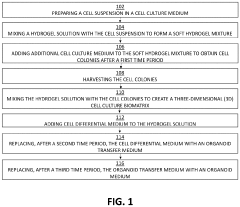
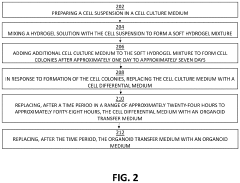
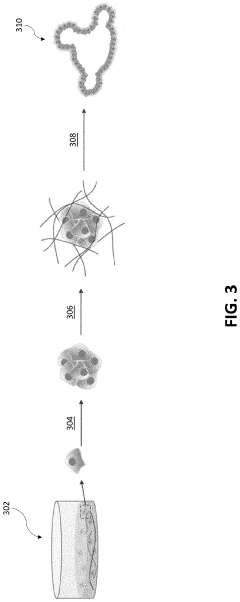
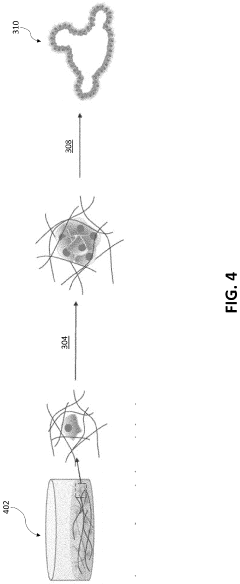
How to Use Hydrogel Matrices for Organoid Culture — Protocols and Viability Metrics
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydrogel Matrices for Organoid Culture: Background and Objectives
Organoid culture technology has evolved significantly over the past decade, representing a revolutionary approach in biomedical research and regenerative medicine. Hydrogel matrices have emerged as critical components in this field, providing three-dimensional microenvironments that mimic native tissue architecture and biochemical cues necessary for organoid development. The evolution of hydrogel technology for organoid culture can be traced back to early extracellular matrix (ECM) research in the 1980s, which laid the groundwork for understanding cell-matrix interactions. However, it was not until the 2010s that significant breakthroughs in stem cell biology and biomaterials science converged to enable reliable organoid culture systems.
The technological trajectory has moved from simple natural hydrogels like Matrigel toward more defined, tunable synthetic and hybrid systems. This progression reflects the growing understanding of the complex interplay between mechanical properties, biochemical signaling, and spatial organization in directing organoid formation and function. Recent advances in biomaterial engineering have enabled unprecedented control over hydrogel properties, allowing researchers to recapitulate tissue-specific microenvironments with increasing fidelity.
Current research trends indicate a shift toward chemically defined hydrogel systems that eliminate batch-to-batch variability and unknown components present in naturally derived matrices. Additionally, there is growing interest in developing dynamic hydrogels that can respond to cellular activities and provide temporal control over matrix properties during organoid development. These innovations address the limitations of static culture systems in modeling complex developmental processes and disease progression.
The primary objective of hydrogel matrix technology for organoid culture is to establish reproducible, scalable, and physiologically relevant in vitro models that accurately recapitulate the structure and function of human tissues. This goal encompasses several specific aims: developing standardized protocols for consistent organoid generation, creating hydrogel systems that support long-term culture and maturation, and establishing reliable metrics for assessing organoid viability and functionality.
Furthermore, the field aims to overcome current technical challenges such as vascularization limitations, size constraints, and maturation barriers that prevent organoids from fully mimicking adult tissue complexity. Advanced hydrogel designs incorporating perfusable channels, degradable components, and spatially patterned bioactive molecules represent promising approaches to address these challenges.
The ultimate technological objective extends beyond basic research applications to clinical translation, including personalized medicine approaches, drug screening platforms, and regenerative therapy development. This requires hydrogel systems that not only support organoid growth but also enable high-throughput analysis, imaging compatibility, and eventual scale-up for therapeutic applications.
The technological trajectory has moved from simple natural hydrogels like Matrigel toward more defined, tunable synthetic and hybrid systems. This progression reflects the growing understanding of the complex interplay between mechanical properties, biochemical signaling, and spatial organization in directing organoid formation and function. Recent advances in biomaterial engineering have enabled unprecedented control over hydrogel properties, allowing researchers to recapitulate tissue-specific microenvironments with increasing fidelity.
Current research trends indicate a shift toward chemically defined hydrogel systems that eliminate batch-to-batch variability and unknown components present in naturally derived matrices. Additionally, there is growing interest in developing dynamic hydrogels that can respond to cellular activities and provide temporal control over matrix properties during organoid development. These innovations address the limitations of static culture systems in modeling complex developmental processes and disease progression.
The primary objective of hydrogel matrix technology for organoid culture is to establish reproducible, scalable, and physiologically relevant in vitro models that accurately recapitulate the structure and function of human tissues. This goal encompasses several specific aims: developing standardized protocols for consistent organoid generation, creating hydrogel systems that support long-term culture and maturation, and establishing reliable metrics for assessing organoid viability and functionality.
Furthermore, the field aims to overcome current technical challenges such as vascularization limitations, size constraints, and maturation barriers that prevent organoids from fully mimicking adult tissue complexity. Advanced hydrogel designs incorporating perfusable channels, degradable components, and spatially patterned bioactive molecules represent promising approaches to address these challenges.
The ultimate technological objective extends beyond basic research applications to clinical translation, including personalized medicine approaches, drug screening platforms, and regenerative therapy development. This requires hydrogel systems that not only support organoid growth but also enable high-throughput analysis, imaging compatibility, and eventual scale-up for therapeutic applications.
Market Demand Analysis for Advanced Organoid Culture Systems
The global organoid culture market is experiencing significant growth, driven by increasing demand for advanced 3D cell culture systems that better mimic in vivo conditions. Current market valuations place this sector at approximately $1.3 billion in 2023, with projections indicating a compound annual growth rate of 22.1% through 2030, potentially reaching $5.2 billion by the end of the decade. This remarkable growth trajectory reflects the expanding applications of organoid technology across multiple industries.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of current demand. These companies are increasingly adopting organoid culture systems to reduce drug development costs and timelines by providing more physiologically relevant models for drug screening and toxicity testing. The ability of hydrogel-based organoid cultures to predict human responses more accurately than traditional 2D cell cultures or animal models is driving substantial investment in this area.
Academic and research institutions constitute the second-largest market segment, with growing interest in using organoid systems for basic research in developmental biology, disease modeling, and regenerative medicine. The demand for standardized, reproducible hydrogel matrices for organoid culture has increased by over 30% in the past three years within this segment.
Biotechnology companies focusing on personalized medicine represent the fastest-growing market segment, with an estimated growth rate of 28% annually. These companies are leveraging patient-derived organoids cultured in specialized hydrogel matrices to develop tailored therapeutic approaches, particularly in oncology and rare genetic disorders.
Regionally, North America currently dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to show the highest growth rate over the next five years, driven by increasing research funding and biotechnology infrastructure development in countries like China, Japan, and South Korea.
Key market drivers include the rising prevalence of chronic diseases necessitating better disease models, increasing R&D investments in regenerative medicine, and growing awareness of the limitations of traditional cell culture and animal models. Additionally, regulatory agencies are increasingly recognizing the value of organoid-based testing systems, potentially accelerating their adoption in regulatory frameworks.
Market challenges include the high cost of specialized hydrogel matrices, technical complexities in maintaining long-term organoid cultures, and the need for standardized protocols to ensure reproducibility across different laboratories and applications. These challenges present significant opportunities for companies developing innovative, cost-effective hydrogel solutions with improved performance metrics and standardized viability assessment methods.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of current demand. These companies are increasingly adopting organoid culture systems to reduce drug development costs and timelines by providing more physiologically relevant models for drug screening and toxicity testing. The ability of hydrogel-based organoid cultures to predict human responses more accurately than traditional 2D cell cultures or animal models is driving substantial investment in this area.
Academic and research institutions constitute the second-largest market segment, with growing interest in using organoid systems for basic research in developmental biology, disease modeling, and regenerative medicine. The demand for standardized, reproducible hydrogel matrices for organoid culture has increased by over 30% in the past three years within this segment.
Biotechnology companies focusing on personalized medicine represent the fastest-growing market segment, with an estimated growth rate of 28% annually. These companies are leveraging patient-derived organoids cultured in specialized hydrogel matrices to develop tailored therapeutic approaches, particularly in oncology and rare genetic disorders.
Regionally, North America currently dominates the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is expected to show the highest growth rate over the next five years, driven by increasing research funding and biotechnology infrastructure development in countries like China, Japan, and South Korea.
Key market drivers include the rising prevalence of chronic diseases necessitating better disease models, increasing R&D investments in regenerative medicine, and growing awareness of the limitations of traditional cell culture and animal models. Additionally, regulatory agencies are increasingly recognizing the value of organoid-based testing systems, potentially accelerating their adoption in regulatory frameworks.
Market challenges include the high cost of specialized hydrogel matrices, technical complexities in maintaining long-term organoid cultures, and the need for standardized protocols to ensure reproducibility across different laboratories and applications. These challenges present significant opportunities for companies developing innovative, cost-effective hydrogel solutions with improved performance metrics and standardized viability assessment methods.
Current Challenges in Hydrogel-Based Organoid Culture Technologies
Despite significant advancements in hydrogel-based organoid culture technologies, several critical challenges persist that limit their widespread application and reproducibility. One of the primary obstacles is the inconsistency in hydrogel composition and mechanical properties across different batches and sources. Natural hydrogels like Matrigel, while biologically relevant, suffer from batch-to-batch variability that can significantly impact organoid formation, growth patterns, and functionality. This variability makes standardization difficult and complicates the comparison of results across different research groups.
The optimization of hydrogel stiffness and degradation kinetics presents another substantial challenge. Organoids require dynamic microenvironments that evolve as they develop, but current hydrogel systems often provide static mechanical properties. The inability to precisely control the temporal changes in matrix stiffness and degradation rates limits the accurate recapitulation of native tissue development processes, potentially affecting organoid maturation and functionality.
Diffusion limitations within hydrogel matrices represent a significant technical hurdle. As organoids grow larger, the transport of nutrients, oxygen, and waste products becomes increasingly restricted, leading to necrotic cores in larger organoids. This diffusion constraint imposes size limitations on cultured organoids and affects their long-term viability, particularly for applications requiring larger tissue constructs.
The integration of vascular-like networks within hydrogel matrices remains largely unresolved. Without proper vascularization, organoids face limitations in size and functionality due to insufficient nutrient supply. Current approaches to introduce perfusable channels or promote angiogenesis within hydrogels are still in early developmental stages and have not been widely implemented in standard organoid culture protocols.
Scalability and reproducibility issues further complicate the translation of hydrogel-based organoid technologies from research to clinical applications. The labor-intensive nature of current protocols, combined with the high costs of specialized hydrogel materials, presents significant barriers to large-scale production and standardization. Additionally, the lack of universally accepted viability metrics and quality control parameters makes it difficult to establish reliable benchmarks for organoid functionality across different systems.
The development of defined, synthetic alternatives to natural hydrogels has progressed, but these systems often lack the complex biochemical cues present in natural matrices. Finding the optimal balance between reproducibility (offered by synthetic systems) and biological relevance (provided by natural matrices) remains an ongoing challenge that requires interdisciplinary approaches combining materials science, tissue engineering, and developmental biology.
The optimization of hydrogel stiffness and degradation kinetics presents another substantial challenge. Organoids require dynamic microenvironments that evolve as they develop, but current hydrogel systems often provide static mechanical properties. The inability to precisely control the temporal changes in matrix stiffness and degradation rates limits the accurate recapitulation of native tissue development processes, potentially affecting organoid maturation and functionality.
Diffusion limitations within hydrogel matrices represent a significant technical hurdle. As organoids grow larger, the transport of nutrients, oxygen, and waste products becomes increasingly restricted, leading to necrotic cores in larger organoids. This diffusion constraint imposes size limitations on cultured organoids and affects their long-term viability, particularly for applications requiring larger tissue constructs.
The integration of vascular-like networks within hydrogel matrices remains largely unresolved. Without proper vascularization, organoids face limitations in size and functionality due to insufficient nutrient supply. Current approaches to introduce perfusable channels or promote angiogenesis within hydrogels are still in early developmental stages and have not been widely implemented in standard organoid culture protocols.
Scalability and reproducibility issues further complicate the translation of hydrogel-based organoid technologies from research to clinical applications. The labor-intensive nature of current protocols, combined with the high costs of specialized hydrogel materials, presents significant barriers to large-scale production and standardization. Additionally, the lack of universally accepted viability metrics and quality control parameters makes it difficult to establish reliable benchmarks for organoid functionality across different systems.
The development of defined, synthetic alternatives to natural hydrogels has progressed, but these systems often lack the complex biochemical cues present in natural matrices. Finding the optimal balance between reproducibility (offered by synthetic systems) and biological relevance (provided by natural matrices) remains an ongoing challenge that requires interdisciplinary approaches combining materials science, tissue engineering, and developmental biology.
Established Protocols for Hydrogel-Based Organoid Culture
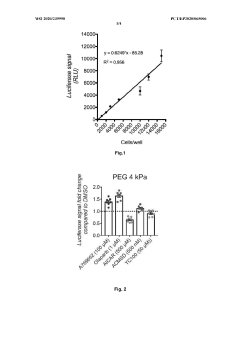
01 Hydrogel compositions for cell viability assessment
Hydrogel matrices can be formulated to support cell growth and provide environments for assessing cell viability. These compositions typically include biocompatible polymers that create three-dimensional networks mimicking natural tissue environments. The matrices allow for nutrient diffusion while maintaining structural integrity, enabling researchers to evaluate cell survival, proliferation, and metabolic activity using various viability metrics such as fluorescence-based assays, metabolic indicators, and morphological assessments.- Hydrogel compositions for cell viability assessment: Hydrogel matrices can be formulated to support cell growth and provide environments for assessing cell viability. These compositions typically include biocompatible polymers that create three-dimensional networks mimicking natural tissue environments. The matrices allow for nutrient diffusion while maintaining structural integrity, enabling researchers to evaluate cell survival, proliferation, and metabolic activity using various viability metrics such as fluorescent dyes, metabolic assays, and imaging techniques.
- Biomedical applications of hydrogel matrices with viability monitoring: Hydrogel matrices are increasingly used in biomedical applications where cell viability monitoring is critical. These applications include tissue engineering, wound healing, drug delivery systems, and regenerative medicine. The hydrogels can be designed with specific mechanical properties and degradation profiles to match target tissues. Viability metrics in these applications often involve quantitative assessment of cell function, integration with host tissue, and long-term survival rates of encapsulated cells or tissues.
- Advanced hydrogel formulations with enhanced cell viability properties: Recent advances in hydrogel technology have led to formulations specifically designed to enhance cell viability. These include the incorporation of growth factors, adhesion molecules, and other bioactive compounds that promote cell survival and function. Some formulations utilize stimuli-responsive elements that can adjust matrix properties in response to cellular needs or external triggers. Viability metrics for these advanced systems often include functional assessments beyond simple live/dead staining, such as protein expression profiles and cellular differentiation markers.
- Analytical methods for assessing cell viability in hydrogel matrices: Various analytical techniques have been developed to assess cell viability within hydrogel matrices. These include non-invasive imaging methods such as confocal microscopy, two-photon microscopy, and bioluminescence imaging that allow for real-time monitoring of cells. Other approaches involve biochemical assays that measure metabolic activity, membrane integrity, or specific cellular functions. These methods must account for the three-dimensional nature of hydrogels and potential interference from the matrix materials themselves.
- Standardization of viability metrics for hydrogel-encapsulated cells: Efforts to standardize viability metrics for cells in hydrogel matrices are essential for comparing results across different research groups and applications. These standardization approaches include developing reference materials, establishing protocol guidelines, and creating quantitative benchmarks for cell performance in various hydrogel environments. Considerations include accounting for diffusion limitations, matrix-specific interactions, and temporal changes in both the cells and the hydrogel structure. Standardized metrics help in regulatory approval processes and translation of hydrogel technologies to clinical applications.
02 Biomedical applications of hydrogel matrices with viability monitoring
Hydrogel matrices are increasingly used in biomedical applications where cell viability monitoring is critical. These applications include tissue engineering, wound healing, drug delivery systems, and regenerative medicine. The hydrogels provide structural support while allowing for the assessment of therapeutic efficacy through various viability metrics. Advanced formulations incorporate bioactive components that enhance cell survival and function, with real-time monitoring capabilities to track treatment outcomes and cellular responses over time.Expand Specific Solutions03 Novel hydrogel formulations with enhanced cell viability properties
Innovative hydrogel formulations have been developed to enhance cell viability and functionality. These formulations incorporate specific polymers, crosslinking agents, and bioactive molecules that create optimal microenvironments for cells. Some formulations include temperature-responsive or pH-sensitive components that allow for controlled gelation and cell encapsulation. The viability metrics for these novel hydrogels often show improved cell survival rates, better nutrient exchange, and enhanced cellular function compared to conventional matrices.Expand Specific Solutions04 Analytical methods for assessing cell viability in hydrogel matrices
Various analytical techniques have been developed to assess cell viability within hydrogel matrices. These methods include live/dead staining, metabolic activity assays (such as MTT and Alamar Blue), confocal microscopy for spatial distribution analysis, and molecular techniques for gene expression profiling. Advanced imaging technologies allow for non-destructive, real-time monitoring of cell behavior within three-dimensional hydrogel environments. These analytical approaches provide comprehensive viability metrics that evaluate multiple aspects of cellular health and function.Expand Specific Solutions05 Hydrogel matrices for long-term cell culture and viability maintenance
Specialized hydrogel matrices have been designed for long-term cell culture applications where maintaining viability over extended periods is crucial. These matrices incorporate sustained-release systems for growth factors, optimized porosity for nutrient diffusion, and degradation kinetics matched to tissue regeneration rates. The viability metrics for these systems focus on long-term survival indicators, functional maintenance of specialized cell types, and preservation of phenotypic characteristics. These hydrogels are particularly valuable for chronic disease modeling, tissue engineering, and transplantation applications.Expand Specific Solutions
Leading Research Groups and Companies in Organoid Culture Field
The organoid culture hydrogel matrices market is in a growth phase, characterized by increasing adoption across research institutions and biotechnology companies. The global market size is expanding rapidly, driven by rising applications in disease modeling, drug discovery, and personalized medicine. Technologically, the field shows moderate maturity with established protocols but continuous innovation. Key players demonstrate varying levels of specialization: Thewell Bioscience and Cell Microsystems focus on advanced hydrogel systems and single-cell isolation technologies, while academic institutions like MIT, EPFL, and University of Pennsylvania contribute fundamental research. Companies like CYTOO and Chuangxin International Biotechnology are developing specialized applications for high-content screening and regenerative medicine. The competitive landscape features collaboration between academic research centers and commercial entities, with increasing focus on standardization of viability metrics and protocol optimization.
École Polytechnique Fédérale de Lausanne
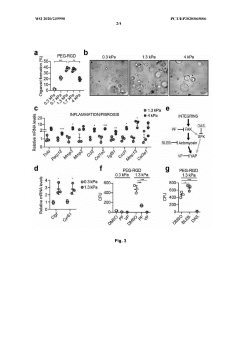
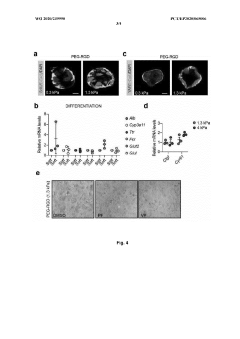
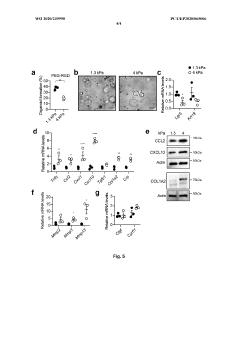
Technical Solution: EPFL has pioneered innovative hydrogel systems for organoid culture based on enzymatically crosslinked polyethylene glycol (PEG) hydrogels. Their approach utilizes factor XIII-catalyzed crosslinking of PEG precursors functionalized with glutamine and lysine peptide substrates, creating physiologically relevant matrices under cell-compatible conditions. EPFL researchers have developed protocols for incorporating various bioactive components, including cell-adhesive peptides (RGD, IKVAV), growth factor-binding domains, and enzymatically degradable crosslinks to create tissue-specific microenvironments. Their system allows precise control of matrix stiffness (0.1-100 kPa) and porosity through adjustment of polymer concentration and crosslinking density. EPFL has established comprehensive viability assessment methods including confocal microscopy with fluorescent viability indicators, automated image analysis for quantifying organoid formation efficiency and morphology, and advanced techniques such as Raman spectroscopy for non-invasive metabolic monitoring. Their protocols detail methods for creating gradient hydrogels with spatially varying mechanical and biochemical properties to study organoid development under heterogeneous conditions, and include specialized techniques for recovering organoids from hydrogels for downstream analysis.
Strengths: Physiologically relevant enzymatic crosslinking; excellent biocompatibility; highly customizable with multiple bioactive components; well-established protocols for various organoid types. Weaknesses: Requires specialized reagents and expertise for hydrogel synthesis; enzymatic crosslinking can be sensitive to environmental conditions; more complex to implement than commercial alternatives.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced hydrogel matrices for organoid culture that utilize tunable synthetic hydrogels with precisely controlled mechanical properties and biochemical compositions. Their approach employs poly(ethylene glycol) (PEG)-based hydrogels modified with cell-adhesive peptides and enzymatically degradable crosslinks to create microenvironments that support organoid growth and differentiation. The system allows independent control of matrix stiffness (ranging from 0.5-50 kPa) and ligand density, enabling researchers to study mechanotransduction effects on organoid development. MIT researchers have also pioneered methods to incorporate dynamic mechanical cues through photodegradable or photoactivatable crosslinks that can be manipulated in real-time during culture. Their protocols include detailed viability assessment using live/dead staining, metabolic activity assays (MTT/MTS), and advanced imaging techniques to quantify organoid formation efficiency and functional maturation.
Strengths: Highly customizable matrices with precise control over mechanical and biochemical properties; ability to introduce dynamic mechanical changes during culture; comprehensive viability metrics. Weaknesses: Requires specialized equipment for photochemical modifications; synthetic systems may lack some complex interactions present in natural matrices; higher technical expertise needed for implementation.
Key Innovations in Hydrogel Matrix Composition and Properties
Hydrogel for stem cell and organoid culture
PatentPendingUS20200239824A1
Innovation
- The use of soft polysaccharide hydrogels with shear-thinning and self-healing properties, capable of conversion to hard polysaccharide hydrogels, which are suitable for injection and exhibit a homogenous matrix structure, allowing for controlled experimental conditions and in vivo applications, incorporating functional ligands and bioactive molecules for enhanced cell culture and organoid formation.
Three dimensional models of tissue fibrosis
PatentWO2020239990A1
Innovation
- A method using chemically defined, mechanically tunable hydrogels with a shear modulus of at least 4 kPa to culture liver organoids, mimicking the stiffness of fibrotic liver tissue, which allows for the formation of three-dimensional cellular models of liver fibrosis and facilitates the screening of compounds for therapeutic efficacy.
Standardization of Organoid Viability Assessment Methods
The standardization of organoid viability assessment methods represents a critical challenge in the field of organoid culture using hydrogel matrices. Currently, the lack of universally accepted protocols for evaluating organoid viability hampers cross-laboratory comparisons and slows scientific progress in this rapidly evolving field.
Various methodologies exist for assessing organoid viability, each with distinct advantages and limitations. Metabolic assays such as MTT, XTT, and ATP-based luminescence tests measure cellular metabolic activity as a proxy for viability. These methods offer quantitative results but may not accurately reflect the functional status of complex three-dimensional organoid structures within hydrogels.
Membrane integrity assays, including trypan blue exclusion, propidium iodide staining, and LIVE/DEAD viability kits, evaluate cell membrane integrity to distinguish between viable and non-viable cells. While these techniques provide visual confirmation of viability, they often require optimization for penetration through hydrogel matrices, which can affect result reliability.
Functional assays that measure specific organoid activities (e.g., hormone secretion, enzyme production) offer more physiologically relevant viability metrics but are typically tissue-specific and challenging to standardize across different organoid types. This creates significant barriers to establishing universal viability assessment protocols.
Image-based analysis methods utilizing confocal microscopy and advanced computational algorithms have emerged as promising approaches for non-destructive, longitudinal viability assessment. These techniques can quantify organoid growth, morphology, and cellular organization within hydrogels, though they require sophisticated equipment and expertise.
The scientific community increasingly recognizes the need for standardized reporting of multiple complementary viability metrics. A comprehensive assessment approach should include: (1) metabolic activity measurements, (2) membrane integrity evaluation, (3) functional assays relevant to the specific organoid type, and (4) morphological analysis through imaging.
Efforts toward standardization should focus on establishing minimum reporting requirements for organoid viability studies, including detailed documentation of hydrogel composition, culture conditions, assessment timepoints, and technical parameters of viability assays. International collaborative initiatives involving academic institutions, industry partners, and regulatory bodies are essential to develop consensus guidelines.
The development of reference materials and positive/negative controls specifically designed for organoid viability assessment in hydrogels would significantly advance standardization efforts. These materials could serve as calibration tools across different laboratories and methodologies, enhancing result comparability and reproducibility in the field.
Various methodologies exist for assessing organoid viability, each with distinct advantages and limitations. Metabolic assays such as MTT, XTT, and ATP-based luminescence tests measure cellular metabolic activity as a proxy for viability. These methods offer quantitative results but may not accurately reflect the functional status of complex three-dimensional organoid structures within hydrogels.
Membrane integrity assays, including trypan blue exclusion, propidium iodide staining, and LIVE/DEAD viability kits, evaluate cell membrane integrity to distinguish between viable and non-viable cells. While these techniques provide visual confirmation of viability, they often require optimization for penetration through hydrogel matrices, which can affect result reliability.
Functional assays that measure specific organoid activities (e.g., hormone secretion, enzyme production) offer more physiologically relevant viability metrics but are typically tissue-specific and challenging to standardize across different organoid types. This creates significant barriers to establishing universal viability assessment protocols.
Image-based analysis methods utilizing confocal microscopy and advanced computational algorithms have emerged as promising approaches for non-destructive, longitudinal viability assessment. These techniques can quantify organoid growth, morphology, and cellular organization within hydrogels, though they require sophisticated equipment and expertise.
The scientific community increasingly recognizes the need for standardized reporting of multiple complementary viability metrics. A comprehensive assessment approach should include: (1) metabolic activity measurements, (2) membrane integrity evaluation, (3) functional assays relevant to the specific organoid type, and (4) morphological analysis through imaging.
Efforts toward standardization should focus on establishing minimum reporting requirements for organoid viability studies, including detailed documentation of hydrogel composition, culture conditions, assessment timepoints, and technical parameters of viability assays. International collaborative initiatives involving academic institutions, industry partners, and regulatory bodies are essential to develop consensus guidelines.
The development of reference materials and positive/negative controls specifically designed for organoid viability assessment in hydrogels would significantly advance standardization efforts. These materials could serve as calibration tools across different laboratories and methodologies, enhancing result comparability and reproducibility in the field.
Ethical and Regulatory Considerations in Organoid Research
The rapid advancement of organoid technology using hydrogel matrices raises significant ethical and regulatory considerations that must be addressed to ensure responsible research and application. Organoids, as three-dimensional cellular structures that mimic organ functionality, blur traditional boundaries between cell cultures and complete organs, creating novel ethical challenges.
Patient consent and ownership of biological materials represent primary ethical concerns. When patient-derived cells are used to create organoids, questions arise regarding informed consent scope, especially for future research applications not initially specified. The potential commercialization of patient-derived organoids further complicates ownership rights and benefit-sharing arrangements.
Privacy considerations become increasingly relevant as organoids derived from specific individuals may contain genetic information that could potentially identify the donor. This raises questions about data protection standards and confidentiality measures necessary when storing, analyzing, and sharing organoid-related information across research institutions.
The development of cerebral organoids presents particularly complex ethical questions. These brain-like structures, while lacking consciousness, raise philosophical and ethical debates about the moral status of increasingly complex neural organoids. Clear guidelines are needed regarding the acceptable complexity levels for cerebral organoid research.
From a regulatory perspective, organoid research exists in a relatively undefined space. Current frameworks for cell cultures and animal testing may not adequately address the unique characteristics of organoids. Regulatory bodies worldwide are working to develop appropriate oversight mechanisms, but significant gaps remain in standardization.
Good Laboratory Practice (GLP) standards for organoid culture using hydrogel matrices require adaptation to ensure consistency and reproducibility. This includes establishing protocols for quality control, documentation requirements, and validation methods specific to hydrogel-based organoid systems.
International harmonization of regulations presents another challenge, as different jurisdictions apply varying standards to organoid research. This regulatory fragmentation complicates multi-center studies and technology transfer, potentially slowing scientific progress and clinical applications.
Looking forward, the field requires development of comprehensive ethical frameworks and regulatory guidelines specifically tailored to organoid research using hydrogel matrices. These should balance scientific advancement with ethical considerations while providing clear direction for researchers, clinicians, and industry stakeholders.
Patient consent and ownership of biological materials represent primary ethical concerns. When patient-derived cells are used to create organoids, questions arise regarding informed consent scope, especially for future research applications not initially specified. The potential commercialization of patient-derived organoids further complicates ownership rights and benefit-sharing arrangements.
Privacy considerations become increasingly relevant as organoids derived from specific individuals may contain genetic information that could potentially identify the donor. This raises questions about data protection standards and confidentiality measures necessary when storing, analyzing, and sharing organoid-related information across research institutions.
The development of cerebral organoids presents particularly complex ethical questions. These brain-like structures, while lacking consciousness, raise philosophical and ethical debates about the moral status of increasingly complex neural organoids. Clear guidelines are needed regarding the acceptable complexity levels for cerebral organoid research.
From a regulatory perspective, organoid research exists in a relatively undefined space. Current frameworks for cell cultures and animal testing may not adequately address the unique characteristics of organoids. Regulatory bodies worldwide are working to develop appropriate oversight mechanisms, but significant gaps remain in standardization.
Good Laboratory Practice (GLP) standards for organoid culture using hydrogel matrices require adaptation to ensure consistency and reproducibility. This includes establishing protocols for quality control, documentation requirements, and validation methods specific to hydrogel-based organoid systems.
International harmonization of regulations presents another challenge, as different jurisdictions apply varying standards to organoid research. This regulatory fragmentation complicates multi-center studies and technology transfer, potentially slowing scientific progress and clinical applications.
Looking forward, the field requires development of comprehensive ethical frameworks and regulatory guidelines specifically tailored to organoid research using hydrogel matrices. These should balance scientific advancement with ethical considerations while providing clear direction for researchers, clinicians, and industry stakeholders.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!