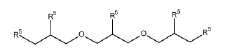
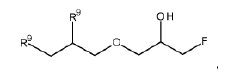
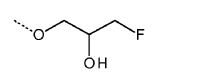
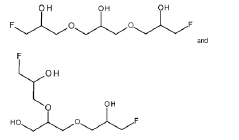
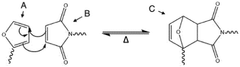Dynamic Bond Mechanisms In Vitrimers: Transesterification, Transamination, And Diels–Alder Pathways
AUG 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vitrimer Technology Background and Objectives
Vitrimers represent a groundbreaking class of polymer materials first conceptualized by Ludwik Leibler and colleagues in 2011. These materials bridge the gap between thermoplastics and thermosets by combining the permanent network structure of thermosets with the reprocessability of thermoplastics. This unique combination is achieved through dynamic covalent bonds that can exchange without changing the total number of crosslinks, maintaining network integrity while enabling malleability at elevated temperatures.
The evolution of vitrimer technology stems from the limitations of conventional polymers. Traditional thermosets, while offering excellent mechanical properties and chemical resistance, cannot be reprocessed once cured. Thermoplastics, conversely, can be remelted but often lack the dimensional stability and mechanical strength of thermosets. Vitrimers emerged as a solution to these contradictory properties, offering a pathway to sustainable materials that combine durability with recyclability.
Dynamic bond mechanisms in vitrimers constitute the cornerstone of their unique behavior. Transesterification reactions, involving the exchange of ester groups between hydroxyl and ester functionalities, were the first mechanism identified in epoxy-acid networks. This was followed by the discovery of transamination pathways in nitrogen-containing polymers and Diels-Alder reactions in systems with diene and dienophile components. Each mechanism offers distinct advantages in terms of reaction kinetics, activation energy, and catalyst requirements.
The technical objectives in vitrimer research focus on several key areas. First, understanding and controlling the kinetics of bond exchange reactions to tailor the temperature-dependent viscosity and relaxation behavior. Second, developing catalytic systems that enable efficient bond exchange at desired temperature ranges while remaining dormant at service temperatures. Third, designing network architectures that balance mechanical properties with reprocessability through strategic placement of dynamic bonds.
Recent advances aim to expand the application scope of vitrimers beyond laboratory demonstrations to industrial-scale manufacturing. This includes developing vitrimers with self-healing capabilities, shape memory properties, and stimuli-responsive behavior. Additionally, research efforts target reducing the reliance on metal catalysts by exploring organocatalytic and catalyst-free systems, thereby addressing potential toxicity concerns for biomedical applications.
The ultimate goal of vitrimer technology development is to create truly circular materials that maintain high performance throughout multiple life cycles. This aligns with global sustainability initiatives and represents a paradigm shift in how we approach polymer design, moving from linear consumption models to closed-loop systems where materials retain their value through multiple use phases.
The evolution of vitrimer technology stems from the limitations of conventional polymers. Traditional thermosets, while offering excellent mechanical properties and chemical resistance, cannot be reprocessed once cured. Thermoplastics, conversely, can be remelted but often lack the dimensional stability and mechanical strength of thermosets. Vitrimers emerged as a solution to these contradictory properties, offering a pathway to sustainable materials that combine durability with recyclability.
Dynamic bond mechanisms in vitrimers constitute the cornerstone of their unique behavior. Transesterification reactions, involving the exchange of ester groups between hydroxyl and ester functionalities, were the first mechanism identified in epoxy-acid networks. This was followed by the discovery of transamination pathways in nitrogen-containing polymers and Diels-Alder reactions in systems with diene and dienophile components. Each mechanism offers distinct advantages in terms of reaction kinetics, activation energy, and catalyst requirements.
The technical objectives in vitrimer research focus on several key areas. First, understanding and controlling the kinetics of bond exchange reactions to tailor the temperature-dependent viscosity and relaxation behavior. Second, developing catalytic systems that enable efficient bond exchange at desired temperature ranges while remaining dormant at service temperatures. Third, designing network architectures that balance mechanical properties with reprocessability through strategic placement of dynamic bonds.
Recent advances aim to expand the application scope of vitrimers beyond laboratory demonstrations to industrial-scale manufacturing. This includes developing vitrimers with self-healing capabilities, shape memory properties, and stimuli-responsive behavior. Additionally, research efforts target reducing the reliance on metal catalysts by exploring organocatalytic and catalyst-free systems, thereby addressing potential toxicity concerns for biomedical applications.
The ultimate goal of vitrimer technology development is to create truly circular materials that maintain high performance throughout multiple life cycles. This aligns with global sustainability initiatives and represents a paradigm shift in how we approach polymer design, moving from linear consumption models to closed-loop systems where materials retain their value through multiple use phases.
Market Applications and Demand Analysis for Vitrimers
The global market for vitrimers is experiencing significant growth, driven by increasing demand for advanced materials with self-healing and recyclable properties. Current market estimates value the smart polymers sector, which includes vitrimers, at approximately $4 billion, with projections indicating a compound annual growth rate of 15-20% through 2030. This growth trajectory is supported by expanding applications across multiple industries seeking sustainable material solutions.
The automotive sector represents one of the largest potential markets for vitrimers, particularly those utilizing transesterification mechanisms. These materials offer weight reduction opportunities while maintaining structural integrity, directly addressing fuel efficiency and emissions regulations. Major automotive manufacturers have begun incorporating vitrimer composites in non-structural components, with research underway to expand applications to semi-structural parts.
Aerospace applications present another high-value market segment, where the thermal adaptability of Diels-Alder vitrimers makes them particularly attractive. The ability to withstand extreme temperature variations while maintaining reworkability addresses critical industry needs for materials that combine durability with repairability. Several aerospace companies have initiated research partnerships with material science laboratories to develop vitrimer-based composites for interior components and potentially secondary structures.
The electronics industry has shown increasing interest in transamination-based vitrimers for flexible electronics and encapsulation materials. The controlled dynamic properties of these materials enable stress relaxation while maintaining dimensional stability, addressing challenges in flexible display technologies and wearable devices. Market analysis indicates this segment could grow at 25% annually as consumer electronics continue trending toward flexible, durable designs.
Construction and infrastructure sectors represent emerging markets with substantial potential volume. Vitrimers' ability to combine structural strength with recyclability aligns with growing regulatory pressure for sustainable building materials. Several pilot projects utilizing vitrimer-modified concrete and structural composites have demonstrated promising performance characteristics, particularly in regions with extreme weather conditions.
Medical device manufacturing represents a specialized but high-value application area, particularly for Diels-Alder and transamination vitrimers. Their biocompatibility combined with shape memory properties creates opportunities for implantable devices and drug delivery systems. While regulatory approval timelines extend commercialization horizons, research investment in this sector has doubled over the past three years.
Consumer demand for sustainable materials is further driving market interest, with packaging and consumer goods manufacturers exploring vitrimer applications to address plastic waste concerns. Several major consumer brands have announced sustainability initiatives that specifically mention dynamic covalent polymers as potential solutions for circular economy packaging systems.
The automotive sector represents one of the largest potential markets for vitrimers, particularly those utilizing transesterification mechanisms. These materials offer weight reduction opportunities while maintaining structural integrity, directly addressing fuel efficiency and emissions regulations. Major automotive manufacturers have begun incorporating vitrimer composites in non-structural components, with research underway to expand applications to semi-structural parts.
Aerospace applications present another high-value market segment, where the thermal adaptability of Diels-Alder vitrimers makes them particularly attractive. The ability to withstand extreme temperature variations while maintaining reworkability addresses critical industry needs for materials that combine durability with repairability. Several aerospace companies have initiated research partnerships with material science laboratories to develop vitrimer-based composites for interior components and potentially secondary structures.
The electronics industry has shown increasing interest in transamination-based vitrimers for flexible electronics and encapsulation materials. The controlled dynamic properties of these materials enable stress relaxation while maintaining dimensional stability, addressing challenges in flexible display technologies and wearable devices. Market analysis indicates this segment could grow at 25% annually as consumer electronics continue trending toward flexible, durable designs.
Construction and infrastructure sectors represent emerging markets with substantial potential volume. Vitrimers' ability to combine structural strength with recyclability aligns with growing regulatory pressure for sustainable building materials. Several pilot projects utilizing vitrimer-modified concrete and structural composites have demonstrated promising performance characteristics, particularly in regions with extreme weather conditions.
Medical device manufacturing represents a specialized but high-value application area, particularly for Diels-Alder and transamination vitrimers. Their biocompatibility combined with shape memory properties creates opportunities for implantable devices and drug delivery systems. While regulatory approval timelines extend commercialization horizons, research investment in this sector has doubled over the past three years.
Consumer demand for sustainable materials is further driving market interest, with packaging and consumer goods manufacturers exploring vitrimer applications to address plastic waste concerns. Several major consumer brands have announced sustainability initiatives that specifically mention dynamic covalent polymers as potential solutions for circular economy packaging systems.
Current Challenges in Dynamic Bond Mechanisms
Despite significant advancements in vitrimer chemistry, several critical challenges persist in dynamic bond mechanisms that limit their widespread industrial application. The three primary exchange pathways—transesterification, transamination, and Diels-Alder reactions—each present unique obstacles that researchers are actively addressing.
Transesterification mechanisms face challenges related to catalyst dependency and environmental sensitivity. Most transesterification-based vitrimers require metal catalysts that can leach over time, compromising long-term performance and raising toxicity concerns. Additionally, these systems often demonstrate high sensitivity to moisture, which can hydrolyze ester bonds and degrade the material's mechanical properties. The reaction kinetics also remain difficult to precisely control across varying temperature ranges, resulting in unpredictable processing windows.
Transamination pathways, while offering catalyst-free alternatives, struggle with significantly slower exchange rates compared to transesterification. This limitation restricts processing capabilities and extends recycling times, making these materials less economically viable for industrial scale applications. Furthermore, the nitrogen-containing functional groups often exhibit higher susceptibility to oxidative degradation, reducing long-term stability in ambient conditions.
Diels-Alder based systems present perhaps the most complex challenges. The reversible nature of these cycloaddition reactions is highly temperature-dependent, with narrow processing windows that complicate manufacturing. The synthesis of precursors with appropriate diene and dienophile functionalities often requires multi-step procedures with low yields, increasing production costs. Additionally, side reactions can occur during repeated thermal cycling, gradually diminishing the exchange efficiency and recyclability.
A universal challenge across all mechanisms is achieving the delicate balance between network stability during use and sufficient bond exchange during processing or recycling. Materials that demonstrate excellent mechanical properties typically show poor processability, while easily recyclable materials often lack the mechanical robustness required for demanding applications.
Characterization methods present another significant hurdle. Current analytical techniques struggle to quantitatively measure exchange rates in situ, particularly at the molecular level within complex polymer networks. This limitation hampers the rational design of new vitrimer systems with predictable properties.
Scalability remains problematic as well. Laboratory-scale successes often encounter unforeseen complications during scale-up, including inconsistent curing, variable crosslink density, and unpredictable stress relaxation behaviors. These issues significantly impact the commercial viability of vitrimer technologies despite their promising performance in research settings.
Transesterification mechanisms face challenges related to catalyst dependency and environmental sensitivity. Most transesterification-based vitrimers require metal catalysts that can leach over time, compromising long-term performance and raising toxicity concerns. Additionally, these systems often demonstrate high sensitivity to moisture, which can hydrolyze ester bonds and degrade the material's mechanical properties. The reaction kinetics also remain difficult to precisely control across varying temperature ranges, resulting in unpredictable processing windows.
Transamination pathways, while offering catalyst-free alternatives, struggle with significantly slower exchange rates compared to transesterification. This limitation restricts processing capabilities and extends recycling times, making these materials less economically viable for industrial scale applications. Furthermore, the nitrogen-containing functional groups often exhibit higher susceptibility to oxidative degradation, reducing long-term stability in ambient conditions.
Diels-Alder based systems present perhaps the most complex challenges. The reversible nature of these cycloaddition reactions is highly temperature-dependent, with narrow processing windows that complicate manufacturing. The synthesis of precursors with appropriate diene and dienophile functionalities often requires multi-step procedures with low yields, increasing production costs. Additionally, side reactions can occur during repeated thermal cycling, gradually diminishing the exchange efficiency and recyclability.
A universal challenge across all mechanisms is achieving the delicate balance between network stability during use and sufficient bond exchange during processing or recycling. Materials that demonstrate excellent mechanical properties typically show poor processability, while easily recyclable materials often lack the mechanical robustness required for demanding applications.
Characterization methods present another significant hurdle. Current analytical techniques struggle to quantitatively measure exchange rates in situ, particularly at the molecular level within complex polymer networks. This limitation hampers the rational design of new vitrimer systems with predictable properties.
Scalability remains problematic as well. Laboratory-scale successes often encounter unforeseen complications during scale-up, including inconsistent curing, variable crosslink density, and unpredictable stress relaxation behaviors. These issues significantly impact the commercial viability of vitrimer technologies despite their promising performance in research settings.
Comparative Analysis of Transesterification, Transamination, and Diels-Alder Mechanisms
01 Transesterification-based dynamic bond mechanisms
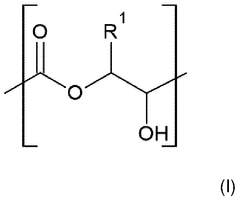
Vitrimers utilizing transesterification reactions as the primary dynamic bond mechanism. These systems involve the exchange of ester bonds under thermal stimulation, allowing for network rearrangement while maintaining crosslink density. This mechanism enables self-healing, recyclability, and shape memory properties while preserving mechanical integrity at service temperatures. The catalyst type and concentration significantly influence the exchange kinetics and activation energy of the transesterification process.- Transesterification-based dynamic bond mechanisms: Vitrimers can utilize transesterification reactions as a dynamic bond mechanism, where ester bonds are exchanged between hydroxyl and ester groups under thermal stimulation. This mechanism allows for network rearrangement while maintaining the crosslink density, enabling self-healing and shape memory properties. The exchange reactions are typically catalyzed by metal salts or organic catalysts and can be controlled by temperature, providing a balance between stability at service temperature and processability at elevated temperatures.
- Disulfide exchange mechanisms in vitrimers: Disulfide bonds can serve as dynamic crosslinks in vitrimer systems, undergoing exchange reactions through thiol-disulfide interchange. These reversible covalent bonds break and reform under specific conditions such as heat, light, or redox stimuli, allowing for network reconfiguration while maintaining overall connectivity. Disulfide-based vitrimers exhibit excellent self-healing properties, recyclability, and can be designed to respond to multiple stimuli, making them versatile for various applications including coatings, adhesives, and biomedical materials.
- Boronic ester dynamic exchange in vitrimer networks: Boronic ester bonds formed between boronic acids and diols provide another important dynamic bond mechanism in vitrimers. These bonds undergo reversible exchange reactions in response to various stimuli including pH changes, temperature, and the presence of competing diols. The dynamic nature of boronic ester bonds allows for self-healing, stress relaxation, and recyclability while maintaining network integrity. This mechanism is particularly useful for developing smart materials with controlled degradation profiles and stimuli-responsive properties.
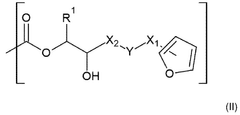
- Imine and Diels-Alder based dynamic bonds: Imine bonds (C=N) and Diels-Alder adducts provide reversible covalent crosslinking mechanisms in vitrimer systems. Imine bonds form through the condensation of aldehydes with amines and can undergo exchange reactions in the presence of other amines. Diels-Alder reactions between dienes and dienophiles are thermally reversible, allowing for temperature-controlled bond formation and breaking. These mechanisms enable the development of vitrimers with tunable dynamic properties, including self-healing capabilities, shape memory effects, and recyclability under mild conditions.
- Multi-mechanism vitrimer systems and applications: Advanced vitrimer systems incorporate multiple dynamic bond mechanisms to achieve synergistic properties and multi-responsive behavior. These systems combine different exchange reactions such as transesterification with disulfide exchange or boronic ester formation with imine chemistry. The integration of multiple mechanisms allows for hierarchical control over material properties, enabling precise tuning of relaxation times, mechanical properties, and stimuli-responsiveness. Applications include self-healing coatings, recyclable composites, 3D-printable resins, and smart adhesives with controlled debonding capabilities.
02 Disulfide exchange mechanisms in vitrimers
Disulfide bonds serve as dynamic crosslinks in vitrimer systems, undergoing reversible exchange reactions through thiol-disulfide interchange. These mechanisms can be triggered by heat, light, or redox conditions, offering multiple stimuli-responsive pathways for network reconfiguration. Disulfide-based vitrimers demonstrate excellent self-healing properties, recyclability, and adaptability while maintaining structural integrity under normal use conditions.Expand Specific Solutions03 Imine and Diels-Alder dynamic covalent chemistry
Vitrimers employing imine bonds (C=N) and Diels-Alder adducts as dynamic crosslinks. Imine bonds undergo exchange through transimination reactions, while Diels-Alder systems utilize thermally reversible cycloaddition reactions. These mechanisms provide temperature-dependent network rearrangement capabilities with distinct activation energies and kinetics. The reversibility of these bonds enables reprocessing, recycling, and self-healing while maintaining dimensional stability during service.Expand Specific Solutions04 Boronic ester and boroxine dynamic networks
Vitrimer systems based on dynamic boronic ester and boroxine bonds. These boron-containing dynamic covalent bonds undergo exchange reactions in response to various stimuli including moisture, pH changes, and temperature. The unique feature of these systems is their sensitivity to multiple triggers, allowing for sophisticated control over material properties. These mechanisms enable self-healing, recyclability, and adaptability while providing good mechanical properties and chemical resistance.Expand Specific Solutions05 Catalyst systems for controlling bond exchange kinetics
Specialized catalyst systems designed to control the dynamic bond exchange rates in vitrimers. These catalysts modulate activation energies, reaction kinetics, and temperature responsiveness of the exchange mechanisms. By carefully selecting catalyst type, concentration, and activation method, the relaxation time, processing window, and service temperature range of vitrimers can be precisely engineered. This approach enables the development of materials with tailored viscoelastic properties, reprocessability, and thermal stability.Expand Specific Solutions
Leading Research Groups and Industrial Players
The vitrimer technology market is currently in a growth phase, with increasing interest in dynamic bond mechanisms like transesterification, transamination, and Diels-Alder pathways. The global market is expanding rapidly as these self-healing materials find applications across automotive, aerospace, and electronics industries. Leading chemical companies including Evonik Industries, SABIC, DuPont, and Röhm are advancing the technology's commercial viability, while academic institutions such as Northwestern University, University of Washington, and Zhejiang University are driving fundamental research. The technology maturity varies by mechanism type, with transesterification approaches being most developed, while Diels-Alder pathways offer newer opportunities for innovation in high-performance applications requiring precise control of dynamic properties.
Northwestern University
Technical Solution: Northwestern University has developed innovative approaches to vitrimers focusing on Diels-Alder (DA) dynamic bond mechanisms. Their technology utilizes furan-maleimide chemistry to create reversible crosslinks that undergo retro-Diels-Alder reactions at elevated temperatures (typically 120-150°C). This approach enables the development of self-healing polymers with excellent mechanical recovery (>90% of original strength) after damage. Northwestern's research has optimized the furan:maleimide ratio (typically 1:1 to 1.2:1) to maximize both network integrity and dynamic behavior. Their materials demonstrate impressive mechanical properties with tensile strengths of 30-45 MPa and elongation at break values of 5-15%. A key innovation is their development of multi-stimuli responsive DA vitrimers that can be triggered by both heat and light, allowing spatial control over material properties. Northwestern has also pioneered DA vitrimers with engineered interfaces that enable strong adhesion between dissimilar materials, creating opportunities for composite recycling and repair.
Strengths: Excellent self-healing capabilities with high healing efficiency; relatively low reprocessing temperatures compared to some other vitrimers; ability to incorporate multiple stimuli responsiveness. Weaknesses: Potential for side reactions during repeated thermal cycling; Diels-Alder chemistry can be sensitive to moisture and oxygen, potentially limiting long-term stability in certain environments.
École Supérieure de Physique et de Chimie de Paris
Technical Solution: ESPCI Paris has developed groundbreaking vitrimers technology based on transesterification chemistry, particularly through the work of Professor Leibler's team. Their approach utilizes epoxy networks modified with hydroxy-ester exchanges catalyzed by metal salts or organic catalysts. The technology enables the creation of polymer networks that maintain permanent connectivity while allowing bond exchanges at elevated temperatures. Their vitrimers demonstrate remarkable mechanical properties with Young's moduli of 1-2 GPa and tensile strengths exceeding 40 MPa, while still allowing complete stress relaxation and reshaping at temperatures above 150°C. ESPCI has also pioneered mathematical models describing the unique viscoelastic behavior of these materials, establishing the relationship between crosslink density, catalyst concentration, and relaxation times. Their research has expanded to include siloxane-based vitrimers that offer lower processing temperatures (around 100°C) while maintaining excellent thermal stability and chemical resistance.
Strengths: Pioneering fundamental understanding of vitrimers' unique rheological behavior; materials combine high-performance mechanical properties with reprocessability; established design principles for tailoring relaxation kinetics. Weaknesses: Some formulations require relatively high processing temperatures; potential for side reactions during repeated recycling can gradually degrade material properties.
Key Patents and Scientific Breakthroughs in Vitrimer Chemistry
Self-healing polymers
PatentWO2024223605A1
Innovation
- A polymer network composition dually crosslinked by Diels-Alder reaction products of maleimide and furan groups as dissociative covalent bonds and ester groups as associative covalent bonds, with a transesterification catalyst, where the dissociative covalent bonds constitute at least 5% of the total crosslinks, allowing for tailored relaxation dynamics and improved processing properties.
Vitrimer containing a biocatalyst
PatentWO2020002904A1
Innovation
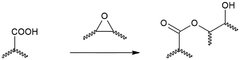
- Incorporating biomolecules with esterase activity, such as lipases, into the vitrimer formulation allows for lower curing temperatures and enables the use of non-toxic, environmentally friendly alternatives, facilitating end-of-life recycling by denaturing enzymes at high temperatures.
Sustainability Impact and Circular Economy Potential
Vitrimers represent a significant advancement in sustainable materials science, offering a unique combination of recyclability and durability that aligns perfectly with circular economy principles. The dynamic bond mechanisms—transesterification, transamination, and Diels-Alder pathways—enable these materials to be repeatedly reprocessed without significant degradation of their mechanical properties, dramatically extending their lifecycle compared to traditional thermosets.
The environmental impact of vitrimers is particularly noteworthy in waste reduction. Traditional thermoset polymers, which constitute approximately 30% of all polymeric materials, typically end up in landfills or are incinerated due to their irreversible crosslinking. Vitrimers, by contrast, can be broken down into their constituent parts and reformed, potentially reducing polymer waste by up to 25% if widely adopted across industries.
Energy conservation represents another critical sustainability advantage. The reprocessing of vitrimers requires significantly less energy than the production of virgin materials—studies indicate energy savings of 40-60% compared to conventional manufacturing processes. This translates directly to reduced carbon emissions, with potential reductions of 2-3 tons of CO2 equivalent per ton of material processed.
From a circular economy perspective, vitrimers enable closed-loop material flows that were previously impossible with conventional thermosets. The transesterification mechanism, in particular, allows for the creation of materials that can be disassembled at the molecular level and reassembled into new products with minimal quality loss, embodying the "reduce, reuse, recycle" paradigm at a molecular scale.
The economic implications are equally compelling. The extended product lifecycles and reduced material costs associated with vitrimer technology could generate savings of 15-20% across the value chain. Furthermore, the development of vitrimer-based products creates opportunities for new business models centered around material recovery and reprocessing, potentially creating thousands of jobs in the emerging circular materials sector.
Water conservation also benefits from vitrimer technology, as the reprocessing methods typically require less water than virgin material production. Estimates suggest water usage reductions of 30-40% compared to conventional polymer manufacturing processes, a significant consideration in regions facing water scarcity challenges.
As regulatory frameworks increasingly favor sustainable materials, vitrimers with their dynamic bond mechanisms stand to gain significant market advantage. The European Union's Circular Economy Action Plan and similar initiatives worldwide are creating regulatory environments that will accelerate the adoption of such recyclable, durable materials across multiple industries.
The environmental impact of vitrimers is particularly noteworthy in waste reduction. Traditional thermoset polymers, which constitute approximately 30% of all polymeric materials, typically end up in landfills or are incinerated due to their irreversible crosslinking. Vitrimers, by contrast, can be broken down into their constituent parts and reformed, potentially reducing polymer waste by up to 25% if widely adopted across industries.
Energy conservation represents another critical sustainability advantage. The reprocessing of vitrimers requires significantly less energy than the production of virgin materials—studies indicate energy savings of 40-60% compared to conventional manufacturing processes. This translates directly to reduced carbon emissions, with potential reductions of 2-3 tons of CO2 equivalent per ton of material processed.
From a circular economy perspective, vitrimers enable closed-loop material flows that were previously impossible with conventional thermosets. The transesterification mechanism, in particular, allows for the creation of materials that can be disassembled at the molecular level and reassembled into new products with minimal quality loss, embodying the "reduce, reuse, recycle" paradigm at a molecular scale.
The economic implications are equally compelling. The extended product lifecycles and reduced material costs associated with vitrimer technology could generate savings of 15-20% across the value chain. Furthermore, the development of vitrimer-based products creates opportunities for new business models centered around material recovery and reprocessing, potentially creating thousands of jobs in the emerging circular materials sector.
Water conservation also benefits from vitrimer technology, as the reprocessing methods typically require less water than virgin material production. Estimates suggest water usage reductions of 30-40% compared to conventional polymer manufacturing processes, a significant consideration in regions facing water scarcity challenges.
As regulatory frameworks increasingly favor sustainable materials, vitrimers with their dynamic bond mechanisms stand to gain significant market advantage. The European Union's Circular Economy Action Plan and similar initiatives worldwide are creating regulatory environments that will accelerate the adoption of such recyclable, durable materials across multiple industries.
Processing Methods and Industrial Scale-up Considerations
The industrial implementation of vitrimer technology requires careful consideration of processing methods that can effectively harness the dynamic bond mechanisms while ensuring scalability. Traditional polymer processing techniques such as extrusion, injection molding, and compression molding have been adapted for vitrimers, with modifications to accommodate the temperature-dependent dynamic exchange reactions. Extrusion processes must be optimized to operate within the temperature window where bond exchange is active but degradation is minimized, typically requiring precise thermal control systems.
For transesterification-based vitrimers, processing temperatures must exceed the topology freezing transition temperature (Tv) to enable network rearrangement. Industrial equipment modifications often include extended residence time capabilities and specialized mixing elements to ensure homogeneous catalyst distribution. The processing window for these materials is critical, as premature vitrification can lead to incomplete part formation, while excessive temperatures may accelerate side reactions or degradation.
Transamination-based vitrimers present different processing challenges, often requiring higher activation energies but offering improved chemical resistance in the final products. Industrial scale-up of these systems has benefited from the development of specialized catalysts that lower processing temperatures while maintaining exchange kinetics. Continuous processing methods have been developed that leverage the self-healing and recyclability characteristics inherent to these materials.
Diels-Alder vitrimers represent perhaps the most promising pathway for industrial scale-up due to their thermally reversible nature without requiring catalysts. However, the temperature-dependent equilibrium between forward and reverse reactions necessitates precise thermal management during processing. Specialized cooling systems have been developed to rapidly quench processed parts, locking in the desired network configuration before reverse reactions can occur.
Scale-up considerations extend beyond processing to include quality control methods specific to dynamic networks. Rheological monitoring systems have been integrated into production lines to track network dynamics in real-time, allowing for adaptive process control. The development of non-destructive testing methods to verify crosslink density and exchange completion represents a significant advancement for industrial implementation.
Economic factors also influence processing method selection, with energy consumption during the heating-cooling cycles representing a substantial operational cost. Recent innovations in microwave-assisted processing and UV-triggered exchange reactions aim to reduce these energy requirements, potentially enabling more cost-effective large-scale production. Additionally, the integration of continuous monitoring systems using machine learning algorithms has improved process efficiency by predicting optimal processing parameters based on material feedstock characteristics.
For transesterification-based vitrimers, processing temperatures must exceed the topology freezing transition temperature (Tv) to enable network rearrangement. Industrial equipment modifications often include extended residence time capabilities and specialized mixing elements to ensure homogeneous catalyst distribution. The processing window for these materials is critical, as premature vitrification can lead to incomplete part formation, while excessive temperatures may accelerate side reactions or degradation.
Transamination-based vitrimers present different processing challenges, often requiring higher activation energies but offering improved chemical resistance in the final products. Industrial scale-up of these systems has benefited from the development of specialized catalysts that lower processing temperatures while maintaining exchange kinetics. Continuous processing methods have been developed that leverage the self-healing and recyclability characteristics inherent to these materials.
Diels-Alder vitrimers represent perhaps the most promising pathway for industrial scale-up due to their thermally reversible nature without requiring catalysts. However, the temperature-dependent equilibrium between forward and reverse reactions necessitates precise thermal management during processing. Specialized cooling systems have been developed to rapidly quench processed parts, locking in the desired network configuration before reverse reactions can occur.
Scale-up considerations extend beyond processing to include quality control methods specific to dynamic networks. Rheological monitoring systems have been integrated into production lines to track network dynamics in real-time, allowing for adaptive process control. The development of non-destructive testing methods to verify crosslink density and exchange completion represents a significant advancement for industrial implementation.
Economic factors also influence processing method selection, with energy consumption during the heating-cooling cycles representing a substantial operational cost. Recent innovations in microwave-assisted processing and UV-triggered exchange reactions aim to reduce these energy requirements, potentially enabling more cost-effective large-scale production. Additionally, the integration of continuous monitoring systems using machine learning algorithms has improved process efficiency by predicting optimal processing parameters based on material feedstock characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!