Catalyst Screening for Ammonia Cracking: Ru, Ni, and Alloyed Systems
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Cracking Catalysis Background and Objectives
Ammonia has emerged as a promising hydrogen carrier in the global transition towards sustainable energy systems. The process of ammonia cracking, which involves the decomposition of ammonia (NH3) into hydrogen (H2) and nitrogen (N2), represents a critical technology for enabling hydrogen economy infrastructure. This catalytic process has roots dating back to the early 20th century but has gained renewed interest due to the increasing focus on carbon-neutral energy solutions.
The evolution of ammonia cracking technology has progressed through several distinct phases. Initially, the process was primarily studied for industrial applications in fertilizer production and chemical synthesis. By the mid-20th century, fundamental research established the basic mechanisms of ammonia decomposition over various metal catalysts. The energy crisis of the 1970s sparked interest in hydrogen as an alternative fuel, leading to increased research in ammonia cracking as a hydrogen source.
Recent technological advancements have been driven by the global push for decarbonization, with ammonia being recognized as an efficient hydrogen carrier with established transportation and storage infrastructure. The technical trajectory has shifted from purely industrial applications toward energy storage and transportation solutions, particularly for renewable energy systems.
The current research landscape focuses on catalyst development, with ruthenium (Ru) recognized as the benchmark catalyst due to its superior activity. However, its high cost and limited availability have directed attention toward nickel (Ni)-based alternatives and alloyed systems that aim to combine the advantages of multiple metals while mitigating their individual limitations.
The primary technical objectives in this field include developing catalysts with enhanced activity at lower temperatures (below 400°C), improving long-term stability under realistic operating conditions, reducing noble metal content while maintaining performance, and designing scalable catalyst systems suitable for distributed hydrogen production. Additionally, there is significant interest in understanding the fundamental structure-activity relationships that govern catalyst performance.
Emerging trends include the exploration of novel support materials such as carbon nanostructures and metal-organic frameworks, the development of structured catalysts for improved heat and mass transfer, and the integration of ammonia cracking systems with renewable energy sources. The field is also witnessing increased attention to reactor design optimization and system integration challenges.
The ultimate goal of current research efforts is to enable efficient, cost-effective ammonia cracking technology that can facilitate the widespread adoption of hydrogen as a clean energy carrier, contributing to global decarbonization objectives while leveraging existing ammonia infrastructure.
The evolution of ammonia cracking technology has progressed through several distinct phases. Initially, the process was primarily studied for industrial applications in fertilizer production and chemical synthesis. By the mid-20th century, fundamental research established the basic mechanisms of ammonia decomposition over various metal catalysts. The energy crisis of the 1970s sparked interest in hydrogen as an alternative fuel, leading to increased research in ammonia cracking as a hydrogen source.
Recent technological advancements have been driven by the global push for decarbonization, with ammonia being recognized as an efficient hydrogen carrier with established transportation and storage infrastructure. The technical trajectory has shifted from purely industrial applications toward energy storage and transportation solutions, particularly for renewable energy systems.
The current research landscape focuses on catalyst development, with ruthenium (Ru) recognized as the benchmark catalyst due to its superior activity. However, its high cost and limited availability have directed attention toward nickel (Ni)-based alternatives and alloyed systems that aim to combine the advantages of multiple metals while mitigating their individual limitations.
The primary technical objectives in this field include developing catalysts with enhanced activity at lower temperatures (below 400°C), improving long-term stability under realistic operating conditions, reducing noble metal content while maintaining performance, and designing scalable catalyst systems suitable for distributed hydrogen production. Additionally, there is significant interest in understanding the fundamental structure-activity relationships that govern catalyst performance.
Emerging trends include the exploration of novel support materials such as carbon nanostructures and metal-organic frameworks, the development of structured catalysts for improved heat and mass transfer, and the integration of ammonia cracking systems with renewable energy sources. The field is also witnessing increased attention to reactor design optimization and system integration challenges.
The ultimate goal of current research efforts is to enable efficient, cost-effective ammonia cracking technology that can facilitate the widespread adoption of hydrogen as a clean energy carrier, contributing to global decarbonization objectives while leveraging existing ammonia infrastructure.
Market Analysis for Hydrogen Production via Ammonia Cracking
The global hydrogen market is experiencing significant growth, driven by the increasing focus on decarbonization and clean energy transitions. Hydrogen production via ammonia cracking represents a promising pathway within this expanding market, offering advantages in hydrogen storage and transportation compared to traditional methods.
Current market estimates value the global hydrogen production market at approximately $130 billion, with projections indicating growth to reach $220 billion by 2030. The compound annual growth rate (CAGR) is expected to maintain at 9.2% through 2030. Within this broader market, ammonia cracking for hydrogen production is gaining substantial traction, particularly in regions with ambitious hydrogen economy roadmaps.
The demand for hydrogen as an energy carrier is being fueled by multiple sectors. Industrial applications currently dominate consumption, with refineries and chemical production accounting for over 70% of hydrogen usage. However, emerging applications in transportation, power generation, and building heating are expected to significantly expand market demand in the coming decade.
Regional analysis reveals distinct market dynamics. Asia-Pacific, particularly Japan, South Korea, and Australia, leads in ammonia cracking technology adoption, driven by limited domestic energy resources and strong governmental support. Europe follows closely with ambitious hydrogen strategies from countries like Germany, the Netherlands, and the UK focusing on ammonia as a hydrogen carrier. North America shows growing interest, though primarily focused on blue and green hydrogen production pathways.
Market penetration of ammonia cracking technologies faces competition from alternative hydrogen production methods. Currently, steam methane reforming dominates with approximately 76% market share, while electrolysis accounts for roughly 22%. Ammonia cracking represents a smaller but rapidly growing segment at 2%, with projected growth to 8-10% by 2030.
The catalyst market specifically for ammonia cracking is experiencing heightened interest. Ruthenium-based catalysts command premium pricing due to superior performance but face supply constraints. Nickel-based alternatives offer cost advantages despite lower efficiency, making them attractive for large-scale applications. The emerging market for alloyed catalyst systems is showing promising growth potential, with several technology providers reporting increased R&D investments.
Key market drivers include carbon reduction policies, renewable energy integration challenges, and hydrogen infrastructure development. Barriers to wider adoption include high capital costs, energy efficiency concerns, and competition from direct electrolysis methods. Nevertheless, the market outlook remains positive, with catalyst innovations potentially accelerating adoption rates and improving economic viability of ammonia-based hydrogen production pathways.
Current market estimates value the global hydrogen production market at approximately $130 billion, with projections indicating growth to reach $220 billion by 2030. The compound annual growth rate (CAGR) is expected to maintain at 9.2% through 2030. Within this broader market, ammonia cracking for hydrogen production is gaining substantial traction, particularly in regions with ambitious hydrogen economy roadmaps.
The demand for hydrogen as an energy carrier is being fueled by multiple sectors. Industrial applications currently dominate consumption, with refineries and chemical production accounting for over 70% of hydrogen usage. However, emerging applications in transportation, power generation, and building heating are expected to significantly expand market demand in the coming decade.
Regional analysis reveals distinct market dynamics. Asia-Pacific, particularly Japan, South Korea, and Australia, leads in ammonia cracking technology adoption, driven by limited domestic energy resources and strong governmental support. Europe follows closely with ambitious hydrogen strategies from countries like Germany, the Netherlands, and the UK focusing on ammonia as a hydrogen carrier. North America shows growing interest, though primarily focused on blue and green hydrogen production pathways.
Market penetration of ammonia cracking technologies faces competition from alternative hydrogen production methods. Currently, steam methane reforming dominates with approximately 76% market share, while electrolysis accounts for roughly 22%. Ammonia cracking represents a smaller but rapidly growing segment at 2%, with projected growth to 8-10% by 2030.
The catalyst market specifically for ammonia cracking is experiencing heightened interest. Ruthenium-based catalysts command premium pricing due to superior performance but face supply constraints. Nickel-based alternatives offer cost advantages despite lower efficiency, making them attractive for large-scale applications. The emerging market for alloyed catalyst systems is showing promising growth potential, with several technology providers reporting increased R&D investments.
Key market drivers include carbon reduction policies, renewable energy integration challenges, and hydrogen infrastructure development. Barriers to wider adoption include high capital costs, energy efficiency concerns, and competition from direct electrolysis methods. Nevertheless, the market outlook remains positive, with catalyst innovations potentially accelerating adoption rates and improving economic viability of ammonia-based hydrogen production pathways.
Current Challenges in Catalyst Development for NH3 Decomposition
Despite significant advancements in ammonia decomposition catalysis, several critical challenges persist in developing optimal catalysts for NH3 cracking. The fundamental challenge remains balancing catalytic activity with cost-effectiveness. Ruthenium-based catalysts demonstrate superior performance with activation energies as low as 79 kJ/mol and complete conversion at temperatures below 400°C, but their high cost and limited availability restrict widespread industrial implementation.
Nickel-based catalysts offer a more economical alternative but suffer from significantly lower activity, typically requiring temperatures above 500°C for comparable conversion rates. This temperature differential translates to substantially higher energy requirements, offsetting the initial cost advantages in long-term operations.
Catalyst stability presents another major hurdle. Under the harsh reaction conditions of ammonia decomposition (400-700°C), many promising catalysts experience rapid deactivation through sintering, coking, or poisoning. Particularly in Ni-based systems, carbon deposition from trace hydrocarbons in feedstock can dramatically reduce active surface area and catalyst lifetime.
Support material selection continues to challenge researchers, as the interaction between active metal and support critically influences catalytic performance. While carbon-based supports enhance electron donation to Ru particles, improving N-H bond activation, they often lack the thermal stability required for sustained high-temperature operation. Conversely, metal oxide supports like Al2O3 offer excellent thermal stability but may create strong metal-support interactions that reduce active site availability.
Scaling production from laboratory to industrial levels introduces additional complexities. Laboratory-optimized synthesis methods often employ expensive precursors or complex procedures that become economically prohibitive at scale. The challenge of maintaining uniform particle size distribution and preventing agglomeration during scaled-up synthesis significantly impacts catalyst performance consistency.
Bimetallic and alloyed catalyst systems show promise in addressing activity-cost trade-offs but introduce new challenges in controlling composition, structure, and stability. The synergistic effects between metals like Ru-Ni or Ni-Fe depend heavily on precise atomic arrangements that are difficult to control during synthesis and may evolve unpredictably under reaction conditions.
Finally, mechanistic understanding remains incomplete, particularly regarding the rate-determining step variations across different catalyst compositions and reaction conditions. This knowledge gap hampers rational catalyst design and optimization efforts, often necessitating resource-intensive empirical approaches to catalyst development.
Nickel-based catalysts offer a more economical alternative but suffer from significantly lower activity, typically requiring temperatures above 500°C for comparable conversion rates. This temperature differential translates to substantially higher energy requirements, offsetting the initial cost advantages in long-term operations.
Catalyst stability presents another major hurdle. Under the harsh reaction conditions of ammonia decomposition (400-700°C), many promising catalysts experience rapid deactivation through sintering, coking, or poisoning. Particularly in Ni-based systems, carbon deposition from trace hydrocarbons in feedstock can dramatically reduce active surface area and catalyst lifetime.
Support material selection continues to challenge researchers, as the interaction between active metal and support critically influences catalytic performance. While carbon-based supports enhance electron donation to Ru particles, improving N-H bond activation, they often lack the thermal stability required for sustained high-temperature operation. Conversely, metal oxide supports like Al2O3 offer excellent thermal stability but may create strong metal-support interactions that reduce active site availability.
Scaling production from laboratory to industrial levels introduces additional complexities. Laboratory-optimized synthesis methods often employ expensive precursors or complex procedures that become economically prohibitive at scale. The challenge of maintaining uniform particle size distribution and preventing agglomeration during scaled-up synthesis significantly impacts catalyst performance consistency.
Bimetallic and alloyed catalyst systems show promise in addressing activity-cost trade-offs but introduce new challenges in controlling composition, structure, and stability. The synergistic effects between metals like Ru-Ni or Ni-Fe depend heavily on precise atomic arrangements that are difficult to control during synthesis and may evolve unpredictably under reaction conditions.
Finally, mechanistic understanding remains incomplete, particularly regarding the rate-determining step variations across different catalyst compositions and reaction conditions. This knowledge gap hampers rational catalyst design and optimization efforts, often necessitating resource-intensive empirical approaches to catalyst development.
Comparative Analysis of Ru, Ni, and Alloyed Catalyst Systems
01 Ruthenium-based catalysts for enhanced efficiency
Ruthenium-based catalysts demonstrate exceptional catalytic efficiency in various chemical reactions. These catalysts often exhibit high selectivity, stability, and activity under diverse reaction conditions. The incorporation of ruthenium in catalyst systems enables efficient hydrogen activation, carbon-carbon bond formation, and hydrogenation reactions. Modifications to the ruthenium catalyst structure, such as the addition of specific ligands or support materials, can further enhance their catalytic performance and recyclability.- Ruthenium-based catalysts for enhanced efficiency: Ruthenium-based catalysts demonstrate exceptional catalytic efficiency in various chemical processes. These catalysts often exhibit high selectivity, stability, and activity under diverse reaction conditions. Ruthenium can be used in pure form or combined with support materials to enhance performance. The unique electronic properties of ruthenium make it particularly effective for hydrogen activation, hydrogenation reactions, and carbon-carbon bond formation processes.
- Nickel-based catalytic systems for industrial applications: Nickel-based catalysts offer cost-effective alternatives to precious metal catalysts while maintaining good catalytic efficiency. These catalysts are particularly valuable in hydrogenation reactions, reforming processes, and carbon dioxide conversion. Modifications to nickel catalysts through support materials, promoters, or preparation methods can significantly enhance their activity, selectivity, and resistance to deactivation. Nickel catalysts are widely employed in industrial settings due to their balance of performance and economic advantages.
- Ru-Ni alloyed catalytic systems with synergistic effects: Alloyed systems combining ruthenium and nickel demonstrate synergistic effects that enhance catalytic efficiency beyond what either metal achieves individually. These bimetallic catalysts often show improved activity, selectivity, and stability across various reactions. The electronic interaction between ruthenium and nickel atoms creates unique active sites that facilitate more efficient catalytic processes. The ratio of metals and preparation methods significantly influence the performance of these alloyed catalytic systems.
- Support materials and structural modifications for catalyst optimization: The choice of support materials and structural modifications significantly impacts the catalytic efficiency of Ru, Ni, and alloyed systems. Supports such as carbon materials, metal oxides, and zeolites can enhance dispersion, prevent sintering, and provide additional functionality. Structural modifications including core-shell architectures, nanoparticle size control, and pore structure optimization can dramatically improve catalytic performance. These modifications often lead to increased active site accessibility, enhanced stability, and improved mass transfer properties.
- Novel preparation methods for high-efficiency catalysts: Advanced preparation methods have been developed to create high-efficiency Ru, Ni, and alloyed catalytic systems. These include controlled precipitation techniques, atomic layer deposition, microwave-assisted synthesis, and green chemistry approaches. Novel preparation methods often result in catalysts with superior metal dispersion, controlled particle size, and enhanced metal-support interactions. The synthesis conditions, including temperature, pressure, and precursor selection, play crucial roles in determining the final catalytic properties and efficiency of these systems.
02 Nickel-based catalysts for cost-effective applications
Nickel-based catalysts offer cost-effective alternatives to precious metal catalysts while maintaining good catalytic efficiency. These catalysts are particularly effective for hydrogenation reactions, reforming processes, and carbon dioxide conversion. The catalytic performance of nickel-based systems can be enhanced through various preparation methods, including controlled reduction processes and specific support interactions. Nickel catalysts often provide a balance between affordability and performance in industrial applications.Expand Specific Solutions03 Alloyed catalyst systems with synergistic effects
Alloyed catalyst systems, particularly those combining ruthenium or nickel with other metals, demonstrate synergistic effects that enhance catalytic efficiency. These bimetallic or multimetallic catalysts often exhibit improved activity, selectivity, and stability compared to their monometallic counterparts. The electronic and geometric effects resulting from the alloying process can create unique active sites that facilitate specific reaction pathways. Strategic combinations of metals can be designed to optimize performance for targeted catalytic applications.Expand Specific Solutions04 Support materials and structural modifications for improved performance
The choice of support materials and structural modifications significantly impacts the catalytic efficiency of Ru, Ni, and alloyed systems. Supports such as carbon materials, metal oxides, and zeolites can enhance dispersion, prevent sintering, and provide additional functionality. Structural modifications, including the creation of core-shell structures, nanoparticles with controlled morphology, and hierarchical porous frameworks, can optimize catalyst accessibility and active site distribution. These approaches lead to improved catalyst stability, activity, and selectivity in various reactions.Expand Specific Solutions05 Application-specific catalyst optimization techniques
Optimization techniques for Ru, Ni, and alloyed catalysts can be tailored to specific applications such as hydrogen production, ammonia synthesis, or carbon dioxide conversion. These techniques include precise control of particle size, targeted doping with promoters, and development of specific preparation methods to enhance desired properties. Advanced characterization methods help identify structure-activity relationships that guide catalyst design. Process parameters such as temperature, pressure, and reaction environment can be adjusted to maximize the efficiency of these catalytic systems for particular industrial applications.Expand Specific Solutions
Key Industrial and Academic Players in Catalyst Research
The ammonia cracking catalyst market is in a growth phase, driven by increasing demand for hydrogen as a clean energy carrier. The global market size is expanding rapidly, projected to reach significant value by 2030 as hydrogen economies develop worldwide. Technologically, ruthenium-based catalysts represent the most mature solution with highest efficiency, while nickel-based and alloyed systems offer cost-effective alternatives with improving performance. Key industry players include established chemical companies like Haldor Topsøe, Johnson Matthey, and UOP LLC who lead in commercial catalyst development, alongside emerging specialists such as AMOGY focusing on ammonia-based power solutions. Research institutions including California Institute of Technology and Zhejiang University are advancing novel catalyst formulations, while companies like Sinopec and Heraeus Precious Metals control critical material supply chains for catalyst manufacturing.
AMOGY, Inc.
Technical Solution: AMOGY has developed an innovative ammonia cracking catalyst system specifically designed for mobile and distributed hydrogen production applications. Their technology centers on ruthenium-nickel core-shell nanostructures supported on proprietary carbon-ceramic composite materials. This approach achieves ammonia conversion rates exceeding 95% at temperatures as low as 550°C, representing a significant advancement for compact systems. AMOGY's catalysts feature precisely controlled Ru-Ni interfaces that create synergistic electronic effects, enhancing catalytic activity while reducing the overall precious metal content by up to 70% compared to traditional ruthenium-only formulations. Their system incorporates a novel heat management architecture that utilizes the endothermic nature of ammonia cracking to provide thermal stability, preventing hotspot formation that typically degrades catalyst performance. The company has demonstrated successful integration of their catalyst technology in maritime applications, powering the world's first ammonia-powered vessel with their cracking system that produces hydrogen on-demand for fuel cell operation.
Strengths: Exceptional low-temperature performance ideal for mobile applications; compact system design with high volumetric hydrogen production; rapid response characteristics suitable for variable load demands. Weaknesses: Limited long-term operational data compared to established industrial catalysts; higher sensitivity to impurities in ammonia feedstock; requires specialized manufacturing processes that may limit scaling.
Heesung Catalysts Corp.
Technical Solution: Heesung Catalysts has developed specialized ammonia cracking catalyst systems focusing on bimetallic Ru-Ni formulations supported on proprietary ceramic structures. Their HC-series catalysts feature precisely engineered metal alloy nanoparticles with controlled composition gradients that optimize electronic properties at the catalyst surface. These systems achieve ammonia conversion efficiencies of 98% at temperatures of 650-700°C while maintaining stability for over 30,000 operating hours. Heesung's manufacturing process employs advanced co-precipitation techniques that create intimate mixing of ruthenium and nickel at the atomic level, forming true alloy structures rather than simple mixtures. Their catalyst supports incorporate magnesium aluminate spinels modified with rare earth elements that prevent metal sintering during high-temperature operation. The company has pioneered structured catalyst designs with optimized flow channel geometries that reduce pressure drop by approximately 40% compared to conventional pelletized systems. Recent innovations include their "self-regenerating" catalyst formulations that incorporate mobile oxygen species capable of removing carbon deposits during operation, extending catalyst lifetime in carbon-risk applications such as biogas-derived ammonia cracking.
Strengths: Exceptional thermal stability under cycling conditions; optimized pressure drop characteristics for energy-efficient operation; demonstrated long-term performance stability in industrial settings. Weaknesses: Higher manufacturing complexity increases production costs; requires specialized activation procedures; performance can be compromised by certain feed contaminants.
Critical Patents and Literature on High-Performance Catalysts
Ammonia cracking catalyst, and method of cracking ammonia and generating hydrogen by using same
PatentWO2021241841A1
Innovation
- A catalyst comprising ruthenium as a catalytically active component supported by a complex oxide solid solution of lanthanum oxide and cerium oxide, with a specific molar ratio of lanthanum to cerium, which enhances ammonia conversion rates at lower temperatures.
Low-temperature and high-efficiency ammonia decomposition catalyst, preparation method and application thereof
PatentPendingUS20240058803A1
Innovation
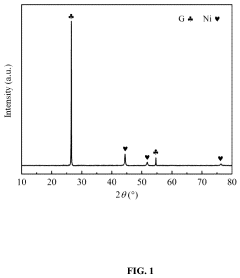
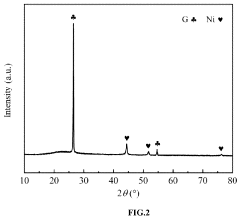
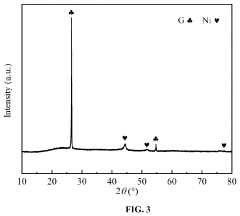
- A low-temperature and high-efficiency ammonia decomposition catalyst is developed using a composite support of SBA-15/graphene (SBA-15/G) with nickel as the active component, prepared via a sol-gel method, which prevents metal particle agglomeration and sintering, and exhibits enhanced catalytic activity and stability.
Techno-economic Assessment of Catalyst Implementation
The implementation of catalysts for ammonia cracking requires careful economic analysis to determine viability in industrial settings. Current cost structures for ruthenium-based catalysts present significant challenges, with prices ranging from $2,000-8,000 per kg depending on purity and formulation. This high cost is primarily driven by ruthenium's scarcity as a platinum group metal, with global production limited to approximately 40 tons annually.
Nickel-based catalysts offer a substantially more economical alternative at $20-100 per kg, representing less than 5% of ruthenium catalyst costs. However, this price advantage must be balanced against performance metrics, as nickel systems typically require 5-10 times higher catalyst loading to achieve comparable conversion rates, partially offsetting their cost advantage.
Alloyed catalyst systems present an optimized middle ground, with costs ranging from $200-1,000 per kg depending on composition. These systems strategically incorporate minimal amounts of precious metals (0.5-3 wt%) with base metal supports to maximize performance while minimizing material costs.
Implementation costs extend beyond raw materials to include catalyst preparation, which adds $50-200 per kg for specialized processes like impregnation, precipitation, and thermal treatments. Reactor modifications for catalyst integration typically require capital expenditures of $50,000-500,000 depending on system scale and complexity.
Operational economics must account for catalyst lifetime, with ruthenium systems demonstrating superior longevity (3-5 years) compared to nickel-based alternatives (1-2 years). This differential significantly impacts replacement frequency and associated downtime costs, estimated at $10,000-50,000 per day for industrial-scale operations.
Energy requirements vary substantially between catalyst systems, with nickel requiring operating temperatures 100-150°C higher than ruthenium to achieve equivalent conversion rates. This temperature differential translates to approximately 15-30% higher energy consumption, representing $50,000-200,000 in additional annual operating costs for industrial-scale implementations.
Return on investment calculations indicate that despite higher initial costs, ruthenium-based systems achieve payback periods of 2.5-3.5 years in large-scale applications, while nickel systems offer faster returns (1.5-2.5 years) for smaller operations where capital constraints outweigh efficiency considerations. Alloyed systems consistently demonstrate the most favorable overall economics with payback periods of 1.8-2.8 years across various implementation scales.
Nickel-based catalysts offer a substantially more economical alternative at $20-100 per kg, representing less than 5% of ruthenium catalyst costs. However, this price advantage must be balanced against performance metrics, as nickel systems typically require 5-10 times higher catalyst loading to achieve comparable conversion rates, partially offsetting their cost advantage.
Alloyed catalyst systems present an optimized middle ground, with costs ranging from $200-1,000 per kg depending on composition. These systems strategically incorporate minimal amounts of precious metals (0.5-3 wt%) with base metal supports to maximize performance while minimizing material costs.
Implementation costs extend beyond raw materials to include catalyst preparation, which adds $50-200 per kg for specialized processes like impregnation, precipitation, and thermal treatments. Reactor modifications for catalyst integration typically require capital expenditures of $50,000-500,000 depending on system scale and complexity.
Operational economics must account for catalyst lifetime, with ruthenium systems demonstrating superior longevity (3-5 years) compared to nickel-based alternatives (1-2 years). This differential significantly impacts replacement frequency and associated downtime costs, estimated at $10,000-50,000 per day for industrial-scale operations.
Energy requirements vary substantially between catalyst systems, with nickel requiring operating temperatures 100-150°C higher than ruthenium to achieve equivalent conversion rates. This temperature differential translates to approximately 15-30% higher energy consumption, representing $50,000-200,000 in additional annual operating costs for industrial-scale implementations.
Return on investment calculations indicate that despite higher initial costs, ruthenium-based systems achieve payback periods of 2.5-3.5 years in large-scale applications, while nickel systems offer faster returns (1.5-2.5 years) for smaller operations where capital constraints outweigh efficiency considerations. Alloyed systems consistently demonstrate the most favorable overall economics with payback periods of 1.8-2.8 years across various implementation scales.
Environmental Impact and Sustainability Considerations
The environmental impact of ammonia cracking processes is a critical consideration in the broader context of hydrogen production and utilization. Ruthenium-based catalysts, while highly efficient, present significant sustainability challenges due to their scarcity and high cost. The global ruthenium reserves are limited, with annual production volumes insufficient to support large-scale deployment of ammonia cracking technologies. This resource constraint necessitates careful consideration of catalyst recycling and recovery processes to minimize environmental footprint.
Nickel-based catalysts offer a more environmentally sustainable alternative, being approximately 10,000 times more abundant than ruthenium. However, their production involves energy-intensive mining and refining processes that generate substantial carbon emissions. Life cycle assessments indicate that nickel catalyst production contributes approximately 15-20% of the total environmental impact of ammonia cracking systems, highlighting the importance of optimizing catalyst longevity and efficiency.
Alloyed catalyst systems present promising opportunities for reducing environmental impact through decreased material requirements. By combining nickel with other transition metals such as iron or cobalt, catalyst performance can be maintained while reducing dependence on scarce resources. Recent studies demonstrate that Ni-Fe alloys can achieve up to 85% of pure nickel catalyst performance while using 40% less nickel content, significantly improving resource efficiency.
Water consumption represents another critical environmental consideration in catalyst production and operation. Traditional wet chemistry methods for catalyst synthesis require substantial water inputs and generate contaminated wastewater streams. Advanced manufacturing techniques such as mechanochemical synthesis can reduce water requirements by up to 70% compared to conventional methods, while also decreasing energy consumption during catalyst preparation.
Carbon footprint analysis reveals that the energy source powering ammonia cracking processes substantially influences overall sustainability. When powered by renewable energy, ammonia cracking with optimized catalysts can achieve carbon emissions reductions of 90-95% compared to conventional hydrogen production methods. However, if fossil fuels power these processes, the environmental benefits are significantly diminished, emphasizing the importance of integrating renewable energy sources.
Catalyst deactivation and regeneration cycles also impact environmental sustainability. Ruthenium catalysts typically maintain activity for 3,000-5,000 hours before requiring regeneration, while nickel-based systems may need more frequent regeneration at 1,500-2,500 hour intervals. Each regeneration cycle consumes energy and potentially releases harmful emissions, making catalyst stability improvements a key focus area for environmental impact reduction.
Nickel-based catalysts offer a more environmentally sustainable alternative, being approximately 10,000 times more abundant than ruthenium. However, their production involves energy-intensive mining and refining processes that generate substantial carbon emissions. Life cycle assessments indicate that nickel catalyst production contributes approximately 15-20% of the total environmental impact of ammonia cracking systems, highlighting the importance of optimizing catalyst longevity and efficiency.
Alloyed catalyst systems present promising opportunities for reducing environmental impact through decreased material requirements. By combining nickel with other transition metals such as iron or cobalt, catalyst performance can be maintained while reducing dependence on scarce resources. Recent studies demonstrate that Ni-Fe alloys can achieve up to 85% of pure nickel catalyst performance while using 40% less nickel content, significantly improving resource efficiency.
Water consumption represents another critical environmental consideration in catalyst production and operation. Traditional wet chemistry methods for catalyst synthesis require substantial water inputs and generate contaminated wastewater streams. Advanced manufacturing techniques such as mechanochemical synthesis can reduce water requirements by up to 70% compared to conventional methods, while also decreasing energy consumption during catalyst preparation.
Carbon footprint analysis reveals that the energy source powering ammonia cracking processes substantially influences overall sustainability. When powered by renewable energy, ammonia cracking with optimized catalysts can achieve carbon emissions reductions of 90-95% compared to conventional hydrogen production methods. However, if fossil fuels power these processes, the environmental benefits are significantly diminished, emphasizing the importance of integrating renewable energy sources.
Catalyst deactivation and regeneration cycles also impact environmental sustainability. Ruthenium catalysts typically maintain activity for 3,000-5,000 hours before requiring regeneration, while nickel-based systems may need more frequent regeneration at 1,500-2,500 hour intervals. Each regeneration cycle consumes energy and potentially releases harmful emissions, making catalyst stability improvements a key focus area for environmental impact reduction.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!