How to Minimize Coking and Sintering Under Cyclic Operation
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Coking and Sintering Challenges in Cyclic Operations
Coking and sintering represent two of the most significant challenges in cyclic operations across various industrial processes, particularly in petrochemical, refining, and catalytic systems. These phenomena occur when carbon deposits accumulate on catalyst surfaces (coking) or when high-temperature conditions cause catalyst particles to agglomerate and lose surface area (sintering). The cyclic nature of operations—involving repeated heating, cooling, oxidation, and reduction cycles—exacerbates these issues compared to steady-state processes.
In petrochemical industries, coking manifests as carbonaceous deposits that block active catalyst sites, reducing catalytic efficiency and necessitating frequent regeneration cycles. These deposits form through complex mechanisms including polymerization, dehydrogenation, and condensation reactions of hydrocarbon intermediates. The severity of coking varies with feedstock composition, operating temperature, and catalyst properties.
Sintering, meanwhile, occurs predominantly during high-temperature phases of cyclic operations when metal particles on catalyst supports become mobile and coalesce. This irreversible process reduces catalyst surface area and porosity, ultimately diminishing catalytic activity. Unlike coking, sintering damage cannot be reversed through standard regeneration procedures, making it a particularly costly form of catalyst deactivation.
The economic impact of these challenges is substantial. Industry reports indicate that coking and sintering account for approximately 20-30% of catalyst replacement costs in refining operations, translating to billions of dollars annually across the sector. Additionally, the energy requirements for more frequent regeneration cycles contribute significantly to operational costs and environmental footprint.
Cyclic temperature variations particularly intensify both phenomena. During temperature ramps, thermal expansion and contraction create mechanical stresses that can accelerate sintering. Similarly, rapid temperature changes can create localized hotspots that promote both coking reactions and sintering events. Studies have shown that the frequency and amplitude of temperature cycling correlate directly with accelerated catalyst deactivation rates.
The interplay between coking and sintering further complicates mitigation efforts. Coke removal often requires oxidative treatments at elevated temperatures, which can inadvertently promote sintering. Conversely, modifications to reduce sintering susceptibility may create surface properties that enhance coking tendencies.
Recent research has identified several critical factors that influence coking and sintering susceptibility under cyclic conditions, including catalyst composition, support material properties, metal dispersion techniques, and operational parameters such as gas composition, pressure fluctuations, and cycle duration. Understanding these factors is essential for developing effective minimization strategies.
In petrochemical industries, coking manifests as carbonaceous deposits that block active catalyst sites, reducing catalytic efficiency and necessitating frequent regeneration cycles. These deposits form through complex mechanisms including polymerization, dehydrogenation, and condensation reactions of hydrocarbon intermediates. The severity of coking varies with feedstock composition, operating temperature, and catalyst properties.
Sintering, meanwhile, occurs predominantly during high-temperature phases of cyclic operations when metal particles on catalyst supports become mobile and coalesce. This irreversible process reduces catalyst surface area and porosity, ultimately diminishing catalytic activity. Unlike coking, sintering damage cannot be reversed through standard regeneration procedures, making it a particularly costly form of catalyst deactivation.
The economic impact of these challenges is substantial. Industry reports indicate that coking and sintering account for approximately 20-30% of catalyst replacement costs in refining operations, translating to billions of dollars annually across the sector. Additionally, the energy requirements for more frequent regeneration cycles contribute significantly to operational costs and environmental footprint.
Cyclic temperature variations particularly intensify both phenomena. During temperature ramps, thermal expansion and contraction create mechanical stresses that can accelerate sintering. Similarly, rapid temperature changes can create localized hotspots that promote both coking reactions and sintering events. Studies have shown that the frequency and amplitude of temperature cycling correlate directly with accelerated catalyst deactivation rates.
The interplay between coking and sintering further complicates mitigation efforts. Coke removal often requires oxidative treatments at elevated temperatures, which can inadvertently promote sintering. Conversely, modifications to reduce sintering susceptibility may create surface properties that enhance coking tendencies.
Recent research has identified several critical factors that influence coking and sintering susceptibility under cyclic conditions, including catalyst composition, support material properties, metal dispersion techniques, and operational parameters such as gas composition, pressure fluctuations, and cycle duration. Understanding these factors is essential for developing effective minimization strategies.
Market Demand for Anti-Coking Solutions in Industrial Processes
The global market for anti-coking solutions in industrial processes has been experiencing significant growth, driven primarily by the increasing demand for operational efficiency and equipment longevity in petrochemical, refining, and chemical processing industries. These sectors face substantial financial losses due to coking and sintering issues during cyclic operations, creating a robust demand for effective preventive solutions.
Petrochemical and refining industries represent the largest market segment, where steam crackers and catalytic reforming units suffer from coking deposits that reduce heat transfer efficiency and catalyst activity. According to industry reports, unplanned shutdowns due to coking issues cost refineries between $500,000 and $1 million per day in lost production, creating a compelling economic case for anti-coking technologies.
The market is further propelled by stringent environmental regulations worldwide that mandate reduced emissions and improved energy efficiency in industrial operations. As cyclic operations become more common due to fluctuating feedstock prices and varying product demand, the need for materials and processes resistant to coking under these challenging conditions has intensified.
Geographically, North America and Europe currently dominate the market for anti-coking solutions, owing to their mature petrochemical industries and stringent regulatory frameworks. However, the Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate as these countries rapidly expand their refining and petrochemical capacities.
The market segmentation reveals distinct categories of anti-coking solutions, including specialized coatings, advanced materials, process additives, and operational optimization services. Among these, surface modification technologies and catalytic coatings have gained significant traction, with annual growth rates exceeding the overall market average.
End-users increasingly demand integrated solutions that not only prevent coking but also offer real-time monitoring capabilities to predict and manage deposition before it becomes problematic. This trend has created new market opportunities for digital service providers who combine materials science with data analytics.
The economic benefits of effective anti-coking solutions extend beyond preventing downtime. Enhanced energy efficiency, extended equipment lifecycle, reduced maintenance costs, and improved product quality collectively represent a compelling value proposition that continues to drive market expansion. Industry analysts project that the global market for anti-coking technologies will maintain strong growth over the next decade as industrial processes become increasingly complex and cyclic operations more prevalent.
Petrochemical and refining industries represent the largest market segment, where steam crackers and catalytic reforming units suffer from coking deposits that reduce heat transfer efficiency and catalyst activity. According to industry reports, unplanned shutdowns due to coking issues cost refineries between $500,000 and $1 million per day in lost production, creating a compelling economic case for anti-coking technologies.
The market is further propelled by stringent environmental regulations worldwide that mandate reduced emissions and improved energy efficiency in industrial operations. As cyclic operations become more common due to fluctuating feedstock prices and varying product demand, the need for materials and processes resistant to coking under these challenging conditions has intensified.
Geographically, North America and Europe currently dominate the market for anti-coking solutions, owing to their mature petrochemical industries and stringent regulatory frameworks. However, the Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate as these countries rapidly expand their refining and petrochemical capacities.
The market segmentation reveals distinct categories of anti-coking solutions, including specialized coatings, advanced materials, process additives, and operational optimization services. Among these, surface modification technologies and catalytic coatings have gained significant traction, with annual growth rates exceeding the overall market average.
End-users increasingly demand integrated solutions that not only prevent coking but also offer real-time monitoring capabilities to predict and manage deposition before it becomes problematic. This trend has created new market opportunities for digital service providers who combine materials science with data analytics.
The economic benefits of effective anti-coking solutions extend beyond preventing downtime. Enhanced energy efficiency, extended equipment lifecycle, reduced maintenance costs, and improved product quality collectively represent a compelling value proposition that continues to drive market expansion. Industry analysts project that the global market for anti-coking technologies will maintain strong growth over the next decade as industrial processes become increasingly complex and cyclic operations more prevalent.
Current Technological Limitations and Global Research Status
Despite significant advancements in catalyst and reactor design, coking and sintering remain persistent challenges in cyclic operations across petrochemical, refining, and energy conversion processes. Current technologies face fundamental limitations in preventing carbon deposition on catalyst surfaces during hydrocarbon processing. Conventional approaches primarily rely on periodic regeneration cycles rather than truly preventing coking formation, resulting in operational inefficiencies and increased maintenance costs.
The global research landscape reveals regional specialization patterns. North American institutions focus predominantly on advanced materials science approaches, with notable work at MIT and Stanford University exploring atomic layer deposition techniques to create protective catalyst coatings. European research centers, particularly in Germany and France, emphasize process optimization strategies, developing sophisticated temperature and pressure control algorithms to minimize coking conditions.
Asian research, led by China and Japan, demonstrates strength in novel catalyst formulations, with significant publications on bimetallic and trimetallic catalyst systems showing enhanced coking resistance. Recent breakthroughs at the Chinese Academy of Sciences have demonstrated promising results with cerium-modified catalysts that exhibit up to 40% reduction in carbon deposition rates under cyclic conditions.
Temperature control remains a critical limitation across all technologies. Current systems struggle to maintain uniform temperature profiles during rapid cycling, creating hotspots that accelerate both coking and sintering mechanisms. Research at Imperial College London has quantified these effects, showing that even 15°C temperature variations can increase coking rates by 25-30% in steam reforming operations.
Regarding sintering prevention, existing technologies face limitations in stabilizing metal nanoparticles under fluctuating redox conditions. The latest research from Tokyo University demonstrates that conventional supports like alumina and silica provide insufficient anchoring for metal particles during rapid cycling between oxidizing and reducing environments.
Computational modeling capabilities represent another limitation. While significant progress has been made in molecular dynamics simulations of coking mechanisms, current models struggle to accurately predict behavior under dynamic conditions. The gap between laboratory research and industrial implementation remains substantial, with many promising technologies confined to TRL levels 3-5.
International collaboration trends show increasing joint ventures between academic institutions and industry partners, particularly in developing in-situ monitoring technologies. These partnerships aim to bridge fundamental research with practical applications, though commercialization timelines typically extend 5-8 years from laboratory demonstration to industrial deployment.
The global research landscape reveals regional specialization patterns. North American institutions focus predominantly on advanced materials science approaches, with notable work at MIT and Stanford University exploring atomic layer deposition techniques to create protective catalyst coatings. European research centers, particularly in Germany and France, emphasize process optimization strategies, developing sophisticated temperature and pressure control algorithms to minimize coking conditions.
Asian research, led by China and Japan, demonstrates strength in novel catalyst formulations, with significant publications on bimetallic and trimetallic catalyst systems showing enhanced coking resistance. Recent breakthroughs at the Chinese Academy of Sciences have demonstrated promising results with cerium-modified catalysts that exhibit up to 40% reduction in carbon deposition rates under cyclic conditions.
Temperature control remains a critical limitation across all technologies. Current systems struggle to maintain uniform temperature profiles during rapid cycling, creating hotspots that accelerate both coking and sintering mechanisms. Research at Imperial College London has quantified these effects, showing that even 15°C temperature variations can increase coking rates by 25-30% in steam reforming operations.
Regarding sintering prevention, existing technologies face limitations in stabilizing metal nanoparticles under fluctuating redox conditions. The latest research from Tokyo University demonstrates that conventional supports like alumina and silica provide insufficient anchoring for metal particles during rapid cycling between oxidizing and reducing environments.
Computational modeling capabilities represent another limitation. While significant progress has been made in molecular dynamics simulations of coking mechanisms, current models struggle to accurately predict behavior under dynamic conditions. The gap between laboratory research and industrial implementation remains substantial, with many promising technologies confined to TRL levels 3-5.
International collaboration trends show increasing joint ventures between academic institutions and industry partners, particularly in developing in-situ monitoring technologies. These partnerships aim to bridge fundamental research with practical applications, though commercialization timelines typically extend 5-8 years from laboratory demonstration to industrial deployment.
Existing Mitigation Strategies for Cyclic Operation Environments
01 Anti-coking and anti-sintering additives for catalysts
Various additives can be incorporated into catalytic systems to prevent or reduce coking and sintering. These additives work by either forming protective layers on catalyst surfaces, neutralizing coke precursors, or enhancing the stability of metal particles at high temperatures. Common anti-coking additives include alkaline earth metals, rare earth elements, and certain transition metals that can modify the electronic properties of the catalyst surface to reduce carbon deposition.- Prevention of coking in catalytic systems: Various methods and compositions are employed to prevent or reduce coking in catalytic systems. These include the use of specific catalyst formulations, coatings, or additives that inhibit carbon deposition on catalyst surfaces. Such approaches help maintain catalyst activity and selectivity by preventing the buildup of carbonaceous deposits that can block active sites and reduce catalytic performance in processes like hydrocarbon conversion and refining operations.
- Anti-sintering technologies for catalysts: Technologies to prevent sintering of catalytic materials focus on stabilizing the catalyst structure at high temperatures. These include the incorporation of thermal stabilizers, specific support materials, and structural promoters that maintain the dispersion of active metal particles. Anti-sintering approaches are crucial for maintaining catalyst surface area and activity during high-temperature operations, particularly in reforming, combustion, and exhaust gas treatment applications.
- Regeneration of deactivated catalysts: Methods for regenerating catalysts that have been deactivated by coking or sintering involve controlled oxidation processes, chemical treatments, or thermal procedures to remove carbon deposits and restore catalyst activity. These regeneration techniques can include in-situ or ex-situ treatments, often using controlled atmospheres, specific temperature profiles, and sometimes chemical agents to effectively remove contaminants while preserving the catalyst structure and active sites.
- Novel catalyst compositions resistant to deactivation: Advanced catalyst compositions are designed with inherent resistance to coking and sintering. These formulations may incorporate specific metals, promoters, or support materials that enhance stability under severe operating conditions. Some compositions feature bimetallic or multimetallic systems, specialized supports with controlled porosity, or novel structures that maintain dispersion and accessibility of active sites even under challenging reaction environments.
- Process modifications to minimize catalyst deactivation: Operational strategies and process modifications can significantly reduce catalyst deactivation from coking and sintering. These include optimized reaction conditions such as temperature profiles, pressure control, feed pretreatment, and the introduction of specific process additives. Continuous or periodic catalyst rejuvenation procedures integrated into the process design can also extend catalyst life and maintain performance in industrial applications.
02 Catalyst regeneration techniques for coked catalysts
Methods for regenerating catalysts that have been deactivated by coking involve controlled oxidation processes to burn off carbon deposits without damaging the catalyst structure. These techniques include temperature-controlled combustion, steam treatment, and selective oxidation using specific gas mixtures. Advanced regeneration approaches may incorporate pulse regeneration, in-situ monitoring of carbon removal, and sequential treatment steps to restore catalyst activity while preserving its structural integrity.Expand Specific Solutions03 Novel catalyst structures resistant to coking and sintering
Innovative catalyst designs with enhanced resistance to coking and sintering include core-shell structures, supported bimetallic nanoparticles, and hierarchical porous materials. These structures provide improved thermal stability, controlled metal-support interactions, and optimized pore architectures that minimize carbon deposition and metal particle migration. Some designs incorporate physical barriers or sacrificial components that preferentially attract coke formation away from active sites.Expand Specific Solutions04 Process modifications to minimize coking and sintering
Operational strategies to reduce coking and sintering in catalytic processes include optimized reaction conditions, controlled feed composition, and modified reactor designs. These approaches involve careful management of temperature profiles, strategic addition of steam or hydrogen to gasify coke precursors, and implementation of fluidized bed or moving bed technologies that allow for continuous catalyst regeneration. Advanced process control systems can monitor early indicators of coking and adjust conditions accordingly.Expand Specific Solutions05 Characterization and modeling of coking and sintering phenomena
Advanced analytical techniques and computational models are used to understand the mechanisms of coking and sintering in catalytic systems. These include in-situ spectroscopy, electron microscopy, temperature-programmed methods, and molecular modeling approaches that can predict carbon formation and metal particle growth. Such tools enable researchers to identify the root causes of catalyst deactivation and develop targeted strategies for extending catalyst lifetime under specific process conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Catalyst Technology
The coking and sintering minimization under cyclic operation landscape is evolving from early-stage research to commercial implementation, with a market expected to grow significantly as refineries face stricter environmental regulations. Technology maturity varies across key players: ExxonMobil Technology & Engineering and UOP LLC lead with advanced catalyst formulations, while China Petroleum & Chemical Corp. (Sinopec) and its Research Institute demonstrate strong innovation in process optimization. Petróleo Brasileiro SA has developed notable anti-coking surface treatments, and Air Liquide offers specialized gas management solutions. Academic-industry partnerships, particularly with Central South University, are accelerating technological breakthroughs in thermal stability and materials science for cyclic operations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a multi-faceted approach to minimize coking and sintering in cyclic operations across their extensive refining and petrochemical operations. Their strategy includes innovative catalyst formulations with enhanced thermal stability, incorporating rare earth modifiers and controlled porosity structures that resist sintering during temperature swings[1]. For steam crackers, Sinopec has implemented advanced coil metallurgy using proprietary alloys with modified surface characteristics that reduce carbon adhesion and metal-catalyzed coking reactions[2]. Their cyclic operation protocols feature carefully optimized heating and cooling rates, with particular attention to transition periods where coking risk is highest. Sinopec's process monitoring systems employ distributed temperature sensors and specialized algorithms to detect early signs of hot spots or flow maldistribution that could accelerate coking. Additionally, they've developed specialized chemical treatment programs that introduce coking inhibitors at strategic points in the process, disrupting the polymerization mechanisms that lead to coke formation[3]. For units requiring periodic decoking, Sinopec has implemented automated systems that optimize the decoking process while minimizing thermal stress on equipment.
Strengths: Comprehensive integration of material science, process engineering, and chemical treatment approaches provides multiple layers of protection. Their extensive operational experience across diverse feedstocks enables rapid problem identification and solution deployment. Weaknesses: Some solutions may be optimized for specific Chinese crude slates and may require adaptation for different feedstocks. Implementation across their massive operational footprint can lead to inconsistent application of best practices between different facilities.
Sinopec Research Institute of Petroleum Processing
Technical Solution: Sinopec Research Institute of Petroleum Processing (RIPP) has developed specialized technologies targeting coking and sintering issues in cyclic operations, particularly for fluid catalytic cracking (FCC) and hydroprocessing units. Their approach includes advanced catalyst design featuring hierarchical pore structures that maintain accessibility during coking, combined with novel metal dispersion techniques that resist sintering during regeneration cycles[1]. RIPP has pioneered the use of nano-engineered catalyst supports with enhanced thermal stability, incorporating stabilizers that maintain surface area even after multiple high-temperature cycles. For reactor systems, they've developed specialized metallurgical solutions including modified stainless steel alloys with reduced nickel content in critical areas to minimize catalytic coking reactions[2]. Their process engineering innovations include optimized fluidization patterns in FCC units that reduce catalyst residence time in high-temperature regions, limiting thermal degradation. RIPP has also implemented advanced regeneration protocols with carefully controlled oxygen profiles that remove coke deposits while minimizing exotherms that could cause sintering[3]. Additionally, they've developed specialized additive packages that can be introduced during operation to passivate metal surfaces and disrupt the free-radical mechanisms that lead to coke formation.
Strengths: Deep specialization in petroleum processing applications provides highly targeted solutions for refinery operations. Their close integration with Sinopec's operational units enables rapid testing and implementation of innovations. Weaknesses: Research focus may be biased toward applications relevant to Chinese refining industry needs. Some technologies may have limited validation outside Sinopec's operational environment, creating uncertainty about performance with different feedstocks or operating conditions.
Key Innovations in Catalyst Stability and Regeneration Methods
Coking reduction in cracking reactors
PatentWO2001021731A1
Innovation
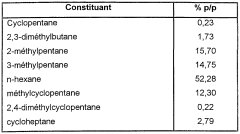
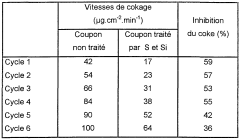
- A process involving a mixture of sulfur and silicon compounds, pretreated with steam, is applied to the metal surfaces of cracking reactors and heat exchangers to inhibit coke formation by using steam as a carrier gas, with the silicon and sulfur compounds being introduced at specific concentrations and temperatures to form a protective layer.
A compound material comprising a metal and nanoparticles and a method for producing the same
PatentInactiveEP2396443A1
Innovation
- A method of producing a composite material with metal crystallites of 1-200 nm size separated by CNTs, achieved through mechanical alloying, which stabilizes metal crystallites and enhances mechanical properties by nano-stabilization, allowing for isotropic distribution and high-temperature stability, and using multi-scroll CNTs with a high aspect ratio to form tangled agglomerates for easier handling and processing.
Economic Impact Analysis of Coking-Related Downtime
Coking and sintering-related downtime in industrial operations represents a significant economic burden across multiple sectors, particularly in petrochemical processing, refining, and chemical manufacturing. The financial implications extend far beyond the immediate costs of equipment repair and replacement. When production units experience unplanned shutdowns due to coking issues, companies face substantial revenue losses from decreased production capacity, typically ranging from $500,000 to $2 million per day depending on facility size and market conditions.
Maintenance costs associated with coking removal and equipment restoration constitute a major expense category. Standard cleaning procedures for moderately coked equipment can cost between $50,000 and $200,000 per cleaning cycle, while severe cases requiring complete disassembly and specialized cleaning techniques may exceed $500,000 per incident. These figures exclude the opportunity costs of extended downtime periods, which average 3-7 days for routine decoking but can extend to 2-3 weeks for severe cases.
Energy efficiency degradation represents another significant economic impact. As coking progresses, heat transfer efficiency typically decreases by 15-30%, resulting in higher energy consumption to maintain production parameters. This translates to increased operational costs of approximately 5-15% depending on energy prices and system design. For large-scale operations, these efficiency losses can amount to millions of dollars annually in additional energy expenditures.
Equipment lifespan reduction further compounds the economic impact. Repeated thermal cycling and coking/decoking processes accelerate metal fatigue and structural degradation. Industry data suggests that severe coking can reduce equipment service life by 20-40%, necessitating premature capital investments in replacement infrastructure. For critical process equipment, these premature replacements can represent costs of $1-10 million depending on complexity and scale.
Product quality issues stemming from coking events also carry significant economic consequences. Contamination from coke particles or process instability during coking-prone operations can lead to off-specification products. The resulting downgrading or disposal of these products typically results in value losses of 30-60% compared to prime-grade materials. For specialty chemical producers, these quality incidents can also damage customer relationships and lead to long-term market share erosion.
Regulatory compliance costs add another dimension to the economic impact analysis. Emissions exceedances during coking-related operational upsets often trigger regulatory penalties, ranging from $10,000 to $100,000 per incident in most jurisdictions. More significantly, repeated non-compliance may result in operating restrictions or mandated capital improvements with costs potentially reaching millions of dollars.
Maintenance costs associated with coking removal and equipment restoration constitute a major expense category. Standard cleaning procedures for moderately coked equipment can cost between $50,000 and $200,000 per cleaning cycle, while severe cases requiring complete disassembly and specialized cleaning techniques may exceed $500,000 per incident. These figures exclude the opportunity costs of extended downtime periods, which average 3-7 days for routine decoking but can extend to 2-3 weeks for severe cases.
Energy efficiency degradation represents another significant economic impact. As coking progresses, heat transfer efficiency typically decreases by 15-30%, resulting in higher energy consumption to maintain production parameters. This translates to increased operational costs of approximately 5-15% depending on energy prices and system design. For large-scale operations, these efficiency losses can amount to millions of dollars annually in additional energy expenditures.
Equipment lifespan reduction further compounds the economic impact. Repeated thermal cycling and coking/decoking processes accelerate metal fatigue and structural degradation. Industry data suggests that severe coking can reduce equipment service life by 20-40%, necessitating premature capital investments in replacement infrastructure. For critical process equipment, these premature replacements can represent costs of $1-10 million depending on complexity and scale.
Product quality issues stemming from coking events also carry significant economic consequences. Contamination from coke particles or process instability during coking-prone operations can lead to off-specification products. The resulting downgrading or disposal of these products typically results in value losses of 30-60% compared to prime-grade materials. For specialty chemical producers, these quality incidents can also damage customer relationships and lead to long-term market share erosion.
Regulatory compliance costs add another dimension to the economic impact analysis. Emissions exceedances during coking-related operational upsets often trigger regulatory penalties, ranging from $10,000 to $100,000 per incident in most jurisdictions. More significantly, repeated non-compliance may result in operating restrictions or mandated capital improvements with costs potentially reaching millions of dollars.
Sustainability Aspects of Extended Catalyst Lifecycle
Extending catalyst lifecycle represents a critical sustainability dimension in industrial processes, particularly when addressing coking and sintering challenges under cyclic operation. The environmental footprint of catalyst production, replacement, and disposal creates significant sustainability concerns that can be mitigated through extended catalyst lifespans.
Reduced raw material consumption stands as a primary sustainability benefit of prolonged catalyst lifecycles. Catalysts often contain precious metals and rare earth elements whose extraction involves energy-intensive mining operations with substantial environmental impacts. By extending catalyst life through minimized coking and sintering, industries can significantly decrease their demand for these finite resources, contributing to conservation efforts and reducing extraction-related environmental degradation.
Energy conservation represents another crucial sustainability advantage. The manufacturing of fresh catalysts requires considerable energy inputs across multiple production stages. When catalysts maintain their activity for extended periods through effective anti-coking and anti-sintering strategies, the frequency of energy-intensive replacement cycles decreases proportionally, resulting in substantial energy savings and reduced carbon emissions throughout the industrial value chain.
Waste reduction constitutes a third major sustainability benefit. Spent catalysts often contain hazardous materials requiring specialized disposal procedures or complex recycling processes. Extended catalyst lifecycles directly translate to reduced waste generation rates, alleviating pressure on waste management systems and minimizing potential environmental contamination risks associated with improper catalyst disposal.
Economic sustainability also improves markedly with extended catalyst performance. The total cost of ownership decreases as replacement frequency diminishes, enhancing process economics and potentially enabling more competitive pricing for end products. This economic advantage can facilitate broader adoption of environmentally superior technologies across various industrial sectors.
Carbon footprint reduction represents perhaps the most significant sustainability impact. By addressing coking and sintering issues effectively, the comprehensive lifecycle emissions associated with catalyst production, transportation, installation, and disposal decrease substantially. This aligns with global decarbonization efforts and supports corporate sustainability goals and regulatory compliance requirements in increasingly carbon-constrained operating environments.
Reduced raw material consumption stands as a primary sustainability benefit of prolonged catalyst lifecycles. Catalysts often contain precious metals and rare earth elements whose extraction involves energy-intensive mining operations with substantial environmental impacts. By extending catalyst life through minimized coking and sintering, industries can significantly decrease their demand for these finite resources, contributing to conservation efforts and reducing extraction-related environmental degradation.
Energy conservation represents another crucial sustainability advantage. The manufacturing of fresh catalysts requires considerable energy inputs across multiple production stages. When catalysts maintain their activity for extended periods through effective anti-coking and anti-sintering strategies, the frequency of energy-intensive replacement cycles decreases proportionally, resulting in substantial energy savings and reduced carbon emissions throughout the industrial value chain.
Waste reduction constitutes a third major sustainability benefit. Spent catalysts often contain hazardous materials requiring specialized disposal procedures or complex recycling processes. Extended catalyst lifecycles directly translate to reduced waste generation rates, alleviating pressure on waste management systems and minimizing potential environmental contamination risks associated with improper catalyst disposal.
Economic sustainability also improves markedly with extended catalyst performance. The total cost of ownership decreases as replacement frequency diminishes, enhancing process economics and potentially enabling more competitive pricing for end products. This economic advantage can facilitate broader adoption of environmentally superior technologies across various industrial sectors.
Carbon footprint reduction represents perhaps the most significant sustainability impact. By addressing coking and sintering issues effectively, the comprehensive lifecycle emissions associated with catalyst production, transportation, installation, and disposal decrease substantially. This aligns with global decarbonization efforts and supports corporate sustainability goals and regulatory compliance requirements in increasingly carbon-constrained operating environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!