How to Design Hydrogel Biosensors: Transduction Mechanisms and Performance Benchmarks
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Biosensor Evolution and Objectives
Hydrogel biosensors have evolved significantly over the past three decades, transforming from simple polymer matrices to sophisticated smart materials capable of detecting various biological analytes with remarkable sensitivity. The journey began in the early 1990s with rudimentary hydrogel-based glucose sensors that relied on basic swelling mechanisms. These early designs laid the foundation for subsequent innovations in material science and sensing technologies.
By the early 2000s, researchers had begun incorporating various functional groups and responsive elements into hydrogels, enabling more specific detection capabilities. The introduction of stimuli-responsive hydrogels marked a pivotal advancement, allowing these materials to react to environmental changes such as pH, temperature, and specific biomolecules. This period also witnessed the integration of hydrogels with electronic components, creating hybrid sensing platforms.
The past decade has seen exponential growth in hydrogel biosensor technology, driven by advancements in nanomaterials, molecular imprinting techniques, and biomimetic approaches. Modern hydrogel biosensors now incorporate sophisticated transduction mechanisms including optical, electrochemical, and mechanical signal conversion pathways. The emergence of 3D printing and microfluidic technologies has further revolutionized fabrication methods, enabling precise control over sensor architecture and functionality.
Current research objectives in hydrogel biosensor design focus on several key areas. First, enhancing sensitivity and specificity to detect biomarkers at increasingly lower concentrations, approaching single-molecule detection in some cases. Second, improving response times to enable real-time monitoring of rapidly changing biological processes. Third, developing robust transduction mechanisms that can reliably convert biological recognition events into measurable signals with minimal interference.
Another critical objective is the creation of multimodal sensing platforms capable of detecting multiple analytes simultaneously, providing comprehensive biological information from a single device. Researchers are also prioritizing biocompatibility and long-term stability for in vivo applications, particularly for continuous health monitoring and implantable devices.
The ultimate goal of current hydrogel biosensor development is to create fully integrated, autonomous sensing systems that combine detection, signal processing, and data transmission capabilities. These next-generation devices aim to bridge the gap between laboratory demonstrations and practical applications in clinical diagnostics, environmental monitoring, and personalized healthcare. The convergence of hydrogel technology with artificial intelligence and Internet of Things (IoT) frameworks represents the frontier of this field, promising unprecedented capabilities in biological sensing and health management.
By the early 2000s, researchers had begun incorporating various functional groups and responsive elements into hydrogels, enabling more specific detection capabilities. The introduction of stimuli-responsive hydrogels marked a pivotal advancement, allowing these materials to react to environmental changes such as pH, temperature, and specific biomolecules. This period also witnessed the integration of hydrogels with electronic components, creating hybrid sensing platforms.
The past decade has seen exponential growth in hydrogel biosensor technology, driven by advancements in nanomaterials, molecular imprinting techniques, and biomimetic approaches. Modern hydrogel biosensors now incorporate sophisticated transduction mechanisms including optical, electrochemical, and mechanical signal conversion pathways. The emergence of 3D printing and microfluidic technologies has further revolutionized fabrication methods, enabling precise control over sensor architecture and functionality.
Current research objectives in hydrogel biosensor design focus on several key areas. First, enhancing sensitivity and specificity to detect biomarkers at increasingly lower concentrations, approaching single-molecule detection in some cases. Second, improving response times to enable real-time monitoring of rapidly changing biological processes. Third, developing robust transduction mechanisms that can reliably convert biological recognition events into measurable signals with minimal interference.
Another critical objective is the creation of multimodal sensing platforms capable of detecting multiple analytes simultaneously, providing comprehensive biological information from a single device. Researchers are also prioritizing biocompatibility and long-term stability for in vivo applications, particularly for continuous health monitoring and implantable devices.
The ultimate goal of current hydrogel biosensor development is to create fully integrated, autonomous sensing systems that combine detection, signal processing, and data transmission capabilities. These next-generation devices aim to bridge the gap between laboratory demonstrations and practical applications in clinical diagnostics, environmental monitoring, and personalized healthcare. The convergence of hydrogel technology with artificial intelligence and Internet of Things (IoT) frameworks represents the frontier of this field, promising unprecedented capabilities in biological sensing and health management.
Market Analysis for Hydrogel-Based Biosensing Applications
The global market for hydrogel-based biosensors is experiencing robust growth, driven by increasing demand for point-of-care diagnostics, continuous health monitoring, and advanced biomedical research tools. Current market valuations place the biosensor industry at approximately $25 billion, with hydrogel-based technologies representing a rapidly expanding segment projected to grow at a compound annual growth rate of 9.8% through 2028.
Healthcare applications dominate the market landscape, accounting for nearly 60% of hydrogel biosensor implementations. Within this sector, glucose monitoring remains the largest application, though emerging applications in continuous monitoring of various biomarkers are gaining significant traction. The pharmaceutical and biotechnology research segment represents the second-largest market, where hydrogel biosensors are increasingly utilized for drug discovery and development processes.
Environmental monitoring applications are emerging as a promising growth area, particularly for detecting water contaminants, agricultural pollutants, and foodborne pathogens. This segment is expected to show the highest growth rate over the next five years as regulatory requirements for environmental safety become more stringent globally.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by increasing healthcare expenditure, expanding biotechnology sectors in China and India, and growing awareness of personalized medicine approaches.
Consumer demand trends indicate a strong preference for non-invasive, continuous monitoring capabilities—a perfect fit for hydrogel technology's inherent advantages. The wearable biosensor segment, where hydrogels offer superior comfort and biocompatibility, is growing at 15.2% annually, outpacing the broader biosensor market.
Key market drivers include the aging global population, rising incidence of chronic diseases requiring continuous monitoring, technological advancements in material sciences, and increasing healthcare costs driving demand for preventative and remote monitoring solutions. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accurate diagnostic capabilities and remote patient monitoring.
Market barriers include regulatory hurdles for medical device approval, concerns regarding long-term stability of hydrogel materials in various environmental conditions, and competition from alternative sensing technologies. Additionally, reimbursement challenges in healthcare systems represent a significant obstacle to widespread adoption in certain applications.
Healthcare applications dominate the market landscape, accounting for nearly 60% of hydrogel biosensor implementations. Within this sector, glucose monitoring remains the largest application, though emerging applications in continuous monitoring of various biomarkers are gaining significant traction. The pharmaceutical and biotechnology research segment represents the second-largest market, where hydrogel biosensors are increasingly utilized for drug discovery and development processes.
Environmental monitoring applications are emerging as a promising growth area, particularly for detecting water contaminants, agricultural pollutants, and foodborne pathogens. This segment is expected to show the highest growth rate over the next five years as regulatory requirements for environmental safety become more stringent globally.
Geographically, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is demonstrating the fastest growth rate, driven by increasing healthcare expenditure, expanding biotechnology sectors in China and India, and growing awareness of personalized medicine approaches.
Consumer demand trends indicate a strong preference for non-invasive, continuous monitoring capabilities—a perfect fit for hydrogel technology's inherent advantages. The wearable biosensor segment, where hydrogels offer superior comfort and biocompatibility, is growing at 15.2% annually, outpacing the broader biosensor market.
Key market drivers include the aging global population, rising incidence of chronic diseases requiring continuous monitoring, technological advancements in material sciences, and increasing healthcare costs driving demand for preventative and remote monitoring solutions. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of rapid, accurate diagnostic capabilities and remote patient monitoring.
Market barriers include regulatory hurdles for medical device approval, concerns regarding long-term stability of hydrogel materials in various environmental conditions, and competition from alternative sensing technologies. Additionally, reimbursement challenges in healthcare systems represent a significant obstacle to widespread adoption in certain applications.
Current Hydrogel Biosensor Technologies and Limitations
Hydrogel biosensors represent a significant advancement in biosensing technology, combining the biocompatibility of hydrogels with various transduction mechanisms to create versatile sensing platforms. Currently, these biosensors employ several key transduction mechanisms including optical, electrochemical, and mechanical methods, each with distinct advantages and limitations.
Optical transduction mechanisms in hydrogel biosensors primarily utilize changes in optical properties such as fluorescence, colorimetric responses, or surface plasmon resonance. These sensors offer high sensitivity and visual readouts but often require sophisticated instrumentation for quantitative analysis. Recent developments have incorporated photonic crystals and quantum dots to enhance sensitivity, though challenges remain in achieving consistent signal stability under varying environmental conditions.
Electrochemical hydrogel biosensors leverage changes in electrical properties when target analytes interact with recognition elements embedded within the hydrogel matrix. These sensors excel in providing rapid, quantitative measurements with relatively simple instrumentation. However, they frequently suffer from electrode fouling during long-term use and interference from electroactive species in complex biological samples, limiting their reliability in clinical applications.
Mechanical transduction mechanisms detect physical changes in hydrogel structures, such as swelling or contraction in response to specific stimuli. While these sensors demonstrate excellent specificity for certain applications, they typically exhibit slower response times compared to other mechanisms and face challenges in miniaturization for portable devices.
A significant limitation across all hydrogel biosensor technologies is the trade-off between sensitivity and response time. Highly sensitive designs often require thicker hydrogel layers, which extend diffusion pathways and consequently increase response times. Conversely, thinner hydrogels provide faster responses but may sacrifice sensitivity and mechanical stability.
Biocompatibility issues present another challenge, particularly for implantable or wearable applications. Despite hydrogels' inherent biocompatibility, long-term stability in biological environments remains problematic due to biofouling, enzymatic degradation, and immune responses that can compromise sensor performance over time.
Manufacturing scalability constitutes a substantial barrier to widespread commercial adoption. Current fabrication methods often involve complex, multi-step processes that are difficult to standardize for mass production. This results in batch-to-batch variability that affects sensor reliability and hampers regulatory approval processes.
The integration of hydrogel biosensors with readout electronics presents additional challenges, especially for point-of-care applications requiring seamless connectivity with smartphones or other portable devices. Current solutions often involve bulky external components that diminish the practical advantages of hydrogel-based sensing platforms.
Optical transduction mechanisms in hydrogel biosensors primarily utilize changes in optical properties such as fluorescence, colorimetric responses, or surface plasmon resonance. These sensors offer high sensitivity and visual readouts but often require sophisticated instrumentation for quantitative analysis. Recent developments have incorporated photonic crystals and quantum dots to enhance sensitivity, though challenges remain in achieving consistent signal stability under varying environmental conditions.
Electrochemical hydrogel biosensors leverage changes in electrical properties when target analytes interact with recognition elements embedded within the hydrogel matrix. These sensors excel in providing rapid, quantitative measurements with relatively simple instrumentation. However, they frequently suffer from electrode fouling during long-term use and interference from electroactive species in complex biological samples, limiting their reliability in clinical applications.
Mechanical transduction mechanisms detect physical changes in hydrogel structures, such as swelling or contraction in response to specific stimuli. While these sensors demonstrate excellent specificity for certain applications, they typically exhibit slower response times compared to other mechanisms and face challenges in miniaturization for portable devices.
A significant limitation across all hydrogel biosensor technologies is the trade-off between sensitivity and response time. Highly sensitive designs often require thicker hydrogel layers, which extend diffusion pathways and consequently increase response times. Conversely, thinner hydrogels provide faster responses but may sacrifice sensitivity and mechanical stability.
Biocompatibility issues present another challenge, particularly for implantable or wearable applications. Despite hydrogels' inherent biocompatibility, long-term stability in biological environments remains problematic due to biofouling, enzymatic degradation, and immune responses that can compromise sensor performance over time.
Manufacturing scalability constitutes a substantial barrier to widespread commercial adoption. Current fabrication methods often involve complex, multi-step processes that are difficult to standardize for mass production. This results in batch-to-batch variability that affects sensor reliability and hampers regulatory approval processes.
The integration of hydrogel biosensors with readout electronics presents additional challenges, especially for point-of-care applications requiring seamless connectivity with smartphones or other portable devices. Current solutions often involve bulky external components that diminish the practical advantages of hydrogel-based sensing platforms.
Established Hydrogel Biosensor Design Approaches
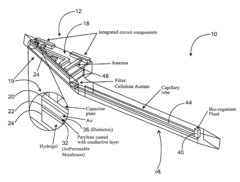
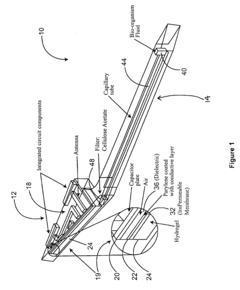
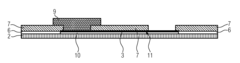
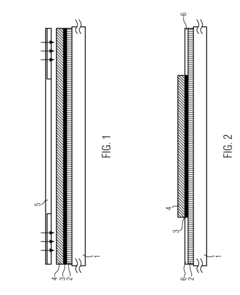
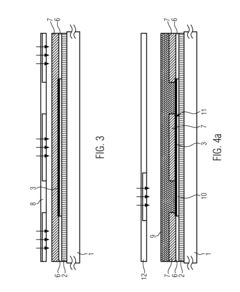
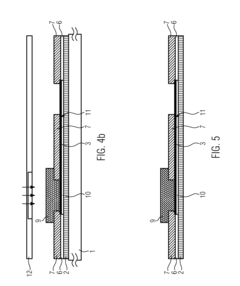
01 Electrochemical transduction mechanisms in hydrogel biosensors
Electrochemical transduction mechanisms are widely used in hydrogel biosensors to convert biological responses into measurable electrical signals. These mechanisms involve the detection of changes in electrical properties such as conductivity, impedance, or potential when target analytes interact with the hydrogel matrix. The incorporation of conductive materials within the hydrogel structure enhances signal transduction efficiency, allowing for real-time monitoring of biological events with high sensitivity and specificity.- Electrochemical transduction mechanisms in hydrogel biosensors: Electrochemical transduction mechanisms are widely used in hydrogel biosensors to convert biological responses into measurable electrical signals. These mechanisms involve the detection of changes in electrical properties such as conductivity, impedance, or potential when target analytes interact with the hydrogel matrix. The incorporation of conductive materials within hydrogels enhances signal transduction efficiency, allowing for real-time monitoring of biological events with high sensitivity and specificity.
- Optical transduction methods for hydrogel-based biosensing: Optical transduction methods in hydrogel biosensors utilize changes in optical properties such as fluorescence, absorbance, or refractive index to detect biological analytes. These biosensors incorporate chromophores or fluorophores within the hydrogel matrix that respond to specific stimuli or target molecules. The resulting optical signals can be measured using various spectroscopic techniques, providing non-invasive and highly sensitive detection capabilities for applications in healthcare monitoring and environmental sensing.
- Performance benchmarking and standardization of hydrogel biosensors: Performance benchmarking of hydrogel biosensors involves systematic evaluation of key parameters including sensitivity, specificity, response time, stability, and reproducibility. Standardized testing protocols are essential for comparing different biosensor designs and ensuring reliable performance across various applications. Advanced analytical methods and statistical tools are employed to establish performance metrics that enable objective assessment of biosensor capabilities and limitations, facilitating their optimization for specific use cases.
- Smart hydrogel materials for enhanced biosensor performance: Smart hydrogel materials with stimuli-responsive properties are being developed to enhance biosensor performance. These advanced hydrogels can undergo reversible physical or chemical changes in response to environmental stimuli such as pH, temperature, or specific biomolecules. By incorporating functional groups, nanoparticles, or biomolecular recognition elements, these materials provide improved sensitivity, selectivity, and response kinetics. The intelligent design of hydrogel composition and structure enables precise control over transduction mechanisms and overall biosensor performance.
- Integration of hydrogel biosensors with data processing systems: Integration of hydrogel biosensors with advanced data processing systems enables real-time analysis and interpretation of complex biosensing data. These integrated systems incorporate signal processing algorithms, machine learning techniques, and cloud connectivity to enhance the accuracy and reliability of biosensor measurements. The combination of innovative hydrogel sensing platforms with sophisticated data analytics facilitates continuous monitoring applications and enables predictive capabilities for early detection of physiological changes or environmental conditions.
02 Optical transduction methods for hydrogel-based biosensing
Optical transduction methods in hydrogel biosensors utilize changes in optical properties such as fluorescence, absorbance, or refractive index to detect biological analytes. These biosensors often incorporate chromophores or fluorophores within the hydrogel matrix that respond to specific biomolecular interactions. The resulting optical signals can be measured using various spectroscopic techniques, providing high sensitivity and the capability for multiplexed detection in complex biological environments.Expand Specific Solutions03 Performance benchmarking methodologies for hydrogel biosensors
Performance benchmarking of hydrogel biosensors involves standardized testing protocols to evaluate key parameters such as sensitivity, specificity, response time, and stability. These methodologies enable objective comparison between different biosensor designs and materials. Advanced statistical analysis techniques are employed to process sensor data and establish performance metrics, facilitating the optimization of biosensor designs for specific applications and ensuring reliable performance across varying environmental conditions.Expand Specific Solutions04 Smart hydrogel materials with enhanced sensing capabilities
Smart hydrogel materials are designed with stimuli-responsive properties that enhance their sensing capabilities in biosensor applications. These advanced materials can undergo reversible physical or chemical changes in response to specific biological targets or environmental conditions. By incorporating functional groups, nanoparticles, or biomolecules within the hydrogel network, these materials achieve improved sensitivity, selectivity, and response times. The intelligent design of these hydrogels enables the development of next-generation biosensors with superior performance characteristics.Expand Specific Solutions05 Integration of hydrogel biosensors with data processing systems
The integration of hydrogel biosensors with advanced data processing systems enables real-time analysis and interpretation of sensor outputs. These integrated systems incorporate signal processing algorithms, machine learning techniques, and cloud connectivity to enhance the functionality of biosensors. By combining hardware and software components, these systems can filter noise, calibrate responses, and extract meaningful information from complex biosensor data, ultimately improving diagnostic accuracy and enabling continuous health monitoring applications.Expand Specific Solutions
Leading Research Groups and Commercial Entities
The hydrogel biosensor market is currently in a growth phase, with increasing applications in healthcare monitoring and diagnostics. The market size is projected to expand significantly due to rising demand for continuous health monitoring solutions and point-of-care diagnostics. Technologically, the field shows moderate maturity with established transduction mechanisms, but considerable innovation potential remains. Key players include medical technology giants like Medtronic, Becton Dickinson, and Philips, who leverage their healthcare infrastructure to commercialize advanced biosensing solutions. Research institutions such as Fraunhofer-Gesellschaft and various universities (Tufts, Texas A&M) are driving fundamental innovations. Specialized companies like Nanowear and Applied Biosensors are focusing on niche applications with cloth nanotechnology and real-time biochemical monitoring respectively, while established electronics manufacturers (STMicroelectronics, NTT) provide essential components for signal processing and connectivity.
Koninklijke Philips NV
Technical Solution: Philips has developed advanced hydrogel-based biosensing platforms for continuous health monitoring applications. Their approach utilizes smart hydrogels with integrated microelectronics for both sensing and wireless data transmission. The transduction mechanisms combine impedance spectroscopy with optical sensing, where hydrogel swelling responses to specific biomarkers create measurable changes in electrical properties and light transmission. Philips' hydrogels incorporate biocompatible synthetic polymers with controlled crosslinking density to optimize response time and sensitivity. Performance benchmarks show detection of inflammatory markers at concentrations as low as 10 ng/mL with response times under 5 minutes. Their technology includes temperature-compensating elements within the hydrogel matrix to maintain consistent performance across physiological temperature ranges (35-40°C). Philips has implemented miniaturized, flexible form factors suitable for wearable applications, with sensors maintaining calibration for up to 7 days of continuous use. The company's integrated approach combines the hydrogel sensing element with low-power electronics and wireless communication capabilities, enabling real-time health monitoring with smartphone connectivity for both clinical and consumer applications.
Strengths: Highly integrated system combining sensing elements with data processing and transmission; excellent form factor design for wearable applications; strong manufacturing capabilities for scaled production. Weaknesses: Higher power requirements compared to purely passive sensors; more complex calibration procedures needed for multi-parameter sensing; limited penetration in implantable medical device markets compared to specialized medical device companies.
Medtronic MiniMed, Inc.
Technical Solution: Medtronic MiniMed has developed advanced hydrogel-based glucose biosensors for continuous glucose monitoring (CGM) systems. Their technology utilizes glucose oxidase enzymes immobilized within specialized hydrogel matrices that maintain enzyme stability while allowing glucose diffusion. The transduction mechanism employs electrochemical detection where glucose oxidation generates electrons that are measured as current proportional to glucose concentration. Their proprietary hydrogel formulations incorporate biocompatible polymers with controlled water content (typically 70-90%) to optimize sensor performance. The company has implemented redundant sensing elements within single sensors to improve accuracy and reliability, achieving mean absolute relative difference (MARD) values of approximately 9-11% in clinical studies. Medtronic's hydrogels are engineered with anti-fouling properties to minimize protein adsorption and extend sensor lifetime to 7+ days with consistent performance. Recent innovations include advanced membrane technologies that regulate analyte diffusion and reduce interference from electroactive substances like acetaminophen.
Strengths: Industry-leading longevity with sensors functioning reliably for 7+ days; excellent biocompatibility with reduced foreign body response; integrated systems approach combining sensor with automated insulin delivery. Weaknesses: Higher manufacturing complexity increases production costs; requires periodic calibration against fingerstick measurements; limited flexibility in form factor due to integrated electronics requirements.
Critical Patents and Innovations in Transduction Mechanisms
Method and apparatus for monitoring biometrical data
PatentInactiveUS20090155918A1
Innovation
- A wireless, powerless biosensor apparatus using a hydrogel with a chemical agent responsive to chemical characteristics, such as pH, that generates a mechanical signal upon contact with biological matter, which is then converted into an electrical signal using a capacitor and inductor circuit, allowing for remote monitoring without the need for a power source.
Method of manufacturing a biosensor
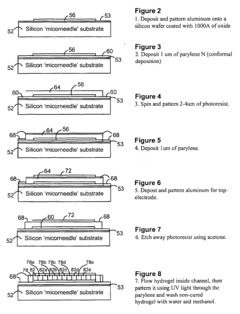
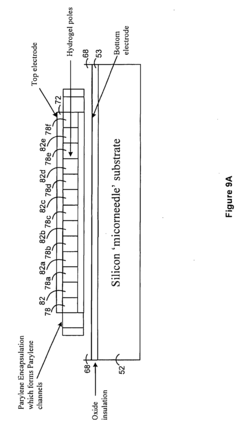
PatentActiveUS20160077427A1
Innovation
- A biosensor design incorporating two photo-definable hydrogel membranes, where the first provides structural rigidity and adhesion, and the second is directly on the electrode layer with an immobilized bio-recognition element, using photo-cross-linking and lithographic patterning to enhance immobilization and sensing properties without forming covalent bonds.
Biocompatibility and Material Selection Considerations
Biocompatibility represents a critical consideration in hydrogel biosensor design, as these devices often interface directly with biological systems. The selection of hydrogel materials must prioritize minimal immune response, reduced protein fouling, and prevention of inflammatory reactions when deployed in vivo. Current research indicates that natural polymers such as alginate, chitosan, and hyaluronic acid demonstrate superior biocompatibility profiles compared to synthetic alternatives, though they often present challenges in mechanical stability and reproducibility.
Material selection for hydrogel biosensors requires balancing multiple competing factors including biocompatibility, mechanical properties, and sensing capabilities. Synthetic polymers like poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poly(acrylamide) offer excellent tunability and reproducibility but may trigger adverse biological responses. Recent advances in hybrid materials combining natural and synthetic components show promising results in achieving optimal performance while maintaining biocompatibility.
Surface modification techniques have emerged as effective strategies to enhance biocompatibility while preserving the desirable properties of synthetic hydrogels. Approaches such as anti-fouling coatings, incorporation of zwitterionic groups, and biomimetic surface patterning significantly reduce protein adsorption and cellular adhesion, extending sensor lifetime and reliability in biological environments. These modifications can be tailored to specific application requirements without compromising sensing performance.
The degradation profile of hydrogel materials represents another crucial consideration, particularly for implantable biosensors. Ideally, degradation rates should match the intended duration of use, with degradation products being non-toxic and easily cleared by physiological processes. Recent research demonstrates that controlled degradation can be engineered through incorporation of enzymatically cleavable crosslinks or hydrolytically susceptible bonds, enabling precise control over sensor lifetime.
Sterilization compatibility must also be evaluated during material selection, as many hydrogels exhibit structural changes or performance degradation when subjected to common sterilization methods. Gamma irradiation, ethylene oxide treatment, and autoclave sterilization each present unique challenges for different hydrogel compositions. Novel approaches utilizing supercritical CO2 and cold plasma treatments show promise for preserving hydrogel integrity while ensuring sterility.
Regulatory considerations increasingly influence material selection decisions, with FDA and international regulatory bodies imposing stringent requirements on materials intended for biological contact. Documentation of biocompatibility testing according to ISO 10993 standards has become standard practice, necessitating comprehensive cytotoxicity, sensitization, and irritation assessments. Forward-looking biosensor designs increasingly incorporate materials with established regulatory precedent to streamline approval processes.
Material selection for hydrogel biosensors requires balancing multiple competing factors including biocompatibility, mechanical properties, and sensing capabilities. Synthetic polymers like poly(ethylene glycol) (PEG), poly(vinyl alcohol) (PVA), and poly(acrylamide) offer excellent tunability and reproducibility but may trigger adverse biological responses. Recent advances in hybrid materials combining natural and synthetic components show promising results in achieving optimal performance while maintaining biocompatibility.
Surface modification techniques have emerged as effective strategies to enhance biocompatibility while preserving the desirable properties of synthetic hydrogels. Approaches such as anti-fouling coatings, incorporation of zwitterionic groups, and biomimetic surface patterning significantly reduce protein adsorption and cellular adhesion, extending sensor lifetime and reliability in biological environments. These modifications can be tailored to specific application requirements without compromising sensing performance.
The degradation profile of hydrogel materials represents another crucial consideration, particularly for implantable biosensors. Ideally, degradation rates should match the intended duration of use, with degradation products being non-toxic and easily cleared by physiological processes. Recent research demonstrates that controlled degradation can be engineered through incorporation of enzymatically cleavable crosslinks or hydrolytically susceptible bonds, enabling precise control over sensor lifetime.
Sterilization compatibility must also be evaluated during material selection, as many hydrogels exhibit structural changes or performance degradation when subjected to common sterilization methods. Gamma irradiation, ethylene oxide treatment, and autoclave sterilization each present unique challenges for different hydrogel compositions. Novel approaches utilizing supercritical CO2 and cold plasma treatments show promise for preserving hydrogel integrity while ensuring sterility.
Regulatory considerations increasingly influence material selection decisions, with FDA and international regulatory bodies imposing stringent requirements on materials intended for biological contact. Documentation of biocompatibility testing according to ISO 10993 standards has become standard practice, necessitating comprehensive cytotoxicity, sensitization, and irritation assessments. Forward-looking biosensor designs increasingly incorporate materials with established regulatory precedent to streamline approval processes.
Performance Metrics and Standardization Frameworks
The establishment of standardized performance metrics for hydrogel biosensors represents a critical step toward their widespread adoption in clinical and commercial applications. Currently, the field suffers from inconsistent reporting practices, making direct comparisons between different sensor designs challenging. Key performance parameters that require standardization include sensitivity, specificity, detection limit, response time, dynamic range, stability, and reproducibility.
Sensitivity metrics should be quantified as the slope of the calibration curve (signal change per unit analyte concentration), allowing for objective comparison across different transduction mechanisms. Detection limits should be reported using the 3σ method, where the limit of detection equals three times the standard deviation of the blank divided by the sensitivity. This approach provides a statistically robust measure of the minimum detectable concentration.
Response time standardization remains particularly problematic, with various definitions currently in use. We propose adopting the t90 value (time to reach 90% of the final signal) as the standard metric, facilitating meaningful comparisons across different sensor architectures. Dynamic range should be expressed as the ratio between the maximum and minimum detectable concentrations, with logarithmic representation for wide-ranging sensors.
Stability assessment frameworks must address both shelf-life and operational stability under relevant environmental conditions. Accelerated aging protocols specific to hydrogel matrices need development, considering their unique degradation mechanisms compared to traditional sensor materials. Cross-reactivity testing should follow a tiered approach, first screening against common interferents, then against application-specific molecules.
Several international organizations are working toward standardization, including the International Organization for Standardization (ISO) Technical Committee 276 on Biotechnology and ASTM International's Committee F40 on Declarable Substances in Materials. The IEEE P2510 working group is developing standards specifically for sensor performance testing in biomedical applications that could be adapted for hydrogel biosensors.
Emerging reference materials for calibration and validation include synthetic analyte mimics with certified concentrations and stability profiles. These materials enable interlaboratory comparisons and quality control procedures essential for clinical translation. Additionally, digital simulation frameworks are being developed to predict sensor performance under various conditions, potentially reducing experimental iterations during the design phase.
Sensitivity metrics should be quantified as the slope of the calibration curve (signal change per unit analyte concentration), allowing for objective comparison across different transduction mechanisms. Detection limits should be reported using the 3σ method, where the limit of detection equals three times the standard deviation of the blank divided by the sensitivity. This approach provides a statistically robust measure of the minimum detectable concentration.
Response time standardization remains particularly problematic, with various definitions currently in use. We propose adopting the t90 value (time to reach 90% of the final signal) as the standard metric, facilitating meaningful comparisons across different sensor architectures. Dynamic range should be expressed as the ratio between the maximum and minimum detectable concentrations, with logarithmic representation for wide-ranging sensors.
Stability assessment frameworks must address both shelf-life and operational stability under relevant environmental conditions. Accelerated aging protocols specific to hydrogel matrices need development, considering their unique degradation mechanisms compared to traditional sensor materials. Cross-reactivity testing should follow a tiered approach, first screening against common interferents, then against application-specific molecules.
Several international organizations are working toward standardization, including the International Organization for Standardization (ISO) Technical Committee 276 on Biotechnology and ASTM International's Committee F40 on Declarable Substances in Materials. The IEEE P2510 working group is developing standards specifically for sensor performance testing in biomedical applications that could be adapted for hydrogel biosensors.
Emerging reference materials for calibration and validation include synthetic analyte mimics with certified concentrations and stability profiles. These materials enable interlaboratory comparisons and quality control procedures essential for clinical translation. Additionally, digital simulation frameworks are being developed to predict sensor performance under various conditions, potentially reducing experimental iterations during the design phase.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!