Mechanistic Kinetic Models For Transesterification In Vitrimer-Type Polymers
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vitrimer Transesterification Kinetics Background and Objectives
Vitrimer polymers, first introduced by Leibler and colleagues in 2011, represent a groundbreaking class of materials that combine the processability of thermoplastics with the mechanical robustness of thermosets. The defining characteristic of vitrimers is their ability to undergo dynamic bond exchange reactions while maintaining network integrity, enabling stress relaxation and reprocessing without compromising structural stability. Among these exchange reactions, transesterification has emerged as one of the most widely studied and implemented mechanisms in vitrimer chemistry.
The evolution of vitrimer technology has been marked by significant advancements in understanding the kinetics of transesterification reactions. Early work focused primarily on qualitative observations of stress relaxation and reprocessing capabilities, while recent research has shifted toward developing quantitative models that can accurately predict material behavior under various conditions. This transition from empirical to theoretical understanding represents a critical step in the maturation of vitrimer technology.
The field has witnessed a progression from simple Arrhenius-based models to more sophisticated approaches that account for complex phenomena such as diffusion limitations, catalyst distribution, and network topology effects. Despite these advances, there remains a significant gap between theoretical predictions and experimental observations, particularly in complex, multi-component systems or under non-equilibrium conditions.
Current research trends indicate growing interest in developing mechanistic kinetic models that can capture the intricacies of transesterification reactions in vitrimer networks. These models aim to provide a more fundamental understanding of the relationship between molecular structure, reaction conditions, and macroscopic material properties. By elucidating these connections, researchers hope to enable more rational design of vitrimer materials with tailored properties for specific applications.
The primary objective of this technical research is to comprehensively analyze the current state of mechanistic kinetic modeling for transesterification reactions in vitrimer-type polymers. This includes evaluating existing models, identifying their strengths and limitations, and exploring potential avenues for improvement. Additionally, we aim to assess how these models can be integrated into material design workflows to accelerate the development of next-generation vitrimers with enhanced performance characteristics.
Secondary objectives include mapping the technological landscape to identify key players and innovation hotspots, analyzing patent trends to understand the commercial trajectory of vitrimer technology, and evaluating the potential impact of advanced kinetic models on emerging application areas such as self-healing materials, recyclable composites, and additive manufacturing.
The evolution of vitrimer technology has been marked by significant advancements in understanding the kinetics of transesterification reactions. Early work focused primarily on qualitative observations of stress relaxation and reprocessing capabilities, while recent research has shifted toward developing quantitative models that can accurately predict material behavior under various conditions. This transition from empirical to theoretical understanding represents a critical step in the maturation of vitrimer technology.
The field has witnessed a progression from simple Arrhenius-based models to more sophisticated approaches that account for complex phenomena such as diffusion limitations, catalyst distribution, and network topology effects. Despite these advances, there remains a significant gap between theoretical predictions and experimental observations, particularly in complex, multi-component systems or under non-equilibrium conditions.
Current research trends indicate growing interest in developing mechanistic kinetic models that can capture the intricacies of transesterification reactions in vitrimer networks. These models aim to provide a more fundamental understanding of the relationship between molecular structure, reaction conditions, and macroscopic material properties. By elucidating these connections, researchers hope to enable more rational design of vitrimer materials with tailored properties for specific applications.
The primary objective of this technical research is to comprehensively analyze the current state of mechanistic kinetic modeling for transesterification reactions in vitrimer-type polymers. This includes evaluating existing models, identifying their strengths and limitations, and exploring potential avenues for improvement. Additionally, we aim to assess how these models can be integrated into material design workflows to accelerate the development of next-generation vitrimers with enhanced performance characteristics.
Secondary objectives include mapping the technological landscape to identify key players and innovation hotspots, analyzing patent trends to understand the commercial trajectory of vitrimer technology, and evaluating the potential impact of advanced kinetic models on emerging application areas such as self-healing materials, recyclable composites, and additive manufacturing.
Market Applications and Demand for Vitrimer Polymers
The global market for vitrimer polymers has been experiencing significant growth due to their unique combination of recyclability and mechanical properties. Current market analysis indicates that the automotive industry represents one of the largest application sectors for vitrimers, with manufacturers seeking lightweight, durable materials that can be easily recycled or repaired. The ability of vitrimers to undergo transesterification reactions enables self-healing properties, making them particularly valuable for components subjected to wear and tear.
Aerospace applications constitute another rapidly expanding market segment, where the high-performance characteristics of vitrimers, including thermal stability and impact resistance, are highly prized. Market research suggests that aerospace manufacturers are increasingly incorporating vitrimer-based composites into non-structural and semi-structural components, with potential expansion into more critical applications as the technology matures.
The construction industry represents a substantial growth opportunity for vitrimer polymers, particularly in sustainable building materials. The demand for environmentally friendly materials with extended lifecycles aligns perfectly with the recyclability and durability of vitrimers. Market trends indicate growing interest in vitrimer-based adhesives, sealants, and composite panels that can be disassembled and repurposed at end-of-life.
Electronics manufacturing presents another promising application area, with vitrimers being explored for flexible electronics, encapsulation materials, and thermally conductive adhesives. The self-healing properties of these materials address a critical need in electronic devices that experience thermal cycling and mechanical stress during operation.
Healthcare applications are emerging as a high-value niche market for vitrimers, particularly in medical devices and implants where biocompatibility combined with mechanical performance is essential. The controlled degradation and remodeling capabilities of certain vitrimer formulations make them attractive for tissue engineering scaffolds and drug delivery systems.
Consumer goods manufacturers are also showing increased interest in vitrimer technology for applications ranging from sporting equipment to household appliances. The ability to create products with extended lifespans through self-healing and recyclability aligns with growing consumer demand for sustainable products.
Market forecasts suggest that the global vitrimer polymer market will continue to expand as manufacturing processes become more scalable and cost-effective. However, widespread adoption faces challenges related to production costs, processing complexity, and the need for standardized testing methodologies to validate long-term performance. The development of more efficient catalysts for transesterification reactions represents a key factor that could accelerate market penetration across these diverse application sectors.
Aerospace applications constitute another rapidly expanding market segment, where the high-performance characteristics of vitrimers, including thermal stability and impact resistance, are highly prized. Market research suggests that aerospace manufacturers are increasingly incorporating vitrimer-based composites into non-structural and semi-structural components, with potential expansion into more critical applications as the technology matures.
The construction industry represents a substantial growth opportunity for vitrimer polymers, particularly in sustainable building materials. The demand for environmentally friendly materials with extended lifecycles aligns perfectly with the recyclability and durability of vitrimers. Market trends indicate growing interest in vitrimer-based adhesives, sealants, and composite panels that can be disassembled and repurposed at end-of-life.
Electronics manufacturing presents another promising application area, with vitrimers being explored for flexible electronics, encapsulation materials, and thermally conductive adhesives. The self-healing properties of these materials address a critical need in electronic devices that experience thermal cycling and mechanical stress during operation.
Healthcare applications are emerging as a high-value niche market for vitrimers, particularly in medical devices and implants where biocompatibility combined with mechanical performance is essential. The controlled degradation and remodeling capabilities of certain vitrimer formulations make them attractive for tissue engineering scaffolds and drug delivery systems.
Consumer goods manufacturers are also showing increased interest in vitrimer technology for applications ranging from sporting equipment to household appliances. The ability to create products with extended lifespans through self-healing and recyclability aligns with growing consumer demand for sustainable products.
Market forecasts suggest that the global vitrimer polymer market will continue to expand as manufacturing processes become more scalable and cost-effective. However, widespread adoption faces challenges related to production costs, processing complexity, and the need for standardized testing methodologies to validate long-term performance. The development of more efficient catalysts for transesterification reactions represents a key factor that could accelerate market penetration across these diverse application sectors.
Current Challenges in Mechanistic Kinetic Modeling
Despite significant advancements in vitrimer polymer research, mechanistic kinetic modeling for transesterification reactions faces several critical challenges. The complexity of these dynamic covalent networks creates substantial difficulties in developing accurate predictive models. Current modeling approaches often fail to account for the heterogeneous nature of the reaction environment within polymer matrices, where local viscosity, crosslinking density, and functional group accessibility vary significantly throughout the material.
A fundamental challenge lies in capturing the temperature-dependent behavior of transesterification reactions. While Arrhenius relationships are commonly employed, they frequently prove inadequate for describing the complex kinetics observed across the broad temperature ranges relevant to vitrimer processing and application. This limitation becomes particularly problematic when modeling the transition between diffusion-controlled and reaction-controlled regimes.
The presence of multiple competing reactions and catalytic pathways further complicates modeling efforts. Most current models oversimplify the reaction landscape by focusing on primary transesterification mechanisms while neglecting side reactions, catalyst degradation, and inhibition effects that significantly influence long-term material behavior. This reductionist approach limits the predictive power of existing models, especially for extended service lifetimes.
Spatial heterogeneity presents another significant obstacle. Current models typically assume homogeneous distribution of reactive groups and catalysts, whereas real vitrimer systems exhibit considerable spatial variation in composition and reactivity. Advanced modeling approaches incorporating spatial resolution remain computationally intensive and practically challenging to implement for complex formulations.
The dynamic nature of network topology during transesterification poses unique challenges for kinetic modeling. As bonds break and reform, the evolving network structure influences subsequent reaction rates in ways that are difficult to capture mathematically. Current models struggle to incorporate these feedback mechanisms between network topology and reaction kinetics.
Validation of kinetic models represents a persistent challenge, with limited experimental techniques available for directly measuring transesterification rates within solid polymer matrices. Researchers often rely on indirect measurements like stress relaxation or creep behavior, which introduce additional complexities in model validation and parameter estimation.
Finally, bridging molecular-scale reaction kinetics with macroscopic material properties remains problematic. Establishing quantitative relationships between transesterification rates and observable material behaviors like stress relaxation, shape memory, and self-healing requires multi-scale modeling approaches that are still in their infancy for vitrimer systems.
A fundamental challenge lies in capturing the temperature-dependent behavior of transesterification reactions. While Arrhenius relationships are commonly employed, they frequently prove inadequate for describing the complex kinetics observed across the broad temperature ranges relevant to vitrimer processing and application. This limitation becomes particularly problematic when modeling the transition between diffusion-controlled and reaction-controlled regimes.
The presence of multiple competing reactions and catalytic pathways further complicates modeling efforts. Most current models oversimplify the reaction landscape by focusing on primary transesterification mechanisms while neglecting side reactions, catalyst degradation, and inhibition effects that significantly influence long-term material behavior. This reductionist approach limits the predictive power of existing models, especially for extended service lifetimes.
Spatial heterogeneity presents another significant obstacle. Current models typically assume homogeneous distribution of reactive groups and catalysts, whereas real vitrimer systems exhibit considerable spatial variation in composition and reactivity. Advanced modeling approaches incorporating spatial resolution remain computationally intensive and practically challenging to implement for complex formulations.
The dynamic nature of network topology during transesterification poses unique challenges for kinetic modeling. As bonds break and reform, the evolving network structure influences subsequent reaction rates in ways that are difficult to capture mathematically. Current models struggle to incorporate these feedback mechanisms between network topology and reaction kinetics.
Validation of kinetic models represents a persistent challenge, with limited experimental techniques available for directly measuring transesterification rates within solid polymer matrices. Researchers often rely on indirect measurements like stress relaxation or creep behavior, which introduce additional complexities in model validation and parameter estimation.
Finally, bridging molecular-scale reaction kinetics with macroscopic material properties remains problematic. Establishing quantitative relationships between transesterification rates and observable material behaviors like stress relaxation, shape memory, and self-healing requires multi-scale modeling approaches that are still in their infancy for vitrimer systems.
Existing Mechanistic Models for Vitrimer Kinetics
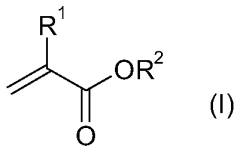
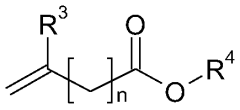
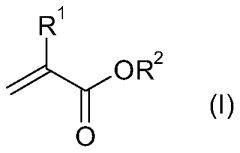
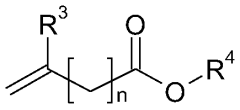
01 Catalyst systems for controlling transesterification kinetics in vitrimers
Various catalyst systems can be employed to control the transesterification reaction kinetics in vitrimer-type polymers. These catalysts influence the exchange rate of ester bonds, which directly affects the material's processability and mechanical properties. Common catalysts include metal-based compounds, organic bases, and enzymes that can accelerate or decelerate the dynamic exchange reactions. The selection of appropriate catalysts allows for precise tuning of the vitrimer's reprocessing temperature and time.- Catalyst effects on transesterification kinetics in vitrimers: Various catalysts can significantly influence the transesterification reaction kinetics in vitrimer-type polymers. These catalysts, including metal-based compounds and organic catalysts, can accelerate the exchange reactions that are fundamental to vitrimer behavior. The catalyst type and concentration directly impact the relaxation time, reprocessability, and self-healing properties of the vitrimer networks. Optimizing catalyst systems allows for precise control over the dynamic bond exchange rate, which determines the material's ability to rearrange its network structure while maintaining dimensional stability.
- Temperature dependence of transesterification in vitrimer networks: The transesterification kinetics in vitrimer-type polymers exhibit strong temperature dependence, which is crucial for their processing and application. At elevated temperatures, the rate of transesterification increases significantly, allowing for network rearrangement and stress relaxation while maintaining the crosslinked structure. This temperature-dependent behavior enables vitrimers to be processed like thermoplastics at high temperatures while retaining the mechanical properties of thermosets at service temperatures. Understanding this relationship is essential for designing vitrimer materials with tailored processing windows and thermal responsiveness.
- Network architecture influence on transesterification dynamics: The molecular architecture of vitrimer networks significantly affects transesterification kinetics and material properties. Factors such as crosslink density, functional group accessibility, and polymer backbone flexibility determine how readily transesterification reactions occur. Strategic design of network structures can enhance or restrict chain mobility, thereby controlling the rate of bond exchange reactions. By manipulating these architectural features, researchers can develop vitrimers with customized relaxation times, mechanical properties, and processing characteristics for specific applications.
- Novel vitrimer compositions with enhanced transesterification properties: Innovative vitrimer compositions have been developed to achieve improved transesterification kinetics and material performance. These include bio-based vitrimers, hybrid organic-inorganic systems, and multi-network architectures. By incorporating specialized functional groups, optimizing ester-to-hydroxyl ratios, or introducing secondary dynamic bonds, these novel compositions offer enhanced reprocessability, mechanical properties, and chemical resistance. Some formulations also feature stimuli-responsive elements that allow for controlled activation of transesterification reactions under specific conditions.
- Characterization methods for transesterification kinetics in vitrimers: Various analytical techniques have been developed to characterize and quantify transesterification kinetics in vitrimer-type polymers. These include rheological measurements to determine relaxation times, spectroscopic methods to track functional group conversions, and mechanical testing to assess reprocessability. Advanced techniques such as stress relaxation analysis, dynamic mechanical analysis, and molecular dynamics simulations provide insights into the relationship between molecular structure and macroscopic properties. These characterization methods are essential for understanding structure-property relationships and optimizing vitrimer formulations for specific applications.
02 Temperature-dependent transesterification behavior in vitrimer networks
The transesterification kinetics in vitrimer-type polymers exhibit strong temperature dependence, which is crucial for their processing and recycling capabilities. At elevated temperatures, the exchange reactions accelerate, allowing the material to flow and be reshaped while maintaining network integrity. This temperature-dependent behavior follows Arrhenius-type kinetics, with activation energies that can be engineered through molecular design. Understanding this relationship enables the development of vitrimers with tailored processing windows and stress relaxation times.Expand Specific Solutions03 Chemical structure effects on transesterification exchange rates
The chemical structure of the polymer backbone and crosslinking units significantly influences the transesterification kinetics in vitrimer systems. Factors such as steric hindrance, hydrogen bonding capabilities, and electronic effects of neighboring groups can either facilitate or impede the exchange reactions. By strategically designing the molecular architecture, researchers can control the activation energy barriers and reaction rates, leading to vitrimers with customized dynamic properties and processing characteristics.Expand Specific Solutions04 Quantification methods for transesterification kinetics in vitrimers
Various analytical techniques have been developed to quantify and characterize the transesterification kinetics in vitrimer-type polymers. These methods include rheological measurements, stress relaxation tests, dynamic mechanical analysis, and spectroscopic techniques. By monitoring the exchange reactions under different conditions, researchers can determine key kinetic parameters such as activation energies, rate constants, and relaxation times. These quantitative assessments are essential for establishing structure-property relationships and optimizing vitrimer formulations for specific applications.Expand Specific Solutions05 Additives for modulating transesterification dynamics in vitrimer composites
Incorporating specific additives into vitrimer formulations offers an effective approach to modulate transesterification dynamics without altering the base polymer structure. These additives can include reactive diluents, phase-transfer agents, plasticizers, and nanofillers that interact with the polymer network to influence the exchange reaction environment. By carefully selecting and dosing these additives, manufacturers can fine-tune the processing characteristics, mechanical properties, and recyclability of vitrimer-based materials and composites.Expand Specific Solutions
Leading Research Groups and Industrial Players
The transesterification in vitrimer-type polymers market is in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global vitrimer market is projected to expand significantly due to demand for recyclable, self-healing materials across industries. Technologically, the field is advancing rapidly with key players at different maturity levels. Leading chemical corporations like BASF, Dow Global Technologies, DuPont, and SABIC are developing commercial applications, while academic institutions including Sichuan University, University of Manchester, and Sorbonne Université are driving fundamental research. Specialized companies such as Jiangsu Feymer Technology and Miracll Chemicals are focusing on niche applications. The collaboration between industry and academia is accelerating the transition from theoretical models to practical applications in sustainable polymer technologies.
SABIC Global Technologies BV
Technical Solution: SABIC Global Technologies has developed sophisticated mechanistic kinetic models for transesterification in vitrimer-type polymers as part of their advanced materials research program. Their approach focuses on establishing quantitative relationships between molecular structure, catalyst activity, and macroscopic material properties. SABIC's models incorporate detailed reaction mechanisms that account for steric effects, electronic factors, and diffusion limitations in polymer networks. They have established mathematical frameworks that predict transesterification rates as functions of temperature, functional group concentration, catalyst loading, and network architecture. Their research has yielded predictive tools for optimizing vitrimer formulations to achieve targeted stress relaxation behaviors while maintaining thermal stability and mechanical integrity. SABIC has implemented advanced characterization techniques including solid-state NMR and synchrotron X-ray scattering to validate their kinetic models at multiple length scales. Their modeling approach emphasizes industrial relevance, with particular focus on how transesterification kinetics influence processing parameters, recycling efficiency, and long-term material performance in demanding applications.
Strengths: Comprehensive approach integrating molecular-level understanding with macroscopic material properties; strong focus on structure-property relationships. Weaknesses: Models may require extensive characterization data for accurate parameterization; some approaches may be optimized for specific chemistry platforms within SABIC's portfolio.
BASF Corp.
Technical Solution: BASF has developed comprehensive mechanistic kinetic models for transesterification in vitrimer-type polymers as part of their sustainable materials initiative. Their approach combines experimental data with computational chemistry to create predictive frameworks applicable across diverse polymer systems. BASF's models incorporate detailed reaction mechanisms including catalyst activation, deactivation pathways, and side reactions that can occur during transesterification processes. They have established structure-activity relationships for various catalyst systems, enabling rational design of transesterification catalysts with optimized activity and selectivity profiles. Their kinetic models feature temperature-dependent parameters that accurately predict material behavior across processing and application conditions. BASF has integrated their transesterification models with rheological predictions to simulate processing behavior during extrusion, injection molding, and other manufacturing techniques. Their approach emphasizes practical industrial applications, with models designed to predict not only reaction kinetics but also resulting material properties such as stress relaxation times, creep resistance, and recyclability potential.
Strengths: Extensive resources for model validation across multiple polymer systems; strong integration between fundamental kinetics and practical material applications. Weaknesses: Models may be optimized for BASF's specific catalyst and polymer systems; some proprietary aspects may limit external validation and academic collaboration.
Key Theoretical Frameworks and Computational Approaches
Method for the reesterification or esterification of side chains in polymers
PatentWO2007063037A2
Innovation
- A process involving the polymerization of (meth)acrylates with a monomer containing a carboxyl group not directly attached to the polymer backbone, followed by transesterification or esterification with an alcohol in the presence of a catalyzing enzyme, allowing for selective and high-conversion modification of side chains without degrading the polymer.
Method for transesterification or esterification of side chain in polymer
PatentInactiveJP2012126919A
Innovation
- A process involving the production of copolymers with carboxyl groups in side chains, followed by transesterification or esterification using enzymes to modify these groups with alcohols, allowing selective modification of side chains without degradation.
Sustainability Impact of Vitrimer Technology
Vitrimer technology represents a significant advancement in sustainable materials science, offering unique environmental benefits through its dynamic covalent bond networks. The inherent recyclability of vitrimers addresses one of the most pressing environmental challenges in polymer science: the accumulation of non-degradable plastic waste. Unlike conventional thermosets that cannot be reprocessed, vitrimers can be repeatedly reshaped and recycled without significant property degradation, potentially reducing landfill waste by up to 30% for applicable polymer categories.
The transesterification mechanisms central to vitrimer chemistry enable material lifespans to be extended considerably. Research indicates that vitrimer-based products can maintain functional performance through multiple recycling cycles, with some systems demonstrating up to 85% property retention after five reprocessing events. This extended lifecycle directly translates to reduced resource consumption and manufacturing energy requirements, with preliminary lifecycle assessments suggesting energy savings of 40-60% compared to traditional polymer production and disposal pathways.
Carbon footprint analyses of vitrimer manufacturing processes show promising results when compared to conventional polymer production. The ability to reprocess these materials at lower temperatures than virgin polymer synthesis contributes to reduced greenhouse gas emissions. Quantitative studies estimate that widespread adoption of vitrimer technology could reduce CO2 emissions in the polymer sector by 15-25%, particularly in industries with high-volume plastic consumption such as automotive and consumer electronics.
Water conservation represents another significant sustainability advantage of vitrimer technology. Traditional polymer recycling often involves water-intensive cleaning processes, while vitrimer reprocessing typically requires minimal water usage. This characteristic becomes increasingly valuable as water scarcity concerns grow globally, with potential water savings of 3-4 gallons per kilogram of recycled material compared to conventional recycling methods.
The economic sustainability of vitrimer technology further enhances its environmental impact. The value proposition of materials that can be repeatedly recycled without quality degradation creates financial incentives for closed-loop material systems. Market analyses project that vitrimer-based circular economy models could generate cost savings of 20-30% in materials-intensive industries while simultaneously reducing environmental impact, creating a rare alignment between economic and ecological interests.
As transesterification kinetics research advances, optimization of catalyst systems and processing conditions continues to improve the energy efficiency of vitrimer recycling. Recent innovations in catalyst design have reduced the reprocessing temperatures by approximately 40°C compared to early vitrimer systems, further enhancing the sustainability profile of these materials through reduced energy consumption during the recycling phase.
The transesterification mechanisms central to vitrimer chemistry enable material lifespans to be extended considerably. Research indicates that vitrimer-based products can maintain functional performance through multiple recycling cycles, with some systems demonstrating up to 85% property retention after five reprocessing events. This extended lifecycle directly translates to reduced resource consumption and manufacturing energy requirements, with preliminary lifecycle assessments suggesting energy savings of 40-60% compared to traditional polymer production and disposal pathways.
Carbon footprint analyses of vitrimer manufacturing processes show promising results when compared to conventional polymer production. The ability to reprocess these materials at lower temperatures than virgin polymer synthesis contributes to reduced greenhouse gas emissions. Quantitative studies estimate that widespread adoption of vitrimer technology could reduce CO2 emissions in the polymer sector by 15-25%, particularly in industries with high-volume plastic consumption such as automotive and consumer electronics.
Water conservation represents another significant sustainability advantage of vitrimer technology. Traditional polymer recycling often involves water-intensive cleaning processes, while vitrimer reprocessing typically requires minimal water usage. This characteristic becomes increasingly valuable as water scarcity concerns grow globally, with potential water savings of 3-4 gallons per kilogram of recycled material compared to conventional recycling methods.
The economic sustainability of vitrimer technology further enhances its environmental impact. The value proposition of materials that can be repeatedly recycled without quality degradation creates financial incentives for closed-loop material systems. Market analyses project that vitrimer-based circular economy models could generate cost savings of 20-30% in materials-intensive industries while simultaneously reducing environmental impact, creating a rare alignment between economic and ecological interests.
As transesterification kinetics research advances, optimization of catalyst systems and processing conditions continues to improve the energy efficiency of vitrimer recycling. Recent innovations in catalyst design have reduced the reprocessing temperatures by approximately 40°C compared to early vitrimer systems, further enhancing the sustainability profile of these materials through reduced energy consumption during the recycling phase.
Experimental Validation Methods for Kinetic Models
Validation of mechanistic kinetic models for transesterification reactions in vitrimer-type polymers requires rigorous experimental methodologies to ensure model accuracy and predictive capability. The primary validation approach involves comparing model predictions with experimental data obtained under controlled conditions across various temperature ranges and catalyst concentrations.
Rheological measurements serve as a fundamental validation technique, where stress relaxation experiments track the dynamic modulus decay over time. These measurements directly correlate with transesterification reaction progress and network rearrangement. Time-temperature superposition principles can be applied to these data to verify the Arrhenius behavior predicted by kinetic models and extract activation energies for comparison with model parameters.
Spectroscopic techniques provide another critical validation pathway. Real-time FTIR spectroscopy enables monitoring of functional group transformations during transesterification, with particular focus on ester carbonyl stretching bands. Similarly, NMR spectroscopy offers quantitative tracking of exchange reactions through peak shifts and integration changes, especially valuable for deuterium-labeled exchange experiments that provide direct evidence of bond exchange kinetics.
Differential scanning calorimetry (DSC) measurements complement these approaches by quantifying reaction enthalpies and activation energies. Isothermal DSC experiments at various temperatures can generate conversion-time data for direct comparison with model predictions, while temperature-ramped experiments provide insights into reaction kinetics across broader temperature ranges.
Advanced microscopy techniques, including AFM and confocal microscopy with fluorescent markers, enable visualization of network rearrangements at micro and nanoscales. These observations provide spatial information about reaction progression that can validate diffusion components in more sophisticated kinetic models.
Model validation quality can be significantly enhanced through designed experiments that systematically vary key parameters like temperature, catalyst concentration, and functional group density. Statistical analysis methods including residual analysis, confidence intervals, and sensitivity analysis should be employed to quantitatively assess model fit quality and parameter uncertainty.
Cross-validation approaches using data partitioning between training and validation sets help prevent overfitting and ensure model robustness. Additionally, validation across different vitrimer chemistries (epoxy-acid, epoxy-anhydride, etc.) tests model transferability and fundamental mechanistic accuracy beyond specific polymer systems.
Rheological measurements serve as a fundamental validation technique, where stress relaxation experiments track the dynamic modulus decay over time. These measurements directly correlate with transesterification reaction progress and network rearrangement. Time-temperature superposition principles can be applied to these data to verify the Arrhenius behavior predicted by kinetic models and extract activation energies for comparison with model parameters.
Spectroscopic techniques provide another critical validation pathway. Real-time FTIR spectroscopy enables monitoring of functional group transformations during transesterification, with particular focus on ester carbonyl stretching bands. Similarly, NMR spectroscopy offers quantitative tracking of exchange reactions through peak shifts and integration changes, especially valuable for deuterium-labeled exchange experiments that provide direct evidence of bond exchange kinetics.
Differential scanning calorimetry (DSC) measurements complement these approaches by quantifying reaction enthalpies and activation energies. Isothermal DSC experiments at various temperatures can generate conversion-time data for direct comparison with model predictions, while temperature-ramped experiments provide insights into reaction kinetics across broader temperature ranges.
Advanced microscopy techniques, including AFM and confocal microscopy with fluorescent markers, enable visualization of network rearrangements at micro and nanoscales. These observations provide spatial information about reaction progression that can validate diffusion components in more sophisticated kinetic models.
Model validation quality can be significantly enhanced through designed experiments that systematically vary key parameters like temperature, catalyst concentration, and functional group density. Statistical analysis methods including residual analysis, confidence intervals, and sensitivity analysis should be employed to quantitatively assess model fit quality and parameter uncertainty.
Cross-validation approaches using data partitioning between training and validation sets help prevent overfitting and ensure model robustness. Additionally, validation across different vitrimer chemistries (epoxy-acid, epoxy-anhydride, etc.) tests model transferability and fundamental mechanistic accuracy beyond specific polymer systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!