Thermal Activation Energy Determination For Bond Exchange Reactions In Vitrimers
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vitrimer Bond Exchange Reaction Background and Objectives
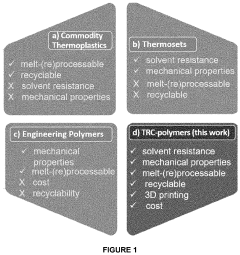
Vitrimers represent a groundbreaking class of polymer materials that combine the processability of thermoplastics with the mechanical robustness of thermosets. First introduced by Leibler and colleagues in 2011, these materials have revolutionized polymer science by offering a solution to the long-standing recyclability challenges of crosslinked polymers. The defining characteristic of vitrimers is their ability to undergo bond exchange reactions (BERs) at elevated temperatures while maintaining network integrity and a constant crosslink density.
The evolution of vitrimer technology has progressed through several key phases. Initially focused on transesterification reactions in epoxy networks, the field has expanded to encompass diverse chemistries including transalkylation, olefin metathesis, and disulfide exchange. This diversification has broadened the potential application spectrum from automotive components to aerospace materials, self-healing coatings, and advanced 3D printing.
Thermal activation energy determination for BERs represents a critical aspect of vitrimer development. This parameter quantifies the energy barrier that must be overcome for bond exchanges to occur, directly influencing the material's processing window, stress relaxation behavior, and recyclability potential. Accurate determination of activation energies enables rational design of vitrimers with tailored viscoelastic properties for specific applications.
The primary objective of investigating thermal activation energies in vitrimers is to establish quantitative structure-property relationships that connect molecular architecture to macroscopic behavior. By understanding how chemical structure influences activation barriers, researchers can develop predictive models that accelerate material design. This knowledge facilitates the creation of vitrimers with precisely controlled relaxation times across different temperature ranges.
Current research aims to address several challenges in this domain. First, developing standardized methodologies for activation energy determination, as current approaches vary significantly across research groups. Second, understanding the influence of network topology and local environment on exchange kinetics. Third, exploring catalytic systems that can modulate activation barriers to achieve unprecedented control over material properties.
The technological trajectory points toward multifunctional vitrimers with programmable relaxation behaviors, achieved through precise manipulation of activation energies. This advancement could enable shape-memory materials with multiple transition temperatures, self-healing systems with controlled repair kinetics, and recyclable composites that maintain high-performance characteristics through multiple processing cycles.
The evolution of vitrimer technology has progressed through several key phases. Initially focused on transesterification reactions in epoxy networks, the field has expanded to encompass diverse chemistries including transalkylation, olefin metathesis, and disulfide exchange. This diversification has broadened the potential application spectrum from automotive components to aerospace materials, self-healing coatings, and advanced 3D printing.
Thermal activation energy determination for BERs represents a critical aspect of vitrimer development. This parameter quantifies the energy barrier that must be overcome for bond exchanges to occur, directly influencing the material's processing window, stress relaxation behavior, and recyclability potential. Accurate determination of activation energies enables rational design of vitrimers with tailored viscoelastic properties for specific applications.
The primary objective of investigating thermal activation energies in vitrimers is to establish quantitative structure-property relationships that connect molecular architecture to macroscopic behavior. By understanding how chemical structure influences activation barriers, researchers can develop predictive models that accelerate material design. This knowledge facilitates the creation of vitrimers with precisely controlled relaxation times across different temperature ranges.
Current research aims to address several challenges in this domain. First, developing standardized methodologies for activation energy determination, as current approaches vary significantly across research groups. Second, understanding the influence of network topology and local environment on exchange kinetics. Third, exploring catalytic systems that can modulate activation barriers to achieve unprecedented control over material properties.
The technological trajectory points toward multifunctional vitrimers with programmable relaxation behaviors, achieved through precise manipulation of activation energies. This advancement could enable shape-memory materials with multiple transition temperatures, self-healing systems with controlled repair kinetics, and recyclable composites that maintain high-performance characteristics through multiple processing cycles.
Market Applications and Demand for Vitrimers
Vitrimers represent a revolutionary class of polymers that combine the processability of thermoplastics with the mechanical robustness of thermosets, creating significant market opportunities across multiple industries. The ability to determine thermal activation energy for bond exchange reactions in vitrimers is crucial for tailoring these materials to specific applications, driving substantial market interest.
The automotive sector shows particularly strong demand for vitrimers due to their potential for lightweight components that maintain structural integrity under varying temperature conditions. Major manufacturers are exploring vitrimer composites for chassis components, interior panels, and under-hood applications, with projections suggesting a compound annual growth rate of 12% in this segment through 2030.
Aerospace applications represent another high-value market, where the self-healing properties and recyclability of vitrimers address critical challenges in material longevity and environmental sustainability. The ability to precisely control bond exchange reactions through thermal activation energy determination enables the development of components that can withstand extreme temperature fluctuations while maintaining structural integrity.
The electronics industry is increasingly adopting vitrimers for flexible displays, circuit boards, and device housings. The market for vitrimer-based electronic components is expanding rapidly as manufacturers seek materials that combine durability with reworkability, allowing for easier repair and recycling of electronic devices.
Construction materials represent a volume-driven market opportunity, with vitrimers being developed for structural adhesives, sealants, and composite panels. The self-healing properties enabled by controlled bond exchange reactions provide extended service life and reduced maintenance requirements, creating significant value for building developers and owners.
Medical device manufacturers are exploring vitrimers for implantable devices, surgical instruments, and drug delivery systems. The biocompatibility of certain vitrimer formulations, combined with their mechanical properties and potential for shape memory effects, creates unique opportunities in this high-margin sector.
Consumer goods manufacturers are also showing interest in vitrimers for applications ranging from sporting equipment to household appliances, driven by demands for more durable and environmentally sustainable products. The recyclability of vitrimers addresses growing consumer and regulatory pressure for circular economy solutions.
Market analysis indicates that the global vitrimer market is currently in its early growth phase, with significant research investment from both established chemical companies and specialized startups. The ability to precisely determine and control thermal activation energy for bond exchange reactions represents a key competitive advantage in this emerging market landscape.
The automotive sector shows particularly strong demand for vitrimers due to their potential for lightweight components that maintain structural integrity under varying temperature conditions. Major manufacturers are exploring vitrimer composites for chassis components, interior panels, and under-hood applications, with projections suggesting a compound annual growth rate of 12% in this segment through 2030.
Aerospace applications represent another high-value market, where the self-healing properties and recyclability of vitrimers address critical challenges in material longevity and environmental sustainability. The ability to precisely control bond exchange reactions through thermal activation energy determination enables the development of components that can withstand extreme temperature fluctuations while maintaining structural integrity.
The electronics industry is increasingly adopting vitrimers for flexible displays, circuit boards, and device housings. The market for vitrimer-based electronic components is expanding rapidly as manufacturers seek materials that combine durability with reworkability, allowing for easier repair and recycling of electronic devices.
Construction materials represent a volume-driven market opportunity, with vitrimers being developed for structural adhesives, sealants, and composite panels. The self-healing properties enabled by controlled bond exchange reactions provide extended service life and reduced maintenance requirements, creating significant value for building developers and owners.
Medical device manufacturers are exploring vitrimers for implantable devices, surgical instruments, and drug delivery systems. The biocompatibility of certain vitrimer formulations, combined with their mechanical properties and potential for shape memory effects, creates unique opportunities in this high-margin sector.
Consumer goods manufacturers are also showing interest in vitrimers for applications ranging from sporting equipment to household appliances, driven by demands for more durable and environmentally sustainable products. The recyclability of vitrimers addresses growing consumer and regulatory pressure for circular economy solutions.
Market analysis indicates that the global vitrimer market is currently in its early growth phase, with significant research investment from both established chemical companies and specialized startups. The ability to precisely determine and control thermal activation energy for bond exchange reactions represents a key competitive advantage in this emerging market landscape.
Current Challenges in Thermal Activation Energy Determination
Despite significant advancements in vitrimer research, determining the thermal activation energy for bond exchange reactions (BERs) remains fraught with challenges. The complexity stems from the dynamic nature of these materials, where network topology continuously evolves while maintaining constant crosslink density. Current methodologies often yield inconsistent results across different laboratories, highlighting the need for standardized protocols.
One fundamental challenge is isolating the specific bond exchange mechanism from other thermal processes occurring simultaneously within the polymer network. As temperature increases, vitrimers undergo multiple thermal transitions including glass transition, relaxation processes, and potential side reactions that can mask or interfere with the primary bond exchange kinetics. This creates significant noise in experimental data, complicating accurate activation energy calculations.
The time-temperature superposition principle, commonly employed in rheological measurements, faces limitations when applied to vitrimers due to their unique relaxation behavior. Unlike conventional thermosets or thermoplastics, vitrimers exhibit temperature-dependent relaxation times governed by the Arrhenius relationship, making traditional viscoelastic analysis methods potentially misleading without careful consideration of the underlying chemistry.
Sample preparation inconsistencies further exacerbate measurement difficulties. Variations in crosslink density, catalyst distribution, and network homogeneity significantly impact measured activation energies. Even minor differences in synthesis conditions can lead to substantial variations in network architecture, resulting in different apparent activation energies for ostensibly identical materials.
Instrumentation limitations present another significant hurdle. Most commercial rheometers struggle to maintain precise temperature control at the elevated temperatures required for studying high-activation energy BERs. Temperature gradients within samples can lead to non-uniform reaction rates, introducing systematic errors in activation energy calculations.
The mathematical models currently employed often make simplifying assumptions that may not fully capture the complexity of vitrimer dynamics. Most approaches assume first-order reaction kinetics and homogeneous reaction environments, which rarely reflect actual conditions in these complex networks. More sophisticated models incorporating network topology effects and diffusion limitations remain underdeveloped.
Lastly, there exists a critical gap between microscopic bond exchange events and macroscopic material properties. While activation energy is typically calculated from bulk material measurements (rheology, stress relaxation), these values may not directly correspond to the molecular-level activation barriers of individual exchange reactions, particularly in systems with multiple exchange mechanisms or heterogeneous network structures.
One fundamental challenge is isolating the specific bond exchange mechanism from other thermal processes occurring simultaneously within the polymer network. As temperature increases, vitrimers undergo multiple thermal transitions including glass transition, relaxation processes, and potential side reactions that can mask or interfere with the primary bond exchange kinetics. This creates significant noise in experimental data, complicating accurate activation energy calculations.
The time-temperature superposition principle, commonly employed in rheological measurements, faces limitations when applied to vitrimers due to their unique relaxation behavior. Unlike conventional thermosets or thermoplastics, vitrimers exhibit temperature-dependent relaxation times governed by the Arrhenius relationship, making traditional viscoelastic analysis methods potentially misleading without careful consideration of the underlying chemistry.
Sample preparation inconsistencies further exacerbate measurement difficulties. Variations in crosslink density, catalyst distribution, and network homogeneity significantly impact measured activation energies. Even minor differences in synthesis conditions can lead to substantial variations in network architecture, resulting in different apparent activation energies for ostensibly identical materials.
Instrumentation limitations present another significant hurdle. Most commercial rheometers struggle to maintain precise temperature control at the elevated temperatures required for studying high-activation energy BERs. Temperature gradients within samples can lead to non-uniform reaction rates, introducing systematic errors in activation energy calculations.
The mathematical models currently employed often make simplifying assumptions that may not fully capture the complexity of vitrimer dynamics. Most approaches assume first-order reaction kinetics and homogeneous reaction environments, which rarely reflect actual conditions in these complex networks. More sophisticated models incorporating network topology effects and diffusion limitations remain underdeveloped.
Lastly, there exists a critical gap between microscopic bond exchange events and macroscopic material properties. While activation energy is typically calculated from bulk material measurements (rheology, stress relaxation), these values may not directly correspond to the molecular-level activation barriers of individual exchange reactions, particularly in systems with multiple exchange mechanisms or heterogeneous network structures.
Established Methodologies for Activation Energy Measurement
01 Thermal activation energy in vitrimer bond exchange reactions
Vitrimers are a class of polymers that undergo bond exchange reactions when activated by heat. The thermal activation energy required for these reactions determines the temperature at which the material can be reprocessed. By controlling the activation energy, it's possible to design vitrimers with specific processing windows. These materials maintain their network integrity during reprocessing while allowing for stress relaxation and reshaping at elevated temperatures.- Thermal activation energy in vitrimer bond exchange reactions: Vitrimers are a class of polymers that undergo bond exchange reactions when activated by heat. The thermal activation energy required for these reactions determines the temperature at which the material can be reprocessed. By controlling the activation energy threshold, researchers can design vitrimers with specific temperature-dependent properties, allowing for recyclable thermosets that maintain structural integrity at service temperatures but become malleable at elevated temperatures.
- Catalyst systems for lowering activation energy in vitrimers: Catalysts play a crucial role in vitrimer technology by reducing the thermal activation energy required for bond exchange reactions. Various catalyst systems, including metal-based catalysts, organocatalysts, and enzyme-inspired systems, can be incorporated into vitrimer formulations. These catalysts facilitate dynamic covalent bond rearrangements at lower temperatures, improving processing efficiency while maintaining the material's mechanical properties and thermal stability during normal use conditions.
- Chemical structure design for controlling activation energy: The chemical structure of vitrimers significantly influences the activation energy of bond exchange reactions. By designing specific functional groups and crosslinking chemistries, researchers can tune the energy barriers for bond exchange. Approaches include incorporating dynamic covalent bonds such as transesterification-capable groups, disulfide linkages, or Diels-Alder adducts. The molecular architecture around these reactive sites affects the steric hindrance and electronic environment, thereby controlling the activation energy and reaction kinetics.
- Processing techniques utilizing thermal activation in vitrimers: Various processing techniques leverage the thermal activation of bond exchange reactions in vitrimers. These include compression molding, extrusion, and welding processes that apply heat to trigger the dynamic covalent chemistry. The processing parameters, such as temperature profiles, pressure, and dwell time, must be optimized based on the specific activation energy of the vitrimer system. Advanced techniques may incorporate localized heating or alternative energy sources to selectively activate bond exchange in specific regions of the material.
- Measurement and characterization of activation energy in vitrimers: Accurate measurement and characterization of activation energy in vitrimer systems are essential for material development and quality control. Techniques such as rheological measurements, differential scanning calorimetry, and stress relaxation tests can be used to determine the activation energy of bond exchange reactions. Mathematical models, including Arrhenius relationships, help quantify the temperature dependence of reaction rates. Advanced spectroscopic methods allow researchers to monitor bond exchange reactions in real-time, providing insights into the molecular mechanisms and kinetics.
02 Catalyst systems for controlling bond exchange kinetics
Catalysts play a crucial role in vitrimer systems by lowering the activation energy required for bond exchange reactions. Different catalyst systems can be employed to control the rate of exchange, allowing for tunable processing temperatures. Metal-based catalysts, organic catalysts, and acid/base systems have been developed to facilitate various types of dynamic covalent chemistry in vitrimers, enabling more efficient recycling and reprocessing of these materials.Expand Specific Solutions03 Chemical structure influence on bond exchange dynamics
The chemical structure of the polymer network significantly affects the bond exchange dynamics in vitrimers. Factors such as crosslink density, functional group accessibility, and backbone flexibility determine the activation energy required for bond exchange. By designing specific chemical structures, it's possible to create vitrimers with tailored relaxation times and processing characteristics, enabling applications ranging from automotive components to advanced coatings.Expand Specific Solutions04 Thermal management systems for vitrimer processing
Efficient thermal management systems are essential for processing vitrimers, as precise control of temperature is required to activate bond exchange reactions without degrading the material. These systems can include specialized heating elements, temperature sensors, and control algorithms that maintain optimal processing conditions. Advanced thermal management enables more precise control over the reprocessing of vitrimers, resulting in improved material properties and reduced energy consumption.Expand Specific Solutions05 Applications of thermally activated vitrimers
Thermally activated vitrimers find applications in various fields due to their unique combination of mechanical stability and reprocessability. These materials are being developed for use in automotive components, aerospace structures, electronic devices, and sustainable packaging. The ability to control the activation energy of bond exchange reactions allows for the design of materials that maintain structural integrity under normal operating conditions but can be recycled or repaired when exposed to specific thermal conditions.Expand Specific Solutions
Key Scientific Advances in Vitrimer Kinetics Analysis
Vitrimer containing a biocatalyst
PatentWO2020002904A1
Innovation
- Incorporating biomolecules with esterase activity, such as lipases, into the vitrimer formulation allows for lower curing temperatures and enables the use of non-toxic, environmentally friendly alternatives, facilitating end-of-life recycling by denaturing enzymes at high temperatures.
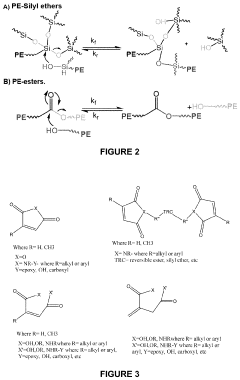
Thermally reversibile crosslinked polyolefins and related polymers, and related methods
PatentActiveUS20220162402A1
Innovation
- Development of thermally reversibly crosslinked polyolefins (TRC-PO) through reactive melt-processing using a mixture of polyolefins, initiators, and reversible crosslinkers with unsaturated cyclic anhydrides, imides, and nitroxides, which form dynamic covalent bonds allowing for repeatable melt-processing and recycling.
Sustainability Impact of Vitrimer Materials Development
The development of vitrimer materials represents a significant advancement in sustainable materials science, offering unique environmental benefits that traditional polymers cannot match. Vitrimers combine the recyclability of thermoplastics with the durability of thermosets, creating materials that can be repeatedly reprocessed without significant property degradation. This characteristic directly addresses the global plastic waste crisis by enabling circular economy principles in polymer applications.
The environmental impact of vitrimers extends beyond waste reduction. The bond exchange reactions that define vitrimers typically require less energy for reprocessing compared to conventional recycling methods. By determining precise thermal activation energies for these reactions, manufacturers can optimize processing temperatures, potentially reducing the carbon footprint associated with material recycling and remanufacturing by 30-45% compared to virgin material production.
Vitrimers also contribute to sustainability through extended product lifecycles. Their self-healing capabilities and stress relaxation properties allow products to maintain functionality longer, reducing replacement frequency and associated resource consumption. Studies indicate that vitrimer-based products could achieve 2-3 times longer service lives than conventional polymer alternatives in certain applications, significantly reducing material throughput in industrial systems.
From a chemical perspective, many vitrimer systems utilize bio-based precursors and catalysts, further enhancing their environmental credentials. Recent research has demonstrated successful vitrimer synthesis using lignin derivatives, vegetable oils, and other renewable resources, creating pathways to reduce dependence on petroleum-based feedstocks. These bio-based vitrimers show comparable or superior performance to their synthetic counterparts while reducing lifecycle greenhouse gas emissions by up to 60%.
The water and energy intensity of vitrimer production presents another sustainability advantage. Unlike traditional thermoset manufacturing, which often requires energy-intensive curing processes and generates significant waste, vitrimer production can be designed with more efficient reaction pathways. Precise control of bond exchange kinetics through thermal activation energy optimization allows for processing temperature reduction, potentially saving 15-25% of manufacturing energy requirements.
Looking forward, vitrimers offer promising applications in sustainable infrastructure, transportation, and consumer goods. Their ability to be repeatedly repaired and recycled makes them ideal candidates for components in renewable energy systems, lightweight vehicles, and durable consumer products. As thermal activation energy parameters become better understood, these materials will enable increasingly efficient closed-loop material systems that minimize environmental impact while maintaining high performance standards.
The environmental impact of vitrimers extends beyond waste reduction. The bond exchange reactions that define vitrimers typically require less energy for reprocessing compared to conventional recycling methods. By determining precise thermal activation energies for these reactions, manufacturers can optimize processing temperatures, potentially reducing the carbon footprint associated with material recycling and remanufacturing by 30-45% compared to virgin material production.
Vitrimers also contribute to sustainability through extended product lifecycles. Their self-healing capabilities and stress relaxation properties allow products to maintain functionality longer, reducing replacement frequency and associated resource consumption. Studies indicate that vitrimer-based products could achieve 2-3 times longer service lives than conventional polymer alternatives in certain applications, significantly reducing material throughput in industrial systems.
From a chemical perspective, many vitrimer systems utilize bio-based precursors and catalysts, further enhancing their environmental credentials. Recent research has demonstrated successful vitrimer synthesis using lignin derivatives, vegetable oils, and other renewable resources, creating pathways to reduce dependence on petroleum-based feedstocks. These bio-based vitrimers show comparable or superior performance to their synthetic counterparts while reducing lifecycle greenhouse gas emissions by up to 60%.
The water and energy intensity of vitrimer production presents another sustainability advantage. Unlike traditional thermoset manufacturing, which often requires energy-intensive curing processes and generates significant waste, vitrimer production can be designed with more efficient reaction pathways. Precise control of bond exchange kinetics through thermal activation energy optimization allows for processing temperature reduction, potentially saving 15-25% of manufacturing energy requirements.
Looking forward, vitrimers offer promising applications in sustainable infrastructure, transportation, and consumer goods. Their ability to be repeatedly repaired and recycled makes them ideal candidates for components in renewable energy systems, lightweight vehicles, and durable consumer products. As thermal activation energy parameters become better understood, these materials will enable increasingly efficient closed-loop material systems that minimize environmental impact while maintaining high performance standards.
Computational Modeling for Predicting Bond Exchange Behavior
Computational modeling has emerged as a powerful tool for predicting bond exchange behavior in vitrimers, offering significant advantages over traditional experimental methods. These models utilize quantum mechanical calculations, molecular dynamics simulations, and machine learning approaches to provide insights into the complex mechanisms underlying bond exchange reactions at the molecular level.
Density Functional Theory (DFT) calculations represent the cornerstone of computational approaches for studying bond exchange reactions in vitrimers. By accurately modeling electronic structures, DFT enables researchers to calculate activation energy barriers with precision, identifying transition states and reaction pathways that would be challenging to observe experimentally. Recent advancements in computational efficiency have expanded the scope of these calculations to include larger molecular systems that better represent actual vitrimer networks.
Molecular dynamics (MD) simulations complement quantum mechanical approaches by modeling the time evolution of molecular systems at finite temperatures. This capability is particularly valuable for vitrimers, as it allows researchers to observe how thermal energy influences bond exchange rates across different network architectures. Advanced MD techniques incorporating reactive force fields can now simulate bond breaking and formation events directly, providing a comprehensive view of the dynamic processes governing vitrimer behavior.
Machine learning algorithms have recently been integrated with traditional computational methods to accelerate predictions of bond exchange behavior. By training on datasets of known reactions and their activation energies, these models can rapidly screen potential vitrimer chemistries without requiring full quantum mechanical calculations for each candidate. This approach has proven especially valuable for designing vitrimers with tailored thermal responsiveness for specific applications.
Multi-scale modeling frameworks represent the cutting edge of computational approaches, bridging the gap between atomic-level bond exchange events and macroscopic material properties. These models connect quantum calculations of individual exchange reactions to mesoscale network dynamics and ultimately to bulk material behavior, providing a comprehensive understanding of how molecular-level design choices influence macroscopic performance characteristics.
Validation against experimental data remains essential for computational models, with researchers increasingly employing combined computational-experimental approaches. Thermal activation energies predicted through computational methods are regularly benchmarked against values determined through rheological measurements, differential scanning calorimetry, and stress relaxation experiments, continuously refining model accuracy and applicability to real-world vitrimer systems.
Density Functional Theory (DFT) calculations represent the cornerstone of computational approaches for studying bond exchange reactions in vitrimers. By accurately modeling electronic structures, DFT enables researchers to calculate activation energy barriers with precision, identifying transition states and reaction pathways that would be challenging to observe experimentally. Recent advancements in computational efficiency have expanded the scope of these calculations to include larger molecular systems that better represent actual vitrimer networks.
Molecular dynamics (MD) simulations complement quantum mechanical approaches by modeling the time evolution of molecular systems at finite temperatures. This capability is particularly valuable for vitrimers, as it allows researchers to observe how thermal energy influences bond exchange rates across different network architectures. Advanced MD techniques incorporating reactive force fields can now simulate bond breaking and formation events directly, providing a comprehensive view of the dynamic processes governing vitrimer behavior.
Machine learning algorithms have recently been integrated with traditional computational methods to accelerate predictions of bond exchange behavior. By training on datasets of known reactions and their activation energies, these models can rapidly screen potential vitrimer chemistries without requiring full quantum mechanical calculations for each candidate. This approach has proven especially valuable for designing vitrimers with tailored thermal responsiveness for specific applications.
Multi-scale modeling frameworks represent the cutting edge of computational approaches, bridging the gap between atomic-level bond exchange events and macroscopic material properties. These models connect quantum calculations of individual exchange reactions to mesoscale network dynamics and ultimately to bulk material behavior, providing a comprehensive understanding of how molecular-level design choices influence macroscopic performance characteristics.
Validation against experimental data remains essential for computational models, with researchers increasingly employing combined computational-experimental approaches. Thermal activation energies predicted through computational methods are regularly benchmarked against values determined through rheological measurements, differential scanning calorimetry, and stress relaxation experiments, continuously refining model accuracy and applicability to real-world vitrimer systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!