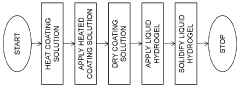
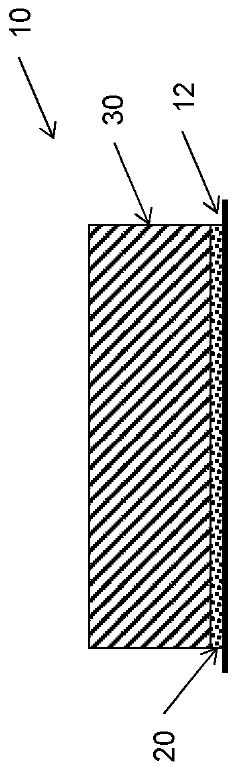
How to Tune Hydrogel Adhesion for Biomedical Applications — Surface Chemistry and Tests
AUG 21, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Adhesion Technology Background and Objectives
Hydrogels have emerged as versatile biomaterials in the biomedical field over the past several decades, evolving from simple water-retaining materials to sophisticated platforms for tissue engineering, drug delivery, and wound healing. The adhesion properties of hydrogels represent a critical aspect of their functionality, particularly at the interface with biological tissues. This technological domain has witnessed significant advancements, transitioning from conventional adhesives that often caused inflammation or toxicity to biocompatible hydrogel systems that can adhere to wet, dynamic biological surfaces.
The evolution of hydrogel adhesion technology has been driven by the increasing demand for minimally invasive medical procedures and the need for materials that can integrate seamlessly with living tissues. Early developments focused primarily on physical entanglement and hydrogen bonding mechanisms, while recent innovations have incorporated sophisticated chemical strategies including dynamic covalent chemistry, mussel-inspired adhesion, and host-guest interactions to enhance adhesive performance in physiological environments.
Current research trends indicate a growing interest in stimuli-responsive adhesive hydrogels that can be activated or deactivated on demand using external triggers such as pH, temperature, light, or electrical signals. This represents a paradigm shift from permanent adhesives to smart, controllable systems that offer unprecedented precision in biomedical applications.
The primary objective of hydrogel adhesion technology development is to create materials that can effectively bond to biological tissues under challenging physiological conditions while maintaining biocompatibility and functional performance. Specific technical goals include enhancing wet adhesion strength, improving mechanical compatibility with soft tissues, ensuring controlled degradation profiles, and developing methods to modulate adhesion properties in response to specific biological cues.
Another critical objective is to establish standardized testing methodologies that accurately predict in vivo performance, as current evaluation protocols often fail to replicate the complex, dynamic nature of biological environments. This includes developing more sophisticated models that account for factors such as surface heterogeneity, presence of biological fluids, and mechanical deformation during tissue movement.
Looking forward, the field aims to bridge the gap between laboratory demonstrations and clinical applications by addressing scalability challenges, regulatory requirements, and integration with existing medical procedures. The ultimate goal is to develop tunable hydrogel adhesives that can be customized for specific biomedical applications, from surgical sealants and wound dressings to tissue engineering scaffolds and drug delivery systems, thereby revolutionizing current medical practices and improving patient outcomes.
The evolution of hydrogel adhesion technology has been driven by the increasing demand for minimally invasive medical procedures and the need for materials that can integrate seamlessly with living tissues. Early developments focused primarily on physical entanglement and hydrogen bonding mechanisms, while recent innovations have incorporated sophisticated chemical strategies including dynamic covalent chemistry, mussel-inspired adhesion, and host-guest interactions to enhance adhesive performance in physiological environments.
Current research trends indicate a growing interest in stimuli-responsive adhesive hydrogels that can be activated or deactivated on demand using external triggers such as pH, temperature, light, or electrical signals. This represents a paradigm shift from permanent adhesives to smart, controllable systems that offer unprecedented precision in biomedical applications.
The primary objective of hydrogel adhesion technology development is to create materials that can effectively bond to biological tissues under challenging physiological conditions while maintaining biocompatibility and functional performance. Specific technical goals include enhancing wet adhesion strength, improving mechanical compatibility with soft tissues, ensuring controlled degradation profiles, and developing methods to modulate adhesion properties in response to specific biological cues.
Another critical objective is to establish standardized testing methodologies that accurately predict in vivo performance, as current evaluation protocols often fail to replicate the complex, dynamic nature of biological environments. This includes developing more sophisticated models that account for factors such as surface heterogeneity, presence of biological fluids, and mechanical deformation during tissue movement.
Looking forward, the field aims to bridge the gap between laboratory demonstrations and clinical applications by addressing scalability challenges, regulatory requirements, and integration with existing medical procedures. The ultimate goal is to develop tunable hydrogel adhesives that can be customized for specific biomedical applications, from surgical sealants and wound dressings to tissue engineering scaffolds and drug delivery systems, thereby revolutionizing current medical practices and improving patient outcomes.
Biomedical Market Demand for Advanced Adhesive Hydrogels
The global market for advanced adhesive hydrogels in biomedical applications is experiencing robust growth, driven by increasing demand for innovative wound care solutions, tissue engineering, drug delivery systems, and implantable medical devices. The market value for medical adhesives reached approximately $11 billion in 2022 and is projected to grow at a compound annual growth rate of 7.2% through 2030, with hydrogel-based adhesives representing one of the fastest-growing segments.
Wound care applications currently dominate the market demand for adhesive hydrogels, accounting for nearly 40% of the total market share. This is primarily due to the rising prevalence of chronic wounds, diabetic ulcers, and surgical wounds, coupled with the growing aging population worldwide. Advanced adhesive hydrogels offer significant advantages in wound management, including maintaining optimal moisture balance, facilitating oxygen exchange, and providing antimicrobial protection.
Tissue engineering represents another rapidly expanding application area, with a market growth rate exceeding 9% annually. The ability of adhesive hydrogels to mimic extracellular matrix properties while providing tunable adhesion to biological tissues makes them ideal scaffolds for tissue regeneration and organ-on-chip technologies. Research institutions and biotechnology companies are increasingly investing in hydrogel-based tissue engineering solutions.
Drug delivery systems utilizing adhesive hydrogels are gaining significant traction in pharmaceutical applications. The market for hydrogel-based drug delivery systems is expected to reach $5.8 billion by 2028, driven by the need for controlled release formulations and targeted therapy approaches. Mucoadhesive hydrogels for oral, nasal, ocular, and transdermal drug delivery are particularly in high demand due to their ability to enhance bioavailability and patient compliance.
Implantable medical devices represent a high-value segment where adhesive hydrogels are increasingly being utilized. The market for hydrogel-coated implants and biosensors is growing at approximately 8.5% annually, with particular emphasis on cardiovascular, orthopedic, and neurological applications. The demand for biocompatible adhesives that can form strong interfaces with living tissues while minimizing foreign body responses is driving innovation in this sector.
Geographically, North America currently leads the market for advanced adhesive hydrogels, accounting for approximately 38% of global demand, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to improving healthcare infrastructure, increasing medical tourism, and growing investment in biomedical research and development.
The market is also being shaped by evolving regulatory requirements and increasing focus on sustainable and biocompatible materials. Healthcare providers and patients are demanding adhesive hydrogels that offer not only superior performance but also reduced environmental impact and enhanced safety profiles, creating opportunities for bio-based and biodegradable formulations.
Wound care applications currently dominate the market demand for adhesive hydrogels, accounting for nearly 40% of the total market share. This is primarily due to the rising prevalence of chronic wounds, diabetic ulcers, and surgical wounds, coupled with the growing aging population worldwide. Advanced adhesive hydrogels offer significant advantages in wound management, including maintaining optimal moisture balance, facilitating oxygen exchange, and providing antimicrobial protection.
Tissue engineering represents another rapidly expanding application area, with a market growth rate exceeding 9% annually. The ability of adhesive hydrogels to mimic extracellular matrix properties while providing tunable adhesion to biological tissues makes them ideal scaffolds for tissue regeneration and organ-on-chip technologies. Research institutions and biotechnology companies are increasingly investing in hydrogel-based tissue engineering solutions.
Drug delivery systems utilizing adhesive hydrogels are gaining significant traction in pharmaceutical applications. The market for hydrogel-based drug delivery systems is expected to reach $5.8 billion by 2028, driven by the need for controlled release formulations and targeted therapy approaches. Mucoadhesive hydrogels for oral, nasal, ocular, and transdermal drug delivery are particularly in high demand due to their ability to enhance bioavailability and patient compliance.
Implantable medical devices represent a high-value segment where adhesive hydrogels are increasingly being utilized. The market for hydrogel-coated implants and biosensors is growing at approximately 8.5% annually, with particular emphasis on cardiovascular, orthopedic, and neurological applications. The demand for biocompatible adhesives that can form strong interfaces with living tissues while minimizing foreign body responses is driving innovation in this sector.
Geographically, North America currently leads the market for advanced adhesive hydrogels, accounting for approximately 38% of global demand, followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years due to improving healthcare infrastructure, increasing medical tourism, and growing investment in biomedical research and development.
The market is also being shaped by evolving regulatory requirements and increasing focus on sustainable and biocompatible materials. Healthcare providers and patients are demanding adhesive hydrogels that offer not only superior performance but also reduced environmental impact and enhanced safety profiles, creating opportunities for bio-based and biodegradable formulations.
Current Challenges in Hydrogel Surface Chemistry
Despite significant advancements in hydrogel technology for biomedical applications, several critical challenges persist in hydrogel surface chemistry that impede optimal adhesion performance. The primary challenge lies in achieving consistent and controlled adhesion across diverse biological environments. Hydrogels often exhibit unpredictable adhesion behavior when transitioning from in vitro testing to in vivo applications, largely due to the complex and dynamic nature of biological systems.
The heterogeneity of target tissues presents another significant obstacle. Different tissues possess unique surface properties, protein compositions, and mechanical characteristics, necessitating customized adhesion strategies. Current hydrogel formulations frequently lack the versatility to adapt to these varied tissue interfaces, resulting in compromised adhesion strength and durability in specific applications.
Maintaining adhesion stability under physiological conditions remains problematic. Hydrogels must withstand exposure to bodily fluids, enzymatic activity, pH variations, and mechanical stresses while preserving their adhesive properties. The degradation of adhesive bonds in biological environments often occurs prematurely, limiting the longevity and reliability of hydrogel-based medical devices and implants.
Biocompatibility concerns further complicate surface chemistry optimization. Chemical modifications that enhance adhesion may simultaneously introduce cytotoxicity or trigger inflammatory responses. Finding the delicate balance between adhesion strength and biocompatibility represents a persistent challenge in hydrogel design, particularly for long-term implantable applications.
The scalability of surface modification techniques poses additional difficulties. Laboratory-scale methods for enhancing hydrogel adhesion frequently encounter obstacles during industrial scale-up, affecting manufacturing consistency and commercial viability. Techniques that work effectively for small samples may prove impractical or prohibitively expensive at production scale.
Characterization and standardization issues also hinder progress in this field. The lack of universally accepted testing protocols for hydrogel adhesion makes comparative analysis challenging. Different research groups employ varied methodologies, resulting in inconsistent data that complicates technology assessment and regulatory approval processes.
Emerging challenges include developing environmentally responsive adhesion mechanisms that can adapt to changing physiological conditions. Creating hydrogels capable of dynamic adhesion—strengthening or weakening bonds in response to specific biological triggers—remains largely unrealized despite its potential to revolutionize targeted drug delivery and tissue engineering applications.
The heterogeneity of target tissues presents another significant obstacle. Different tissues possess unique surface properties, protein compositions, and mechanical characteristics, necessitating customized adhesion strategies. Current hydrogel formulations frequently lack the versatility to adapt to these varied tissue interfaces, resulting in compromised adhesion strength and durability in specific applications.
Maintaining adhesion stability under physiological conditions remains problematic. Hydrogels must withstand exposure to bodily fluids, enzymatic activity, pH variations, and mechanical stresses while preserving their adhesive properties. The degradation of adhesive bonds in biological environments often occurs prematurely, limiting the longevity and reliability of hydrogel-based medical devices and implants.
Biocompatibility concerns further complicate surface chemistry optimization. Chemical modifications that enhance adhesion may simultaneously introduce cytotoxicity or trigger inflammatory responses. Finding the delicate balance between adhesion strength and biocompatibility represents a persistent challenge in hydrogel design, particularly for long-term implantable applications.
The scalability of surface modification techniques poses additional difficulties. Laboratory-scale methods for enhancing hydrogel adhesion frequently encounter obstacles during industrial scale-up, affecting manufacturing consistency and commercial viability. Techniques that work effectively for small samples may prove impractical or prohibitively expensive at production scale.
Characterization and standardization issues also hinder progress in this field. The lack of universally accepted testing protocols for hydrogel adhesion makes comparative analysis challenging. Different research groups employ varied methodologies, resulting in inconsistent data that complicates technology assessment and regulatory approval processes.
Emerging challenges include developing environmentally responsive adhesion mechanisms that can adapt to changing physiological conditions. Creating hydrogels capable of dynamic adhesion—strengthening or weakening bonds in response to specific biological triggers—remains largely unrealized despite its potential to revolutionize targeted drug delivery and tissue engineering applications.
Current Surface Modification Approaches and Testing Methods
01 Hydrogel composition for enhanced adhesion
Specific compositions of hydrogels can be formulated to enhance adhesive properties. These compositions typically include polymers such as polyacrylamide, polyvinyl alcohol, or polyethylene glycol that create strong interfacial bonds. The addition of functional groups like carboxyl, hydroxyl, or amine groups can further improve adhesion through chemical interactions with surfaces. These specially designed hydrogels maintain their adhesive properties even in wet environments, making them suitable for various biomedical applications.- Hydrogel composition for enhanced adhesion: Specific hydrogel compositions can be formulated to enhance adhesion properties. These compositions typically include polymers such as polyvinyl alcohol, polyacrylic acid, or chitosan that provide strong adhesive characteristics. The addition of cross-linking agents helps to optimize the balance between cohesion and adhesion, while maintaining the hydrogel's water content. These specialized formulations enable the hydrogel to adhere effectively to various surfaces, including biological tissues and synthetic materials.
- Surface modification techniques for improved adhesion: Various surface modification techniques can be employed to improve the adhesion of hydrogels. These include plasma treatment, chemical functionalization, and micro/nano-texturing of the hydrogel surface. Such modifications increase the surface energy and create additional binding sites for interfacial interactions. By altering the surface properties, these techniques enhance the adhesive strength of hydrogels without significantly affecting their bulk properties, resulting in stronger and more durable adhesion to target surfaces.
- Bioadhesive hydrogels for medical applications: Bioadhesive hydrogels are specifically designed for medical applications where adhesion to biological tissues is critical. These hydrogels incorporate biomimetic adhesive components inspired by natural adhesion mechanisms, such as those found in mussels or geckos. The incorporation of catechol groups, peptide sequences, or other biologically active molecules enhances tissue adhesion while maintaining biocompatibility. These materials are particularly valuable for wound dressings, tissue engineering scaffolds, and drug delivery systems that require prolonged contact with biological surfaces.
- Stimuli-responsive adhesive hydrogels: Stimuli-responsive adhesive hydrogels can change their adhesion properties in response to external stimuli such as temperature, pH, light, or electrical signals. These smart materials can transition between adhesive and non-adhesive states on demand, offering controlled attachment and detachment capabilities. The responsive behavior is typically achieved through incorporation of thermosensitive polymers, pH-sensitive functional groups, or photosensitive moieties. This dynamic adhesion control is particularly valuable for applications requiring temporary adhesion or sequential attachment to different surfaces.
- Nanocomposite hydrogels with enhanced adhesion: Nanocomposite hydrogels incorporate nanomaterials such as nanoparticles, nanoclays, or nanofibers to enhance their adhesive properties. These nanomaterials create additional physical and chemical interactions at the interface, significantly improving adhesion strength. The nanofillers can also reinforce the hydrogel network, preventing cohesive failure during adhesion testing. By carefully selecting the type, size, and concentration of nanomaterials, the adhesion properties can be tailored for specific applications while maintaining other desirable hydrogel characteristics such as swelling capacity and mechanical strength.
02 Surface modification techniques for improved hydrogel adhesion
Various surface modification techniques can be employed to enhance the adhesion of hydrogels to different substrates. These include plasma treatment, UV irradiation, chemical etching, and the application of coupling agents. Such modifications create reactive sites on the substrate surface that can form covalent or non-covalent bonds with the hydrogel. These techniques are particularly useful for improving adhesion to challenging surfaces such as metals, ceramics, or hydrophobic polymers.Expand Specific Solutions03 Bioadhesive hydrogels for medical applications
Bioadhesive hydrogels are specifically designed for medical applications where adhesion to biological tissues is required. These hydrogels often incorporate biomimetic adhesive components inspired by natural adhesives like those found in mussels or geckos. They may contain catechol groups, peptide sequences, or other biocompatible adhesive moieties that enable strong but gentle adhesion to tissues. These materials are particularly valuable for wound dressings, tissue engineering scaffolds, and drug delivery systems that need to remain in place on or within the body.Expand Specific Solutions04 Stimuli-responsive adhesive hydrogels
Stimuli-responsive hydrogels can change their adhesive properties in response to external triggers such as temperature, pH, light, or electrical signals. These smart materials can switch between adhesive and non-adhesive states on demand, allowing for controlled attachment and detachment. For example, some hydrogels become more adhesive when heated above body temperature and less adhesive when cooled. This reversible adhesion is particularly useful for applications requiring temporary bonding or for creating devices that can be easily removed without damaging surrounding tissues.Expand Specific Solutions05 Nanocomposite hydrogels with enhanced adhesion
Incorporating nanoparticles or nanomaterials into hydrogels can significantly enhance their adhesive properties. Nanocomposite hydrogels may contain materials such as silica nanoparticles, carbon nanotubes, graphene, or clay nanoplatelets that increase the mechanical strength and adhesion of the hydrogel matrix. These nanomaterials can create additional physical entanglements, hydrogen bonding sites, or electrostatic interactions that strengthen the adhesive interface. The resulting nanocomposite hydrogels often demonstrate superior adhesion strength, durability, and resistance to environmental factors compared to conventional hydrogels.Expand Specific Solutions
Leading Research Groups and Companies in Adhesive Hydrogels
The hydrogel adhesion technology for biomedical applications is currently in a growth phase, with an estimated market size of $2-3 billion annually and expanding at 8-10% CAGR. The competitive landscape features established medical device companies like Ethicon Endo-Surgery and Covidien alongside specialized players such as SentryX BV and Actamax Surgical Materials. Academic institutions including MIT, University of Rochester, and Zhejiang University are driving fundamental research advancements. Technical maturity varies across applications, with wound closure solutions being more developed than drug delivery systems. Leading companies are focusing on surface chemistry optimization to enhance biocompatibility, adhesion strength, and controlled degradation properties, with recent innovations addressing tissue-specific adhesion challenges in wet biological environments.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced hydrogel adhesion systems utilizing dynamic covalent chemistry for tissue-specific applications. Their approach involves incorporating catechol-based adhesive moieties inspired by mussel adhesive proteins, which form strong bonds with tissue surfaces under wet conditions. MIT researchers have engineered hydrogels with tunable mechanical properties and degradation rates by incorporating dual-crosslinking networks that respond to physiological stimuli. Their testing methodology includes comprehensive mechanical characterization through lap shear, peel, and tensile tests under various physiological conditions, as well as in vitro and in vivo biocompatibility assessments. MIT has also pioneered the development of tough adhesive hydrogels that maintain strong adhesion even when stretched, using a combination of covalent bonds and energy-dissipating mechanisms to prevent interfacial crack propagation.
Strengths: Superior wet adhesion strength that outperforms commercial tissue adhesives; highly tunable mechanical properties to match specific tissue requirements; excellent biocompatibility with minimal inflammatory response. Weaknesses: Complex synthesis procedures may limit scalability; some formulations require UV activation which limits application in certain surgical settings; potential long-term stability concerns in dynamic physiological environments.
Ningbo Institute of Industrial Technology
Technical Solution: Ningbo Institute of Industrial Technology has developed innovative hydrogel adhesion systems focusing on nano-composite approaches for enhanced biomedical applications. Their technology incorporates carefully engineered nanoparticles (including silica, graphene oxide, and clay nanosheets) into hydrogel matrices to significantly enhance mechanical properties and adhesion strength. Their researchers have pioneered the use of mussel-inspired polydopamine surface modifications combined with these nanocomposite structures to create hydrogels with exceptional wet adhesion properties. The institute has developed specialized testing methodologies including underwater adhesion strength measurements, cyclic loading tests, and long-term stability assessments under physiological conditions. Their approach includes precise control of surface charge distribution and hydrophilic/hydrophobic balance to optimize tissue interactions for specific applications. Recent innovations include stimuli-responsive hydrogels that can modulate their adhesion properties in response to environmental changes (pH, temperature, or enzymatic activity), allowing for controlled attachment and detachment in clinical settings. The institute has also developed specialized surface characterization techniques to correlate nanoscale surface features with macroscale adhesion performance.
Strengths: Exceptional mechanical reinforcement through nanocomposite structures; superior wet adhesion performance compared to conventional hydrogels; stimuli-responsive behavior enables smart adhesion control. Weaknesses: Potential concerns regarding long-term fate of nanoparticles in vivo; more complex manufacturing processes compared to conventional hydrogels; possible trade-offs between enhanced mechanical properties and biocompatibility in some formulations.
Key Innovations in Hydrogel-Tissue Interface Engineering
Hydrogel containing surface-treated nanofibers and method for preparing same
PatentWO2015174643A1
Innovation
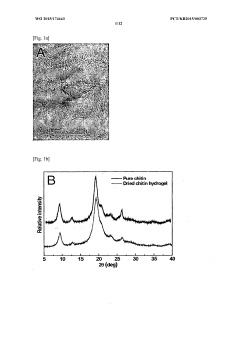
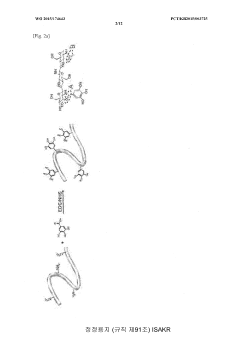
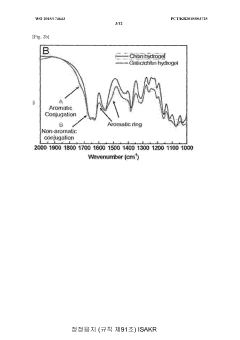
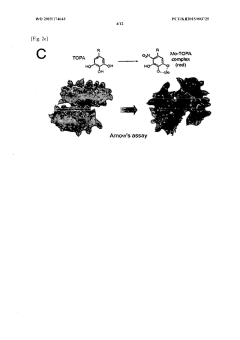
- Development of a bioadhesive hydrogel containing surface-treated chitin or chitosan nanofibers with dihydroxyphenyl or trihydroxyphenyl residues, or tannic acid, which provides enhanced adhesive properties and minimal side effects by forming covalent bonds with biological tissues.
Adherence enhancing coating for plastic surfaces
PatentWO2023191681A1
Innovation
- A surface coating method involving a heated solution of a gelling agent, sulfate salt, and water is applied to the plastic surface, dried to form a pre-coated surface, and then a hydrogel is applied, which adheres strongly to the coated surface without altering the hydrogel material.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount when developing hydrogel adhesives for biomedical applications. The interaction between hydrogel materials and biological tissues must be carefully evaluated to ensure patient safety and treatment efficacy. Comprehensive biocompatibility testing protocols, including cytotoxicity, sensitization, irritation, and systemic toxicity assessments, are essential before any clinical implementation can be considered.
Material selection plays a critical role in determining the biocompatibility profile of hydrogel adhesives. Natural polymers such as hyaluronic acid, alginate, and collagen generally exhibit superior biocompatibility compared to synthetic alternatives, though they often present challenges in mechanical stability and adhesion strength. Synthetic polymers like polyethylene glycol (PEG) and polyvinyl alcohol (PVA) offer tunable properties but require careful modification to minimize immune responses.
The degradation kinetics of hydrogel adhesives must align with the intended application timeline. Degradation products should be non-toxic and easily cleared by the body's natural processes. Recent research has focused on developing hydrogels with controlled degradation profiles that match tissue healing rates, thereby minimizing the risk of chronic inflammation or foreign body reactions.
Surface chemistry modifications significantly impact the safety profile of hydrogel adhesives. Functional groups exposed at the hydrogel-tissue interface can trigger immune responses or promote bacterial adhesion. Strategies such as incorporating antimicrobial peptides, zwitterionic moieties, or anti-fouling components have shown promise in reducing infection risks while maintaining adhesive properties.
Inflammatory responses represent a major safety concern for implantable hydrogel adhesives. Standard testing protocols include acute and chronic inflammation assessments, foreign body reaction evaluations, and granulation tissue formation analyses. Advanced in vitro models utilizing patient-derived cells and organoids are increasingly being employed to predict patient-specific inflammatory responses before clinical testing.
Sterilization compatibility presents another critical consideration, as many hydrogels are sensitive to conventional sterilization methods. Gamma irradiation, ethylene oxide treatment, and supercritical CO2 sterilization can alter the chemical structure and mechanical properties of hydrogels, potentially compromising both safety and functionality. Developing sterilization-resistant formulations remains an active area of research.
Regulatory pathways for hydrogel adhesives vary depending on their intended use, with more stringent requirements for long-term implantable materials compared to topical applications. The FDA and equivalent international bodies typically require extensive preclinical safety data, including biocompatibility testing according to ISO 10993 standards, before approving clinical trials.
Material selection plays a critical role in determining the biocompatibility profile of hydrogel adhesives. Natural polymers such as hyaluronic acid, alginate, and collagen generally exhibit superior biocompatibility compared to synthetic alternatives, though they often present challenges in mechanical stability and adhesion strength. Synthetic polymers like polyethylene glycol (PEG) and polyvinyl alcohol (PVA) offer tunable properties but require careful modification to minimize immune responses.
The degradation kinetics of hydrogel adhesives must align with the intended application timeline. Degradation products should be non-toxic and easily cleared by the body's natural processes. Recent research has focused on developing hydrogels with controlled degradation profiles that match tissue healing rates, thereby minimizing the risk of chronic inflammation or foreign body reactions.
Surface chemistry modifications significantly impact the safety profile of hydrogel adhesives. Functional groups exposed at the hydrogel-tissue interface can trigger immune responses or promote bacterial adhesion. Strategies such as incorporating antimicrobial peptides, zwitterionic moieties, or anti-fouling components have shown promise in reducing infection risks while maintaining adhesive properties.
Inflammatory responses represent a major safety concern for implantable hydrogel adhesives. Standard testing protocols include acute and chronic inflammation assessments, foreign body reaction evaluations, and granulation tissue formation analyses. Advanced in vitro models utilizing patient-derived cells and organoids are increasingly being employed to predict patient-specific inflammatory responses before clinical testing.
Sterilization compatibility presents another critical consideration, as many hydrogels are sensitive to conventional sterilization methods. Gamma irradiation, ethylene oxide treatment, and supercritical CO2 sterilization can alter the chemical structure and mechanical properties of hydrogels, potentially compromising both safety and functionality. Developing sterilization-resistant formulations remains an active area of research.
Regulatory pathways for hydrogel adhesives vary depending on their intended use, with more stringent requirements for long-term implantable materials compared to topical applications. The FDA and equivalent international bodies typically require extensive preclinical safety data, including biocompatibility testing according to ISO 10993 standards, before approving clinical trials.
Regulatory Pathway for Adhesive Hydrogel Medical Devices
The regulatory landscape for adhesive hydrogel medical devices presents a complex pathway that manufacturers must navigate to bring their products to market. In the United States, the Food and Drug Administration (FDA) classifies these devices based on their intended use, risk profile, and level of invasiveness. Most adhesive hydrogels fall under Class II (moderate risk) or Class III (high risk) categories, depending on their specific application and duration of contact with the body.
For Class II hydrogel adhesives, manufacturers typically pursue the 510(k) premarket notification pathway, demonstrating substantial equivalence to a legally marketed predicate device. This requires comprehensive biocompatibility testing according to ISO 10993 standards, with particular emphasis on cytotoxicity, sensitization, and irritation testing for skin-contacting adhesives.
Class III devices, such as implantable hydrogel adhesives or those used in critical applications like neurological or cardiovascular procedures, require the more rigorous Premarket Approval (PMA) pathway. This demands extensive clinical trials to demonstrate safety and efficacy, significantly extending the development timeline and increasing costs.
In the European market, adhesive hydrogel devices must comply with the Medical Device Regulation (MDR 2017/745), which has replaced the previous Medical Device Directive. The MDR imposes stricter requirements for clinical evaluation, post-market surveillance, and technical documentation. Manufacturers must obtain CE marking through assessment by a Notified Body, with the classification similarly dependent on the device's risk profile.
The regulatory requirements for biocompatibility testing are particularly stringent for adhesive hydrogels due to their direct contact with tissues. Testing protocols must address not only the base hydrogel material but also any adhesion-enhancing components, crosslinking agents, and degradation products. Leachable and extractable testing is critical to identify potential toxic compounds that might migrate from the device to the patient.
For novel adhesive mechanisms or chemistries, regulatory bodies often require additional specialized testing beyond standard protocols. This may include custom mechanical testing to evaluate adhesion strength under physiological conditions, degradation kinetics in relevant biological environments, and long-term stability studies.
Post-market surveillance requirements have become increasingly important in both US and EU regulatory frameworks. Manufacturers must implement robust systems to monitor device performance, collect adverse event data, and conduct periodic safety update reports. For adhesive hydrogels with novel tuning mechanisms, this surveillance is crucial to identify any unexpected long-term effects or failure modes.
Regulatory strategies should be integrated early in the development process of tunable adhesive hydrogels, as design choices significantly impact the regulatory pathway. Manufacturers should engage with regulatory bodies through pre-submission consultations to clarify specific requirements and potentially streamline the approval process for these innovative biomaterials.
For Class II hydrogel adhesives, manufacturers typically pursue the 510(k) premarket notification pathway, demonstrating substantial equivalence to a legally marketed predicate device. This requires comprehensive biocompatibility testing according to ISO 10993 standards, with particular emphasis on cytotoxicity, sensitization, and irritation testing for skin-contacting adhesives.
Class III devices, such as implantable hydrogel adhesives or those used in critical applications like neurological or cardiovascular procedures, require the more rigorous Premarket Approval (PMA) pathway. This demands extensive clinical trials to demonstrate safety and efficacy, significantly extending the development timeline and increasing costs.
In the European market, adhesive hydrogel devices must comply with the Medical Device Regulation (MDR 2017/745), which has replaced the previous Medical Device Directive. The MDR imposes stricter requirements for clinical evaluation, post-market surveillance, and technical documentation. Manufacturers must obtain CE marking through assessment by a Notified Body, with the classification similarly dependent on the device's risk profile.
The regulatory requirements for biocompatibility testing are particularly stringent for adhesive hydrogels due to their direct contact with tissues. Testing protocols must address not only the base hydrogel material but also any adhesion-enhancing components, crosslinking agents, and degradation products. Leachable and extractable testing is critical to identify potential toxic compounds that might migrate from the device to the patient.
For novel adhesive mechanisms or chemistries, regulatory bodies often require additional specialized testing beyond standard protocols. This may include custom mechanical testing to evaluate adhesion strength under physiological conditions, degradation kinetics in relevant biological environments, and long-term stability studies.
Post-market surveillance requirements have become increasingly important in both US and EU regulatory frameworks. Manufacturers must implement robust systems to monitor device performance, collect adverse event data, and conduct periodic safety update reports. For adhesive hydrogels with novel tuning mechanisms, this surveillance is crucial to identify any unexpected long-term effects or failure modes.
Regulatory strategies should be integrated early in the development process of tunable adhesive hydrogels, as design choices significantly impact the regulatory pathway. Manufacturers should engage with regulatory bodies through pre-submission consultations to clarify specific requirements and potentially streamline the approval process for these innovative biomaterials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!