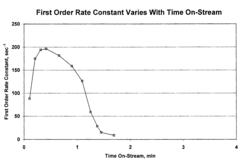
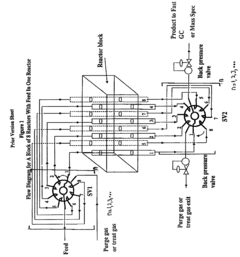
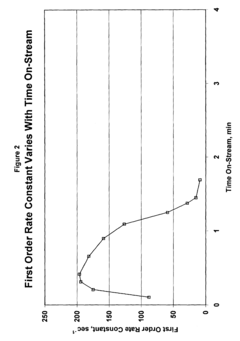
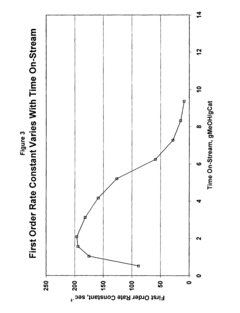
Catalyst Screening For Vitrimer Exchange: High-Throughput Reaction-Rate Assays
AUG 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vitrimer Exchange Catalysis Background and Objectives
Vitrimers represent a groundbreaking class of polymer materials that combine the processability of thermoplastics with the mechanical robustness of thermosets. First introduced by Leibler and colleagues in 2011, these materials feature dynamic covalent bonds that enable network rearrangement while maintaining crosslink density. The evolution of vitrimer technology has progressed from initial proof-of-concept systems to increasingly sophisticated materials with tailored properties for specific applications.
The catalysis of exchange reactions in vitrimers plays a pivotal role in determining their processing characteristics and mechanical behavior. Early vitrimer systems relied primarily on transesterification reactions catalyzed by metal salts or organic bases. Subsequent developments have expanded the repertoire of exchange chemistries to include disulfide exchange, imine exchange, and boronic ester exchange, among others, each requiring specific catalytic systems.
Current trends in vitrimer technology focus on achieving precise control over exchange kinetics, enabling materials with programmable relaxation times and mechanical properties. The field is moving toward catalytic systems that can be externally triggered or regulated, allowing for spatiotemporal control over material properties. Additionally, there is growing interest in developing catalysts that enable exchange reactions under milder conditions or with enhanced selectivity.
The primary objective of high-throughput catalyst screening for vitrimer exchange reactions is to systematically identify and optimize catalytic systems that can efficiently promote dynamic bond exchange while meeting application-specific requirements. This includes identifying catalysts with optimal activity, selectivity, and stability under various processing and service conditions.
Specific technical goals include developing standardized methodologies for quantifying catalyst performance in vitrimer systems, establishing structure-activity relationships for different catalyst classes, and creating predictive models to guide catalyst design. Furthermore, the research aims to identify catalysts that can operate effectively across a broad temperature range, exhibit minimal leaching or degradation, and maintain activity over multiple processing cycles.
Another critical objective is to develop catalytic systems that enable orthogonal control over different exchange reactions within the same material, potentially allowing for hierarchical structuring and multi-responsive behavior. Additionally, there is significant interest in catalysts that can be incorporated into existing industrial polymer processing workflows without requiring specialized equipment or extensive modifications to established manufacturing protocols.
The ultimate goal is to establish a comprehensive catalyst library with well-characterized performance metrics, enabling materials scientists and engineers to rationally select optimal catalytic systems for specific vitrimer applications ranging from automotive components and electronics to biomedical devices and sustainable packaging materials.
The catalysis of exchange reactions in vitrimers plays a pivotal role in determining their processing characteristics and mechanical behavior. Early vitrimer systems relied primarily on transesterification reactions catalyzed by metal salts or organic bases. Subsequent developments have expanded the repertoire of exchange chemistries to include disulfide exchange, imine exchange, and boronic ester exchange, among others, each requiring specific catalytic systems.
Current trends in vitrimer technology focus on achieving precise control over exchange kinetics, enabling materials with programmable relaxation times and mechanical properties. The field is moving toward catalytic systems that can be externally triggered or regulated, allowing for spatiotemporal control over material properties. Additionally, there is growing interest in developing catalysts that enable exchange reactions under milder conditions or with enhanced selectivity.
The primary objective of high-throughput catalyst screening for vitrimer exchange reactions is to systematically identify and optimize catalytic systems that can efficiently promote dynamic bond exchange while meeting application-specific requirements. This includes identifying catalysts with optimal activity, selectivity, and stability under various processing and service conditions.
Specific technical goals include developing standardized methodologies for quantifying catalyst performance in vitrimer systems, establishing structure-activity relationships for different catalyst classes, and creating predictive models to guide catalyst design. Furthermore, the research aims to identify catalysts that can operate effectively across a broad temperature range, exhibit minimal leaching or degradation, and maintain activity over multiple processing cycles.
Another critical objective is to develop catalytic systems that enable orthogonal control over different exchange reactions within the same material, potentially allowing for hierarchical structuring and multi-responsive behavior. Additionally, there is significant interest in catalysts that can be incorporated into existing industrial polymer processing workflows without requiring specialized equipment or extensive modifications to established manufacturing protocols.
The ultimate goal is to establish a comprehensive catalyst library with well-characterized performance metrics, enabling materials scientists and engineers to rationally select optimal catalytic systems for specific vitrimer applications ranging from automotive components and electronics to biomedical devices and sustainable packaging materials.
Market Analysis for Vitrimer Materials and Applications
The global vitrimer materials market is experiencing significant growth, driven by increasing demand for advanced polymers with self-healing and recyclable properties. Current market valuations indicate that the smart polymers sector, which includes vitrimers, is expanding at a compound annual growth rate of approximately 22% and is projected to reach substantial market value by 2028. This growth trajectory is particularly notable in regions with strong manufacturing bases such as North America, Europe, and East Asia.
Vitrimers represent a revolutionary class of polymers that combine the processability of thermoplastics with the mechanical strength and chemical resistance of thermosets. This unique combination addresses critical market needs across multiple industries seeking sustainable material solutions. The automotive sector has emerged as a primary adopter, incorporating vitrimers into lightweight components to improve fuel efficiency while maintaining structural integrity. Additionally, aerospace applications are gaining momentum as manufacturers seek materials that can withstand extreme conditions while offering repairability.
The electronics industry presents another substantial market opportunity, with vitrimers being explored for flexible displays, circuit boards, and protective coatings. Their ability to be reworked and repaired aligns perfectly with the industry's push toward reducing electronic waste. Similarly, the construction sector is investigating vitrimers for smart building materials that can adapt to environmental changes and self-heal when damaged.
Healthcare applications represent a high-value niche market, with vitrimers being developed for medical devices, implants, and drug delivery systems. The biocompatibility and tunable properties of certain vitrimer formulations make them particularly attractive for these applications, commanding premium pricing in the marketplace.
Market analysis reveals that end-users are primarily concerned with performance reliability, processing ease, and total lifecycle costs when considering vitrimer adoption. The ability to efficiently screen catalysts for vitrimer exchange reactions directly addresses these concerns by enabling manufacturers to optimize formulations for specific applications. High-throughput reaction-rate assays are therefore positioned as critical enabling technologies that can accelerate market penetration by reducing development cycles and improving performance predictability.
Competitive analysis indicates that major chemical companies and specialized polymer manufacturers are investing heavily in vitrimer research and development. Strategic partnerships between material suppliers, catalyst developers, and end-users are becoming increasingly common as the industry recognizes the need for collaborative innovation to overcome technical challenges and scale production.
Vitrimers represent a revolutionary class of polymers that combine the processability of thermoplastics with the mechanical strength and chemical resistance of thermosets. This unique combination addresses critical market needs across multiple industries seeking sustainable material solutions. The automotive sector has emerged as a primary adopter, incorporating vitrimers into lightweight components to improve fuel efficiency while maintaining structural integrity. Additionally, aerospace applications are gaining momentum as manufacturers seek materials that can withstand extreme conditions while offering repairability.
The electronics industry presents another substantial market opportunity, with vitrimers being explored for flexible displays, circuit boards, and protective coatings. Their ability to be reworked and repaired aligns perfectly with the industry's push toward reducing electronic waste. Similarly, the construction sector is investigating vitrimers for smart building materials that can adapt to environmental changes and self-heal when damaged.
Healthcare applications represent a high-value niche market, with vitrimers being developed for medical devices, implants, and drug delivery systems. The biocompatibility and tunable properties of certain vitrimer formulations make them particularly attractive for these applications, commanding premium pricing in the marketplace.
Market analysis reveals that end-users are primarily concerned with performance reliability, processing ease, and total lifecycle costs when considering vitrimer adoption. The ability to efficiently screen catalysts for vitrimer exchange reactions directly addresses these concerns by enabling manufacturers to optimize formulations for specific applications. High-throughput reaction-rate assays are therefore positioned as critical enabling technologies that can accelerate market penetration by reducing development cycles and improving performance predictability.
Competitive analysis indicates that major chemical companies and specialized polymer manufacturers are investing heavily in vitrimer research and development. Strategic partnerships between material suppliers, catalyst developers, and end-users are becoming increasingly common as the industry recognizes the need for collaborative innovation to overcome technical challenges and scale production.
Current Challenges in Catalyst Screening for Vitrimers
The catalyst screening process for vitrimers faces significant challenges that impede efficient development of these innovative materials. Traditional screening methods often rely on time-consuming mechanical testing or complex rheological measurements, creating bottlenecks in the discovery pipeline. These conventional approaches typically require large sample quantities and extensive preparation time, limiting throughput and increasing development costs.
A fundamental challenge lies in the correlation between catalyst performance and the dynamic exchange reactions that define vitrimer behavior. Catalysts that excel in model compound studies frequently demonstrate different efficacy when incorporated into complex polymer networks, creating a disconnect between laboratory screening and practical application. This discrepancy stems from the complex microenvironment within polymer matrices, where catalyst mobility, accessibility, and local concentration significantly impact exchange kinetics.
Temperature dependence presents another critical challenge, as catalyst performance often varies dramatically across different thermal conditions. Most screening protocols evaluate catalysts at a single temperature point, failing to capture the Arrhenius parameters necessary for predicting performance across the broad temperature ranges encountered in real-world applications. This limitation creates significant blind spots in catalyst selection.
The diversity of exchange chemistries in vitrimers further complicates screening efforts. Different bond exchange mechanisms—transesterification, disulfide exchange, imine exchange, among others—require specialized screening approaches. A catalyst highly effective for one chemistry may prove ineffective for another, necessitating tailored screening methodologies for each exchange mechanism rather than a universal approach.
Quantification challenges persist throughout the screening process. Current methods struggle to provide precise, reproducible kinetic parameters that correlate directly with material performance. The relationship between microscopic exchange rates and macroscopic material properties remains poorly understood, making it difficult to translate screening results into predictive models for material behavior.
Miniaturization and parallelization of screening assays represent significant technical hurdles. While high-throughput approaches have revolutionized other areas of materials science, their application to vitrimer catalyst screening remains limited by the need for specialized equipment and analytical techniques capable of measuring dynamic bond exchange in real-time across multiple samples simultaneously.
Lastly, the multifunctional nature of many catalysts introduces complexity in performance evaluation. Beyond accelerating exchange reactions, catalysts may influence network formation, alter mechanical properties, or impact material stability. Comprehensive screening must account for these secondary effects, requiring multidimensional analysis rather than simple rate measurements.
A fundamental challenge lies in the correlation between catalyst performance and the dynamic exchange reactions that define vitrimer behavior. Catalysts that excel in model compound studies frequently demonstrate different efficacy when incorporated into complex polymer networks, creating a disconnect between laboratory screening and practical application. This discrepancy stems from the complex microenvironment within polymer matrices, where catalyst mobility, accessibility, and local concentration significantly impact exchange kinetics.
Temperature dependence presents another critical challenge, as catalyst performance often varies dramatically across different thermal conditions. Most screening protocols evaluate catalysts at a single temperature point, failing to capture the Arrhenius parameters necessary for predicting performance across the broad temperature ranges encountered in real-world applications. This limitation creates significant blind spots in catalyst selection.
The diversity of exchange chemistries in vitrimers further complicates screening efforts. Different bond exchange mechanisms—transesterification, disulfide exchange, imine exchange, among others—require specialized screening approaches. A catalyst highly effective for one chemistry may prove ineffective for another, necessitating tailored screening methodologies for each exchange mechanism rather than a universal approach.
Quantification challenges persist throughout the screening process. Current methods struggle to provide precise, reproducible kinetic parameters that correlate directly with material performance. The relationship between microscopic exchange rates and macroscopic material properties remains poorly understood, making it difficult to translate screening results into predictive models for material behavior.
Miniaturization and parallelization of screening assays represent significant technical hurdles. While high-throughput approaches have revolutionized other areas of materials science, their application to vitrimer catalyst screening remains limited by the need for specialized equipment and analytical techniques capable of measuring dynamic bond exchange in real-time across multiple samples simultaneously.
Lastly, the multifunctional nature of many catalysts introduces complexity in performance evaluation. Beyond accelerating exchange reactions, catalysts may influence network formation, alter mechanical properties, or impact material stability. Comprehensive screening must account for these secondary effects, requiring multidimensional analysis rather than simple rate measurements.
Methodologies for High-Throughput Reaction-Rate Assays
01 Metal-based catalysts for vitrimer exchange reactions
Metal-based catalysts, particularly transition metals, are effective in accelerating vitrimer exchange reactions. These catalysts can significantly enhance the reaction rate by lowering the activation energy required for bond exchange. Various metal compounds including zinc, tin, and titanium complexes have been shown to catalyze transesterification and other exchange reactions in vitrimers, leading to improved processing characteristics and faster stress relaxation times.- Metal-based catalysts for vitrimer exchange reactions: Metal-based catalysts, particularly transition metals, are effective in accelerating vitrimer exchange reactions. These catalysts can significantly enhance the reaction rate by lowering the activation energy required for bond exchange. Various metal compounds including zinc, tin, and titanium complexes have been shown to catalyze transesterification and other exchange reactions in vitrimers, allowing for faster network rearrangement while maintaining the material's structural integrity at elevated temperatures.
- Organic catalysts for controlling exchange reaction kinetics: Organic catalysts offer precise control over vitrimer exchange reaction rates. These include nitrogen-containing compounds like amines, imidazoles, and organophosphorus compounds that can catalyze various exchange reactions including transesterification and disulfide exchange. The advantage of organic catalysts lies in their tunability, allowing researchers to adjust reaction rates by modifying catalyst structure or concentration, which is crucial for tailoring vitrimer properties for specific applications.
- Temperature-dependent catalytic systems for vitrimers: Temperature-responsive catalytic systems enable controlled exchange reaction rates in vitrimers. These systems incorporate catalysts that become activated at specific temperature thresholds, allowing the material to maintain stability at service temperatures while enabling rapid network rearrangement at elevated temperatures. This approach provides vitrimers with excellent dimensional stability during normal use while facilitating processing, recycling, and self-healing capabilities when heat is applied.
- Acid-base catalysts for accelerating exchange reactions: Acid and base catalysts play a significant role in controlling vitrimer exchange reaction rates. Strong acids like p-toluenesulfonic acid or Lewis acids can effectively catalyze transesterification reactions, while bases such as tertiary amines or metal hydroxides catalyze other exchange mechanisms. The selection of appropriate acid or base catalysts allows for fine-tuning of reaction kinetics, which directly impacts the vitrimer's processing characteristics, stress relaxation behavior, and recyclability properties.
- Catalyst immobilization techniques for controlled reaction rates: Immobilization techniques for catalysts in vitrimer systems provide enhanced control over exchange reaction rates. By anchoring catalysts to polymer backbones or incorporating them within specific domains of the network, their activity can be regulated more precisely. This approach prevents catalyst leaching, extends material lifespan, and enables the creation of vitrimers with spatially controlled dynamic properties. Immobilized catalysts can maintain activity through multiple processing cycles while offering improved stability compared to free catalysts.
02 Organic catalysts for controlling exchange reaction kinetics
Organic catalysts offer precise control over vitrimer exchange reaction rates. These include nitrogen-containing compounds such as amines, imidazoles, and organophosphorus compounds that can catalyze various exchange reactions including transesterification and disulfide exchange. The advantage of organic catalysts lies in their tunability, allowing for adjustment of reaction rates based on catalyst concentration and structure, which enables better control of vitrimer processing and mechanical properties.Expand Specific Solutions03 Temperature-dependent catalytic systems for vitrimer exchange
Temperature plays a crucial role in catalytic vitrimer exchange reactions. Certain catalyst systems are designed to activate at specific temperature thresholds, allowing for controlled reaction rates during processing and application. These temperature-dependent catalytic systems enable vitrimers to maintain stability at service temperatures while allowing for rapid exchange reactions and reprocessability at elevated temperatures, effectively combining the benefits of thermosets and thermoplastics.Expand Specific Solutions04 Acid-base catalysts for accelerating bond exchange
Acid and base catalysts are effective in promoting various exchange reactions in vitrimer systems. Strong acids can catalyze transesterification and other exchange mechanisms, while bases can facilitate nucleophilic attacks in exchange processes. The combination of acid-base catalysts can create synergistic effects, significantly increasing reaction rates. These catalysts are particularly useful for controlling the dynamic behavior of vitrimers during processing and recycling stages.Expand Specific Solutions05 Novel catalyst delivery systems for controlled reaction rates
Innovative catalyst delivery systems have been developed to precisely control vitrimer exchange reaction rates. These include encapsulated catalysts, latent catalysts that activate under specific conditions, and catalyst immobilization techniques. Such systems allow for spatial and temporal control of the exchange reactions, enabling the production of vitrimers with gradient properties or programmable behavior. These advanced delivery methods enhance processing capabilities while maintaining the desired mechanical properties in the final material.Expand Specific Solutions
Leading Research Groups and Companies in Vitrimer Catalysis
The catalyst screening for vitrimer exchange technology is currently in an early growth phase, characterized by intensive research and development activities. The market for vitrimer materials is expanding rapidly, driven by increasing demand for recyclable and self-healing polymers across automotive, aerospace, and electronics industries. Key players demonstrate varying levels of technical maturity, with major petrochemical corporations like China Petroleum & Chemical Corp., ExxonMobil Technology & Engineering, and BASF leading industrial applications, while research institutions such as CNRS and Beijing University of Chemical Technology focus on fundamental catalyst innovations. Academic-industrial partnerships between universities (Carnegie Mellon, Harvard) and chemical companies are accelerating commercialization efforts, with high-throughput screening methodologies emerging as critical tools for identifying optimal catalysts for specific vitrimer applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced high-throughput catalyst screening platform specifically for vitrimer exchange reactions. Their technology employs parallel microreactors with integrated spectroscopic analysis capabilities to simultaneously evaluate multiple catalyst candidates under varying reaction conditions. The system utilizes automated liquid handling robots coupled with real-time monitoring via FTIR and Raman spectroscopy to track bond exchange kinetics. Sinopec's approach incorporates machine learning algorithms to analyze reaction rate data and identify structure-activity relationships among catalyst candidates. Their platform can process up to 96 different catalyst formulations in a single experimental run, dramatically accelerating the discovery process for optimal catalysts in vitrimer applications. The system also features controlled environmental chambers that can simulate industrial conditions, ensuring practical applicability of screening results.
Strengths: Exceptional throughput capacity with parallel testing capabilities; Integration of advanced spectroscopic techniques for real-time monitoring; Sophisticated data analysis through machine learning. Weaknesses: High initial investment costs; Requires specialized technical expertise to operate effectively; May have limitations in accurately simulating all industrial-scale reaction conditions.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has pioneered a comprehensive catalyst screening methodology for vitrimer exchange reactions that combines high-throughput experimentation with computational modeling. Their approach utilizes custom-designed microfluidic devices that enable precise control over reaction parameters while minimizing reagent consumption. The CNRS system incorporates in-situ monitoring via fluorescence spectroscopy and rheological measurements to track both chemical exchange rates and resulting mechanical property changes in real-time. A distinguishing feature of their technology is the integration of quantum chemical calculations to predict catalyst activity based on electronic structure, which guides experimental design and accelerates the discovery process. The platform employs automated sampling and analysis systems capable of handling diverse catalyst classes including Lewis acids, transesterification catalysts, and novel organometallic complexes. CNRS researchers have successfully applied this methodology to develop catalysts for both polyester and epoxy-based vitrimers, demonstrating its versatility across different dynamic covalent chemistries.
Strengths: Sophisticated integration of experimental and computational approaches; Excellent capability to correlate catalyst activity with mechanical property development; Versatility across multiple vitrimer chemistries. Weaknesses: Complex system architecture requiring interdisciplinary expertise; Higher analytical complexity compared to purely experimental approaches; Potential computational model limitations for novel catalyst structures.
Key Catalytic Mechanisms and Structure-Activity Relationships
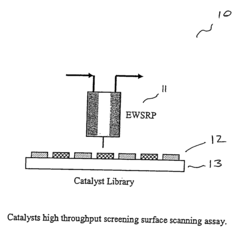
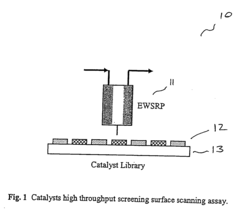
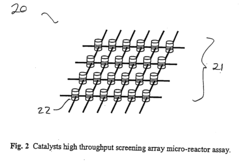
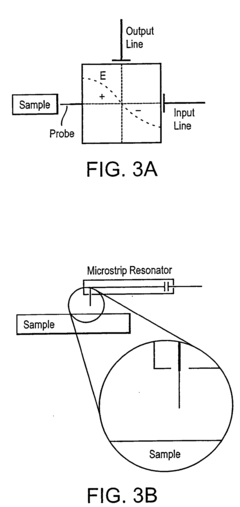
High throughput screening of catalysts using spin resonance
PatentInactiveUS20060160136A1
Innovation
- Development of an evanescent wave probe capable of performing electron spin resonance (ESR) and nuclear magnetic resonance (NMR) spectroscopy for localized detection in catalyst libraries and micro-reactor arrays, allowing for spatially resolved and high sensitivity analysis.
Method and apparatus for high throughput catalysts screening and optimization
PatentInactiveUS7390664B2
Innovation
- A system with multiple reactors, selection valve systems, and detection devices that allow for simultaneous testing, initialization, regeneration, and reactivation of catalysts while maintaining constant TOS, enabling comparison of catalyst activity and selectivity, and allowing different treatment processes for each reactor.
Sustainability Aspects of Vitrimer Catalyst Development
The development of sustainable catalysts for vitrimer exchange reactions represents a critical frontier in green chemistry and materials science. Current catalyst screening methodologies, while effective for identifying high-performance candidates, often overlook crucial sustainability parameters that will determine their long-term viability in industrial applications.
Environmental impact assessment of vitrimer catalysts must consider their entire lifecycle, from raw material extraction to end-of-life disposal. Many high-throughput screening protocols prioritize reaction rates and conversion efficiency without adequately addressing toxicity profiles, bioaccumulation potential, or persistence in environmental systems. Recent studies indicate that certain metal-based catalysts demonstrating excellent transesterification activity also present significant ecotoxicological concerns.
Renewable feedstock utilization presents a promising avenue for sustainable catalyst development. Bio-derived organocatalysts, particularly those based on modified amino acids and peptide structures, have shown encouraging preliminary results in vitrimer exchange reactions. These catalysts offer biodegradability advantages while maintaining acceptable reaction kinetics, though their thermal stability remains a challenge for high-temperature applications.
Energy efficiency considerations must be integrated into catalyst screening protocols. Traditional high-throughput methods often require energy-intensive conditions that may not reflect practical manufacturing scenarios. Developing screening assays that operate under ambient or near-ambient conditions would better identify catalysts with favorable energy profiles while maintaining industrial relevance.
Circular economy principles should guide next-generation catalyst design. Recyclability and recovery efficiency represent key sustainability metrics that current screening methodologies rarely address. Immobilized catalyst systems and magnetic recovery techniques show particular promise for vitrimer applications, enabling multiple reaction cycles without significant activity loss.
Regulatory compliance and safety considerations will increasingly influence catalyst selection. As global chemical regulations tighten, particularly regarding persistent, bioaccumulative, and toxic substances, screening protocols must incorporate predictive toxicology assessments. This proactive approach would identify potential regulatory barriers early in the development process.
Economic sustainability remains essential for industrial adoption. Cost-benefit analyses incorporating raw material availability, synthesis complexity, and catalyst longevity should complement technical performance metrics in screening protocols. Catalysts derived from earth-abundant elements offer particular advantages in this regard, though their activity profiles often require optimization.
Environmental impact assessment of vitrimer catalysts must consider their entire lifecycle, from raw material extraction to end-of-life disposal. Many high-throughput screening protocols prioritize reaction rates and conversion efficiency without adequately addressing toxicity profiles, bioaccumulation potential, or persistence in environmental systems. Recent studies indicate that certain metal-based catalysts demonstrating excellent transesterification activity also present significant ecotoxicological concerns.
Renewable feedstock utilization presents a promising avenue for sustainable catalyst development. Bio-derived organocatalysts, particularly those based on modified amino acids and peptide structures, have shown encouraging preliminary results in vitrimer exchange reactions. These catalysts offer biodegradability advantages while maintaining acceptable reaction kinetics, though their thermal stability remains a challenge for high-temperature applications.
Energy efficiency considerations must be integrated into catalyst screening protocols. Traditional high-throughput methods often require energy-intensive conditions that may not reflect practical manufacturing scenarios. Developing screening assays that operate under ambient or near-ambient conditions would better identify catalysts with favorable energy profiles while maintaining industrial relevance.
Circular economy principles should guide next-generation catalyst design. Recyclability and recovery efficiency represent key sustainability metrics that current screening methodologies rarely address. Immobilized catalyst systems and magnetic recovery techniques show particular promise for vitrimer applications, enabling multiple reaction cycles without significant activity loss.
Regulatory compliance and safety considerations will increasingly influence catalyst selection. As global chemical regulations tighten, particularly regarding persistent, bioaccumulative, and toxic substances, screening protocols must incorporate predictive toxicology assessments. This proactive approach would identify potential regulatory barriers early in the development process.
Economic sustainability remains essential for industrial adoption. Cost-benefit analyses incorporating raw material availability, synthesis complexity, and catalyst longevity should complement technical performance metrics in screening protocols. Catalysts derived from earth-abundant elements offer particular advantages in this regard, though their activity profiles often require optimization.
Scalability and Industrial Implementation Considerations
The scalability of high-throughput catalyst screening methodologies for vitrimer exchange reactions presents significant opportunities for industrial implementation. Current laboratory-scale screening approaches must be evaluated for their potential to operate at production scales. The transition from microliter reaction volumes to industrial batch sizes requires careful engineering considerations regarding mixing efficiency, heat transfer, and reaction homogeneity. These factors can significantly impact catalyst performance metrics when scaling up from high-throughput screening platforms to industrial processes.
Equipment compatibility represents another critical consideration. Industrial implementation necessitates robust automation systems capable of handling larger material quantities while maintaining the precision and reproducibility achieved in laboratory screening. Specialized reactor designs may be required to accommodate the unique rheological properties of vitrimer materials during processing. Additionally, in-line monitoring technologies must be adapted to provide real-time feedback on exchange reactions at industrial scales, potentially requiring different analytical approaches than those used in high-throughput screening.
Economic feasibility remains paramount for successful industrial adoption. The cost-benefit analysis must account for catalyst loading requirements, reaction time optimization, and energy consumption across different production volumes. High-performing catalysts identified through screening must demonstrate cost-effectiveness when implemented at scale, considering both initial investment and operational expenses. Furthermore, recovery and recycling systems for expensive catalysts should be developed to improve process economics and sustainability.
Regulatory compliance and safety considerations become increasingly important at industrial scales. Catalyst systems that perform well in controlled laboratory environments must be evaluated for their safety profiles in production settings, including potential for dust formation, thermal stability under prolonged operation, and compatibility with existing industrial safety protocols. Environmental impact assessments must address waste streams, emissions, and potential catalyst leaching in final products.
Process integration represents the final hurdle for industrial implementation. High-throughput screening results must translate into practical manufacturing workflows that align with existing production capabilities. This includes considerations for continuous vs. batch processing options, integration with current manufacturing lines, and development of standard operating procedures. Pilot-scale testing serves as a critical intermediate step to validate screening results and refine process parameters before full industrial deployment, helping to identify and mitigate unforeseen challenges in scaling catalyst-mediated vitrimer exchange reactions.
Equipment compatibility represents another critical consideration. Industrial implementation necessitates robust automation systems capable of handling larger material quantities while maintaining the precision and reproducibility achieved in laboratory screening. Specialized reactor designs may be required to accommodate the unique rheological properties of vitrimer materials during processing. Additionally, in-line monitoring technologies must be adapted to provide real-time feedback on exchange reactions at industrial scales, potentially requiring different analytical approaches than those used in high-throughput screening.
Economic feasibility remains paramount for successful industrial adoption. The cost-benefit analysis must account for catalyst loading requirements, reaction time optimization, and energy consumption across different production volumes. High-performing catalysts identified through screening must demonstrate cost-effectiveness when implemented at scale, considering both initial investment and operational expenses. Furthermore, recovery and recycling systems for expensive catalysts should be developed to improve process economics and sustainability.
Regulatory compliance and safety considerations become increasingly important at industrial scales. Catalyst systems that perform well in controlled laboratory environments must be evaluated for their safety profiles in production settings, including potential for dust formation, thermal stability under prolonged operation, and compatibility with existing industrial safety protocols. Environmental impact assessments must address waste streams, emissions, and potential catalyst leaching in final products.
Process integration represents the final hurdle for industrial implementation. High-throughput screening results must translate into practical manufacturing workflows that align with existing production capabilities. This includes considerations for continuous vs. batch processing options, integration with current manufacturing lines, and development of standard operating procedures. Pilot-scale testing serves as a critical intermediate step to validate screening results and refine process parameters before full industrial deployment, helping to identify and mitigate unforeseen challenges in scaling catalyst-mediated vitrimer exchange reactions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!