environmental conditions (pH, temperature, ionic strength) that must be precisely maintained throughout the manufacturing process, adding complexity to reactor design and process control systems.
Quality control represents another dimension of the scalability challenge. Chemical crosslinking methods benefit from established analytical techniques for verification, while enzymatic approaches often require more sophisticated and expensive testing protocols. This disparity in quality assurance capabilities can significantly impact regulatory approval timelines and manufacturing costs.
Equipment compatibility also varies significantly across crosslinking approaches. Many existing pharmaceutical and medical device manufacturing facilities are optimized for chemical processes, requiring substantial capital investment to accommodate enzymatic or specialized physical crosslinking methods. This infrastructure limitation often drives initial commercialization strategies toward chemically crosslinked hydrogel systems despite potential performance advantages of alternative approaches.
Sterilization compatibility further complicates manufacturing scale-up. Chemical crosslinkers typically produce materials compatible with standard sterilization techniques (ethylene oxide, gamma irradiation), while physically and enzymatically crosslinked hydrogels may require more specialized approaches that add complexity to the manufacturing process and supply chain management.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!