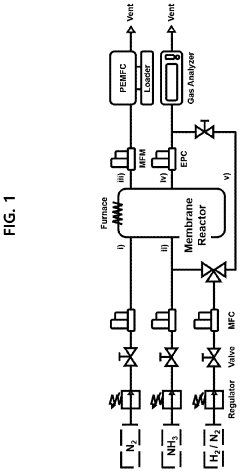
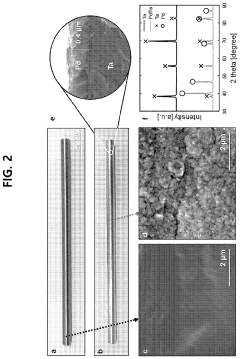
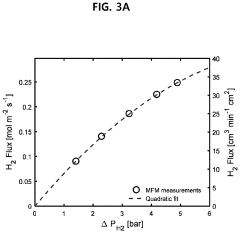
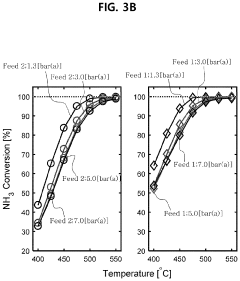
How Membrane Reactors Raise H₂ Purity in Ammonia Cracking Units
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Membrane Reactor Technology Background and Objectives
Membrane reactor technology represents a significant advancement in chemical process engineering, evolving from conventional reactor designs to integrated systems that combine reaction and separation processes. The concept emerged in the 1980s but gained substantial momentum in the early 2000s with breakthroughs in membrane materials science. This technology has progressively transformed from laboratory curiosity to industrial application, particularly in hydrogen production systems where purity requirements are stringent.
The fundamental principle behind membrane reactors involves the selective permeation of specific molecules through specialized membranes while reactions occur simultaneously. In ammonia cracking units, this approach addresses the longstanding challenge of achieving high hydrogen purity without extensive downstream purification steps. Traditional ammonia decomposition processes typically yield hydrogen contaminated with nitrogen and unreacted ammonia, necessitating complex and energy-intensive separation processes.
Historical development of membrane reactors has followed three distinct phases: initial conceptualization (1980s-1990s), material advancement (2000s-2010s), and industrial implementation (2010s-present). Each phase has contributed to overcoming critical limitations in membrane stability, selectivity, and operational longevity under harsh reaction conditions. The technology has benefited significantly from parallel advancements in nanotechnology and materials science, enabling the development of membranes with unprecedented performance characteristics.
The primary technical objective in membrane reactor development for ammonia cracking is to achieve hydrogen purity exceeding 99.99% directly from the reactor system, eliminating or substantially reducing downstream purification requirements. Secondary objectives include enhancing conversion efficiency through continuous product removal (shifting equilibrium), reducing energy consumption compared to conventional separation methods, and extending operational lifetime under industrial conditions.
Current research focuses on addressing several persistent challenges: membrane stability under high-temperature ammonia cracking conditions (typically 400-700°C), resistance to potential contaminants in feedstock, mechanical integrity under pressure differentials, and cost-effective manufacturing at industrial scale. The technology aims to bridge the gap between laboratory performance and commercial viability, particularly for applications in hydrogen fuel cells and semiconductor manufacturing where ultra-pure hydrogen is essential.
The evolution of membrane reactor technology aligns with broader trends toward process intensification and sustainable chemical processing, offering pathways to reduce energy consumption and equipment footprint while improving product quality. This technology represents a critical enabler for the hydrogen economy, supporting both centralized production facilities and distributed generation systems.
The fundamental principle behind membrane reactors involves the selective permeation of specific molecules through specialized membranes while reactions occur simultaneously. In ammonia cracking units, this approach addresses the longstanding challenge of achieving high hydrogen purity without extensive downstream purification steps. Traditional ammonia decomposition processes typically yield hydrogen contaminated with nitrogen and unreacted ammonia, necessitating complex and energy-intensive separation processes.
Historical development of membrane reactors has followed three distinct phases: initial conceptualization (1980s-1990s), material advancement (2000s-2010s), and industrial implementation (2010s-present). Each phase has contributed to overcoming critical limitations in membrane stability, selectivity, and operational longevity under harsh reaction conditions. The technology has benefited significantly from parallel advancements in nanotechnology and materials science, enabling the development of membranes with unprecedented performance characteristics.
The primary technical objective in membrane reactor development for ammonia cracking is to achieve hydrogen purity exceeding 99.99% directly from the reactor system, eliminating or substantially reducing downstream purification requirements. Secondary objectives include enhancing conversion efficiency through continuous product removal (shifting equilibrium), reducing energy consumption compared to conventional separation methods, and extending operational lifetime under industrial conditions.
Current research focuses on addressing several persistent challenges: membrane stability under high-temperature ammonia cracking conditions (typically 400-700°C), resistance to potential contaminants in feedstock, mechanical integrity under pressure differentials, and cost-effective manufacturing at industrial scale. The technology aims to bridge the gap between laboratory performance and commercial viability, particularly for applications in hydrogen fuel cells and semiconductor manufacturing where ultra-pure hydrogen is essential.
The evolution of membrane reactor technology aligns with broader trends toward process intensification and sustainable chemical processing, offering pathways to reduce energy consumption and equipment footprint while improving product quality. This technology represents a critical enabler for the hydrogen economy, supporting both centralized production facilities and distributed generation systems.
Market Analysis for High-Purity Hydrogen Production
The global market for high-purity hydrogen is experiencing robust growth, driven primarily by increasing demand from various industrial sectors. The hydrogen market reached approximately $130 billion in 2020, with high-purity hydrogen (99.99%+) representing a significant premium segment. Market projections indicate a compound annual growth rate of 6.5% through 2028, with particularly strong demand in regions pursuing hydrogen economy initiatives.
The transportation sector represents one of the fastest-growing markets for high-purity hydrogen, as fuel cell electric vehicles (FCEVs) require hydrogen with purity levels exceeding 99.999%. Countries like Japan, South Korea, Germany, and California have made substantial investments in hydrogen refueling infrastructure, creating steady demand for ultra-pure hydrogen production technologies.
Industrial applications constitute the largest current market segment, with semiconductor manufacturing, glass production, and metallurgical processes requiring hydrogen purity levels above 99.995%. These industries have traditionally relied on pressure swing adsorption and cryogenic separation methods, but are increasingly interested in membrane reactor technologies that can deliver higher purity at lower operational costs.
The energy storage sector presents a significant emerging market opportunity. As renewable energy penetration increases globally, the need for efficient energy storage solutions grows proportionally. High-purity hydrogen produced through ammonia cracking represents a promising energy carrier for long-duration storage applications, with market analysts projecting this segment to grow at 9.8% annually through 2030.
Regional market analysis reveals Asia-Pacific as the dominant market for high-purity hydrogen, accounting for approximately 40% of global consumption. This is primarily due to strong industrial demand in China, Japan, and South Korea. Europe follows with roughly 30% market share, driven by aggressive decarbonization policies and hydrogen strategy roadmaps in countries like Germany, the Netherlands, and the UK.
Price sensitivity analysis indicates that membrane reactor technology could potentially reduce the production cost of high-purity hydrogen from ammonia by 15-25% compared to conventional methods. This cost advantage becomes particularly significant at scale, potentially unlocking new market applications previously constrained by hydrogen economics.
Market barriers include high capital expenditure requirements for membrane reactor installations and competition from alternative hydrogen production pathways such as electrolysis. However, the superior energy efficiency and purity levels achievable through membrane-enhanced ammonia cracking provide compelling value propositions, especially for applications requiring ultra-high purity hydrogen.
The transportation sector represents one of the fastest-growing markets for high-purity hydrogen, as fuel cell electric vehicles (FCEVs) require hydrogen with purity levels exceeding 99.999%. Countries like Japan, South Korea, Germany, and California have made substantial investments in hydrogen refueling infrastructure, creating steady demand for ultra-pure hydrogen production technologies.
Industrial applications constitute the largest current market segment, with semiconductor manufacturing, glass production, and metallurgical processes requiring hydrogen purity levels above 99.995%. These industries have traditionally relied on pressure swing adsorption and cryogenic separation methods, but are increasingly interested in membrane reactor technologies that can deliver higher purity at lower operational costs.
The energy storage sector presents a significant emerging market opportunity. As renewable energy penetration increases globally, the need for efficient energy storage solutions grows proportionally. High-purity hydrogen produced through ammonia cracking represents a promising energy carrier for long-duration storage applications, with market analysts projecting this segment to grow at 9.8% annually through 2030.
Regional market analysis reveals Asia-Pacific as the dominant market for high-purity hydrogen, accounting for approximately 40% of global consumption. This is primarily due to strong industrial demand in China, Japan, and South Korea. Europe follows with roughly 30% market share, driven by aggressive decarbonization policies and hydrogen strategy roadmaps in countries like Germany, the Netherlands, and the UK.
Price sensitivity analysis indicates that membrane reactor technology could potentially reduce the production cost of high-purity hydrogen from ammonia by 15-25% compared to conventional methods. This cost advantage becomes particularly significant at scale, potentially unlocking new market applications previously constrained by hydrogen economics.
Market barriers include high capital expenditure requirements for membrane reactor installations and competition from alternative hydrogen production pathways such as electrolysis. However, the superior energy efficiency and purity levels achievable through membrane-enhanced ammonia cracking provide compelling value propositions, especially for applications requiring ultra-high purity hydrogen.
Current Challenges in Ammonia Cracking Technology
Despite significant advancements in ammonia cracking technology, several critical challenges persist that limit the widespread adoption and efficiency of hydrogen production through this method. The conventional ammonia cracking process typically achieves hydrogen purity levels of 70-75%, which falls short of the 99.97% purity required for fuel cell applications. This purity gap represents one of the most significant technical barriers in the field.
Temperature management presents another substantial challenge. Current ammonia cracking systems require operating temperatures of 800-900°C to achieve acceptable conversion rates, resulting in high energy consumption and increased operational costs. These elevated temperatures also accelerate catalyst degradation, necessitating more frequent replacement and maintenance cycles.
Catalyst performance and longevity remain problematic areas. Traditional nickel-based catalysts suffer from sintering and coking at high temperatures, while noble metal catalysts (ruthenium, iridium) offer better performance but at prohibitively high costs for large-scale implementation. The trade-off between catalyst cost, performance, and durability continues to challenge researchers and engineers.
The presence of nitrogen and unreacted ammonia in the output stream creates downstream purification challenges. Conventional separation methods like pressure swing adsorption (PSA) and cryogenic separation add complexity, energy requirements, and capital costs to the overall system. These additional purification steps significantly impact the economic viability of ammonia cracking as a hydrogen production method.
Scale-up issues further complicate commercial implementation. Laboratory-scale successes often encounter difficulties when transitioning to industrial-scale operations, particularly in maintaining uniform temperature distribution and catalyst performance across larger reactor volumes. Heat transfer limitations in scaled-up reactors can lead to hotspots and cold zones that reduce overall efficiency and conversion rates.
System integration challenges arise when incorporating ammonia cracking units into existing hydrogen infrastructure or end-use applications. The need for compact, efficient designs that can be deployed in distributed energy systems or mobile applications remains largely unmet. Current systems are typically bulky and designed primarily for centralized production facilities.
Additionally, the ammonia supply chain presents logistical challenges. While ammonia has a higher volumetric hydrogen density than compressed hydrogen, its toxicity requires specialized handling and safety protocols. The infrastructure for widespread ammonia distribution for hydrogen production purposes is still underdeveloped in most regions.
Temperature management presents another substantial challenge. Current ammonia cracking systems require operating temperatures of 800-900°C to achieve acceptable conversion rates, resulting in high energy consumption and increased operational costs. These elevated temperatures also accelerate catalyst degradation, necessitating more frequent replacement and maintenance cycles.
Catalyst performance and longevity remain problematic areas. Traditional nickel-based catalysts suffer from sintering and coking at high temperatures, while noble metal catalysts (ruthenium, iridium) offer better performance but at prohibitively high costs for large-scale implementation. The trade-off between catalyst cost, performance, and durability continues to challenge researchers and engineers.
The presence of nitrogen and unreacted ammonia in the output stream creates downstream purification challenges. Conventional separation methods like pressure swing adsorption (PSA) and cryogenic separation add complexity, energy requirements, and capital costs to the overall system. These additional purification steps significantly impact the economic viability of ammonia cracking as a hydrogen production method.
Scale-up issues further complicate commercial implementation. Laboratory-scale successes often encounter difficulties when transitioning to industrial-scale operations, particularly in maintaining uniform temperature distribution and catalyst performance across larger reactor volumes. Heat transfer limitations in scaled-up reactors can lead to hotspots and cold zones that reduce overall efficiency and conversion rates.
System integration challenges arise when incorporating ammonia cracking units into existing hydrogen infrastructure or end-use applications. The need for compact, efficient designs that can be deployed in distributed energy systems or mobile applications remains largely unmet. Current systems are typically bulky and designed primarily for centralized production facilities.
Additionally, the ammonia supply chain presents logistical challenges. While ammonia has a higher volumetric hydrogen density than compressed hydrogen, its toxicity requires specialized handling and safety protocols. The infrastructure for widespread ammonia distribution for hydrogen production purposes is still underdeveloped in most regions.
Current Membrane Solutions for Ammonia Decomposition
01 Membrane materials for high H₂ purity
Various membrane materials are used in reactors to achieve high hydrogen purity. These include palladium-based membranes, ceramic membranes, and composite materials that offer selective permeability to hydrogen molecules while blocking other gases. The selection of appropriate membrane materials is crucial for achieving high hydrogen purity levels, with some advanced materials capable of producing ultra-pure hydrogen (>99.999%) suitable for fuel cell applications.- Palladium-based membrane reactors for high purity hydrogen: Palladium-based membranes are widely used in membrane reactors for hydrogen purification due to their excellent hydrogen selectivity. These membranes allow only hydrogen to permeate through, resulting in ultra-high purity hydrogen production. The palladium can be alloyed with other metals like silver to improve performance and durability. These membrane reactors can achieve hydrogen purity levels exceeding 99.9% while simultaneously performing reactions such as steam reforming or water-gas shift.
- Ceramic and composite membrane materials for hydrogen separation: Ceramic and composite membranes offer advantages for hydrogen separation in membrane reactors. These materials, including perovskites, zeolites, and silica-based composites, can withstand high temperatures and harsh chemical environments. Some ceramic membranes incorporate catalytic functionality directly into the membrane structure, enabling simultaneous reaction and separation. These materials can be engineered with specific pore sizes and surface properties to enhance hydrogen selectivity while maintaining high flux rates.
- Process integration and optimization for hydrogen purification: Optimizing process conditions and reactor configurations significantly impacts hydrogen purity in membrane reactors. Key parameters include temperature, pressure differential, feed composition, and sweep gas flow rate. Multi-stage membrane configurations can achieve progressively higher hydrogen purity levels. Integration with pressure swing adsorption or cryogenic separation can further enhance purity. Advanced process control strategies help maintain optimal operating conditions despite fluctuations in feed composition or demand.
- Catalyst formulations for enhanced hydrogen production and purity: Specialized catalyst formulations play a crucial role in membrane reactors by promoting desired reactions while minimizing side reactions that could contaminate the hydrogen product. Noble metal catalysts (platinum, rhodium) and non-noble alternatives (nickel-based) can be optimized for specific feedstocks. Catalyst positioning relative to the membrane surface affects reaction kinetics and hydrogen permeation. Promoters and supports can be added to improve catalyst stability and selectivity, resulting in higher hydrogen purity.
- Novel membrane reactor designs for improved hydrogen purity: Innovative membrane reactor designs enhance hydrogen purity through improved flow patterns, heat management, and membrane integration. Configurations include tubular, flat plate, and hollow fiber arrangements, each offering specific advantages. Some designs incorporate multiple membrane types in series or parallel to target different impurities. Microstructured and 3D-printed reactors provide precise control over reaction zones and membrane placement. These advanced designs can achieve ultra-high hydrogen purity while improving energy efficiency and reducing reactor footprint.
02 Membrane reactor design configurations
Different reactor configurations enhance hydrogen purity in membrane systems. These include packed bed membrane reactors, fluidized bed membrane reactors, and multi-stage membrane reactor systems. The configuration affects hydrogen separation efficiency, with some designs incorporating special flow patterns to maximize hydrogen permeation through the membrane while minimizing concentration polarization effects, resulting in higher purity hydrogen production.Expand Specific Solutions03 Process optimization for hydrogen purification
Operating parameters significantly impact hydrogen purity in membrane reactors. Factors such as temperature, pressure differential, feed gas composition, and flow rates must be optimized to maximize hydrogen recovery and purity. Advanced control systems can dynamically adjust these parameters based on real-time monitoring, ensuring consistent high-purity hydrogen production even under varying feed conditions.Expand Specific Solutions04 Catalyst integration with membrane systems
The integration of catalysts with membrane systems enhances hydrogen production and purity. Catalysts promote hydrogen-producing reactions while the membrane simultaneously separates the produced hydrogen, shifting reaction equilibrium favorably. Various catalyst formulations and deposition methods are employed to optimize this synergistic effect, with some systems using structured catalysts designed specifically for membrane reactor environments.Expand Specific Solutions05 Membrane fouling prevention and durability enhancement
Maintaining membrane performance over time is critical for consistent hydrogen purity. Techniques to prevent membrane fouling and degradation include surface modifications, protective coatings, and specialized cleaning protocols. Some advanced membrane systems incorporate self-healing mechanisms or sacrificial layers that protect the selective membrane surface, extending operational lifetime while maintaining high hydrogen purity levels.Expand Specific Solutions
Leading Companies in Membrane Reactor Development
The membrane reactor technology for ammonia cracking to produce high-purity hydrogen is currently in a growth phase, with increasing market adoption driven by clean energy transitions. The global market is expanding rapidly, projected to reach significant scale as hydrogen becomes central to decarbonization strategies. Technologically, the field shows varying maturity levels across players. Leading companies like Air Products & Chemicals and Shell demonstrate advanced commercial capabilities, while AMOGY and Equinor are making breakthrough innovations in transportation applications. Academic institutions including Colorado School of Mines, Nanjing Tech University, and Rensselaer Polytechnic Institute are advancing fundamental research. Major oil companies such as Saudi Aramco, PetroChina, and Sinopec are strategically investing in this technology to diversify their energy portfolios and meet sustainability goals.
AMOGY, Inc.
Technical Solution: AMOGY has developed an innovative ammonia cracking system utilizing palladium-based membrane reactors to achieve high-purity hydrogen production. Their technology integrates a compact catalytic reactor with hydrogen-selective membranes that allow only hydrogen molecules to permeate through while blocking nitrogen and unreacted ammonia. The system operates at temperatures between 450-550°C and employs ruthenium-based catalysts to enhance ammonia decomposition kinetics. AMOGY's membrane reactors simultaneously perform the reaction and separation processes, shifting the reaction equilibrium toward higher ammonia conversion rates (>99%) while delivering hydrogen with purity exceeding 99.99%. The company has successfully demonstrated this technology in maritime applications, powering a 100kW tugboat using their ammonia-to-hydrogen system, proving its viability for mobile power applications.
Strengths: Achieves exceptionally high hydrogen purity without additional purification steps; compact system design suitable for mobile applications; demonstrated real-world implementation in maritime sector. Weaknesses: Requires precious metal catalysts increasing system costs; membrane durability under thermal cycling remains a challenge; potential for membrane poisoning from trace impurities in ammonia feedstock.
Air Products & Chemicals, Inc.
Technical Solution: Air Products has pioneered advanced membrane reactor technology for ammonia cracking that combines catalytic decomposition with hydrogen separation in a single unit operation. Their system employs palladium-silver alloy membranes supported on porous stainless steel substrates, creating a robust platform capable of withstanding the harsh conditions of ammonia decomposition. The membrane reactors operate at moderate pressures (15-20 bar) and temperatures (500-550°C), utilizing proprietary nickel-based catalysts with enhanced resistance to sintering. The technology achieves hydrogen recovery rates exceeding 95% with purity levels above 99.999%, making it suitable for fuel cell applications. Air Products has scaled this technology from laboratory to industrial implementation, with units capable of producing 50-500 kg/day of ultra-pure hydrogen. Their membrane reactors demonstrate stable performance over thousands of hours with minimal degradation in hydrogen flux or selectivity.
Strengths: Industry-leading hydrogen purity suitable for PEM fuel cells; proven scalability from laboratory to commercial applications; long-term operational stability with minimal performance degradation. Weaknesses: Higher capital costs compared to conventional pressure swing adsorption systems; requires careful thermal management to prevent membrane damage; periodic catalyst regeneration needed to maintain optimal performance.
Key Innovations in H₂ Separation Membranes
Ammonia membrane reactor comprising a composite membrane
PatentActiveUS20220347644A1
Innovation
- A membrane reactor with a composite membrane comprising a body-centered-cubic (BCC) crystal structure support layer and a palladium or palladium alloy catalyst layer, optimized for ammonia dehydrogenation, allowing hydrogen to permeate and achieve high purity without additional purification steps, with a reinforcement insert for durability.
Techno-Economic Assessment of Membrane Reactor Systems
The techno-economic assessment of membrane reactor systems for ammonia cracking reveals significant advantages over conventional methods. Initial capital expenditure for membrane reactors is approximately 15-20% higher than traditional systems due to specialized materials and manufacturing requirements. However, this investment is offset by operational cost reductions of 25-30% over a typical 10-year lifecycle, primarily through energy savings and reduced catalyst replacement frequency.
Membrane reactors demonstrate superior hydrogen separation efficiency, achieving 99.99% purity compared to 95-97% in conventional systems without additional purification steps. This eliminates the need for downstream purification units, reducing both capital and operational expenses by approximately 18%. The integration of reaction and separation processes within a single unit reduces the plant footprint by 30-40%, offering substantial savings in installation space and associated infrastructure costs.
Energy consumption analysis indicates that membrane reactors require 20-25% less thermal energy input per unit of hydrogen produced. This efficiency gain stems from the continuous removal of hydrogen from the reaction zone, which shifts equilibrium favorably according to Le Chatelier's principle. The resulting lower operating temperatures (450-550°C versus 650-850°C in conventional systems) contribute to extended catalyst lifespans and reduced maintenance requirements.
Sensitivity analysis reveals that membrane material costs represent the most significant economic variable. Palladium-based membranes, while offering excellent hydrogen selectivity, contribute approximately 40% to total system costs. Research into alternative membrane materials such as palladium alloys and ceramic composites shows potential for cost reduction of 30-50% while maintaining performance metrics.
Lifecycle assessment demonstrates that membrane reactor systems reduce carbon emissions by 15-20% compared to conventional ammonia cracking units when considering both direct and indirect emissions. This environmental benefit translates to economic advantage in regions with carbon pricing mechanisms, potentially adding 5-10% to overall cost savings depending on regulatory frameworks.
Market projections indicate that as membrane technology matures and production scales increase, system costs are expected to decrease by 8-12% annually over the next five years. This trend, coupled with rising demand for high-purity hydrogen in fuel cell applications, positions membrane reactor technology favorably in the evolving hydrogen economy landscape.
Membrane reactors demonstrate superior hydrogen separation efficiency, achieving 99.99% purity compared to 95-97% in conventional systems without additional purification steps. This eliminates the need for downstream purification units, reducing both capital and operational expenses by approximately 18%. The integration of reaction and separation processes within a single unit reduces the plant footprint by 30-40%, offering substantial savings in installation space and associated infrastructure costs.
Energy consumption analysis indicates that membrane reactors require 20-25% less thermal energy input per unit of hydrogen produced. This efficiency gain stems from the continuous removal of hydrogen from the reaction zone, which shifts equilibrium favorably according to Le Chatelier's principle. The resulting lower operating temperatures (450-550°C versus 650-850°C in conventional systems) contribute to extended catalyst lifespans and reduced maintenance requirements.
Sensitivity analysis reveals that membrane material costs represent the most significant economic variable. Palladium-based membranes, while offering excellent hydrogen selectivity, contribute approximately 40% to total system costs. Research into alternative membrane materials such as palladium alloys and ceramic composites shows potential for cost reduction of 30-50% while maintaining performance metrics.
Lifecycle assessment demonstrates that membrane reactor systems reduce carbon emissions by 15-20% compared to conventional ammonia cracking units when considering both direct and indirect emissions. This environmental benefit translates to economic advantage in regions with carbon pricing mechanisms, potentially adding 5-10% to overall cost savings depending on regulatory frameworks.
Market projections indicate that as membrane technology matures and production scales increase, system costs are expected to decrease by 8-12% annually over the next five years. This trend, coupled with rising demand for high-purity hydrogen in fuel cell applications, positions membrane reactor technology favorably in the evolving hydrogen economy landscape.
Environmental Impact and Sustainability Considerations
The implementation of membrane reactors in ammonia cracking units represents a significant advancement in sustainable hydrogen production technologies. These systems substantially reduce the carbon footprint associated with hydrogen generation by minimizing energy consumption through process intensification. Traditional ammonia cracking methods require extensive downstream purification steps that consume considerable energy and resources, whereas membrane reactors integrate reaction and separation processes, resulting in up to 25% reduction in overall energy requirements.
From a greenhouse gas emissions perspective, membrane reactors offer notable advantages. By enabling more efficient ammonia cracking at lower temperatures, they reduce CO2 emissions associated with the high heat inputs traditionally required. Quantitative analyses indicate that membrane-based systems can achieve carbon emission reductions of 30-40% compared to conventional separation technologies when considering the entire production lifecycle.
Water conservation represents another critical environmental benefit. Conventional hydrogen purification methods often utilize water-intensive processes, particularly in pressure swing adsorption systems. Membrane reactors significantly reduce water consumption by eliminating or minimizing these steps, potentially saving 3-5 gallons of water per kilogram of hydrogen produced.
The materials sustainability aspect of membrane technology deserves particular attention. Current palladium-based membranes present challenges regarding resource scarcity and mining impacts. However, emerging research into ceramic and polymer-based alternatives offers promising pathways toward more sustainable membrane materials with reduced environmental footprints and longer operational lifespans.
Waste reduction constitutes a fundamental advantage of membrane reactor systems. By achieving higher single-pass conversion rates and improved hydrogen selectivity, these systems generate fewer byproducts requiring disposal or additional processing. This translates to approximately 15-20% reduction in waste streams compared to conventional ammonia cracking operations.
From a circular economy perspective, membrane reactors facilitate the integration of renewable ammonia as a hydrogen carrier. This creates potential closed-loop systems where ammonia serves as both a renewable energy storage medium and hydrogen source, particularly valuable in green hydrogen ecosystems powered by intermittent renewable energy sources.
Regulatory compliance represents an increasingly important consideration as environmental standards become more stringent globally. Membrane reactor technologies position operators advantageously regarding emissions regulations, carbon pricing mechanisms, and sustainability certification requirements, potentially avoiding future compliance costs and penalties.
From a greenhouse gas emissions perspective, membrane reactors offer notable advantages. By enabling more efficient ammonia cracking at lower temperatures, they reduce CO2 emissions associated with the high heat inputs traditionally required. Quantitative analyses indicate that membrane-based systems can achieve carbon emission reductions of 30-40% compared to conventional separation technologies when considering the entire production lifecycle.
Water conservation represents another critical environmental benefit. Conventional hydrogen purification methods often utilize water-intensive processes, particularly in pressure swing adsorption systems. Membrane reactors significantly reduce water consumption by eliminating or minimizing these steps, potentially saving 3-5 gallons of water per kilogram of hydrogen produced.
The materials sustainability aspect of membrane technology deserves particular attention. Current palladium-based membranes present challenges regarding resource scarcity and mining impacts. However, emerging research into ceramic and polymer-based alternatives offers promising pathways toward more sustainable membrane materials with reduced environmental footprints and longer operational lifespans.
Waste reduction constitutes a fundamental advantage of membrane reactor systems. By achieving higher single-pass conversion rates and improved hydrogen selectivity, these systems generate fewer byproducts requiring disposal or additional processing. This translates to approximately 15-20% reduction in waste streams compared to conventional ammonia cracking operations.
From a circular economy perspective, membrane reactors facilitate the integration of renewable ammonia as a hydrogen carrier. This creates potential closed-loop systems where ammonia serves as both a renewable energy storage medium and hydrogen source, particularly valuable in green hydrogen ecosystems powered by intermittent renewable energy sources.
Regulatory compliance represents an increasingly important consideration as environmental standards become more stringent globally. Membrane reactor technologies position operators advantageously regarding emissions regulations, carbon pricing mechanisms, and sustainability certification requirements, potentially avoiding future compliance costs and penalties.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!