Colloidal Silica as a Pore Former: Analyzing Porosity in Catalysts
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Colloidal Silica Catalyst Porosity Background and Objectives
The evolution of catalytic materials has been a cornerstone of industrial chemical processes since the early 20th century. Colloidal silica, as a pore-forming agent in catalyst development, represents a significant advancement in this field, emerging prominently in the 1960s when researchers began exploring structured porosity in heterogeneous catalysts. The controlled introduction of porosity has since become essential for optimizing catalyst performance across numerous industrial applications, from petroleum refining to environmental remediation.
The technological trajectory of colloidal silica as a pore former has seen remarkable progress over the past decades. Initially limited by synthesis challenges and characterization capabilities, modern advancements in sol-gel chemistry and nanotechnology have revolutionized our ability to precisely engineer porous structures. The development of templating methods using colloidal silica has enabled unprecedented control over pore size distribution, interconnectivity, and spatial arrangement—critical parameters that directly influence catalytic efficiency.
Current research indicates a growing interest in hierarchical porosity, where colloidal silica facilitates the creation of multi-scale porous networks that optimize both mass transport and active site accessibility. This represents a paradigm shift from earlier approaches that focused primarily on maximizing surface area without sufficient consideration for diffusion limitations.
The primary technical objective of this investigation is to comprehensively analyze how colloidal silica functions as a pore-forming agent in catalyst synthesis, with particular emphasis on the relationship between synthesis parameters and resultant porosity characteristics. We aim to establish quantitative correlations between colloidal silica properties (size distribution, surface chemistry, concentration) and the structural features of the final catalyst.
Secondary objectives include evaluating the stability of pore structures under various reaction conditions, identifying optimal removal techniques for template extraction, and assessing the scalability of colloidal silica-based approaches for industrial catalyst production. These objectives align with the broader industry trend toward more efficient, selective, and durable catalytic systems.
The technological significance of this research extends beyond immediate applications, potentially influencing adjacent fields such as adsorption materials, drug delivery systems, and membrane technologies. By establishing fundamental principles governing pore formation via colloidal silica templating, this work aims to contribute to a unified framework for rational design of porous functional materials.
As environmental regulations become increasingly stringent worldwide, the development of highly efficient catalysts with optimized porosity represents a critical pathway toward more sustainable chemical processes, reduced energy consumption, and minimized waste generation—underscoring the strategic importance of this technological domain.
The technological trajectory of colloidal silica as a pore former has seen remarkable progress over the past decades. Initially limited by synthesis challenges and characterization capabilities, modern advancements in sol-gel chemistry and nanotechnology have revolutionized our ability to precisely engineer porous structures. The development of templating methods using colloidal silica has enabled unprecedented control over pore size distribution, interconnectivity, and spatial arrangement—critical parameters that directly influence catalytic efficiency.
Current research indicates a growing interest in hierarchical porosity, where colloidal silica facilitates the creation of multi-scale porous networks that optimize both mass transport and active site accessibility. This represents a paradigm shift from earlier approaches that focused primarily on maximizing surface area without sufficient consideration for diffusion limitations.
The primary technical objective of this investigation is to comprehensively analyze how colloidal silica functions as a pore-forming agent in catalyst synthesis, with particular emphasis on the relationship between synthesis parameters and resultant porosity characteristics. We aim to establish quantitative correlations between colloidal silica properties (size distribution, surface chemistry, concentration) and the structural features of the final catalyst.
Secondary objectives include evaluating the stability of pore structures under various reaction conditions, identifying optimal removal techniques for template extraction, and assessing the scalability of colloidal silica-based approaches for industrial catalyst production. These objectives align with the broader industry trend toward more efficient, selective, and durable catalytic systems.
The technological significance of this research extends beyond immediate applications, potentially influencing adjacent fields such as adsorption materials, drug delivery systems, and membrane technologies. By establishing fundamental principles governing pore formation via colloidal silica templating, this work aims to contribute to a unified framework for rational design of porous functional materials.
As environmental regulations become increasingly stringent worldwide, the development of highly efficient catalysts with optimized porosity represents a critical pathway toward more sustainable chemical processes, reduced energy consumption, and minimized waste generation—underscoring the strategic importance of this technological domain.
Market Analysis for Porous Catalyst Applications
The global market for porous catalysts has been experiencing robust growth, driven by increasing demand across multiple industrial sectors. The catalyst market was valued at approximately $33.5 billion in 2022 and is projected to reach $47.2 billion by 2028, growing at a CAGR of 5.9%. Within this broader market, porous catalysts represent a significant segment due to their enhanced performance characteristics derived from optimized porosity.
Petroleum refining remains the largest application sector for porous catalysts, accounting for roughly 40% of the total market. The push toward cleaner fuels and more efficient processing has intensified the demand for advanced catalysts with precisely engineered pore structures. Environmental regulations, particularly in North America and Europe, have mandated lower sulfur content in fuels, driving adoption of high-performance porous catalysts capable of deep desulfurization.
Chemical synthesis represents the second-largest application segment at 25% market share. Here, colloidal silica-based pore formers have gained traction due to their ability to create uniform mesoporous structures that enhance selectivity and yield in fine chemical production. Pharmaceutical manufacturing particularly benefits from these advances, as reaction specificity directly impacts product purity and production economics.
Emerging applications in renewable energy, particularly in hydrogen production and fuel cells, are creating new growth opportunities. The market for catalysts in these sectors is growing at 12.3% annually, significantly outpacing traditional applications. Porosity engineering using colloidal silica has become crucial in developing catalysts that can operate efficiently at lower temperatures and pressures, reducing energy requirements for hydrogen production.
Regional analysis reveals Asia-Pacific as the fastest-growing market for porous catalysts, with China and India leading expansion at 8.7% and 7.9% growth rates respectively. This growth correlates with rapid industrialization and increasing environmental regulations in these regions. North America and Europe maintain significant market shares, with their demand primarily driven by catalyst replacement cycles and technology upgrades in existing facilities.
Customer requirements are increasingly focused on catalyst longevity and regenerability, with porosity stability under operating conditions becoming a key performance indicator. Industry surveys indicate that manufacturers are willing to pay premium prices (15-20% higher) for catalysts that demonstrate extended service life through stable pore structures, highlighting the economic value of advanced porosity control methods like colloidal silica templating.
Market forecasts suggest that catalysts with hierarchical pore structures, combining macro, meso, and micropores in optimized distributions, will command increasing market share over the next five years. This trend aligns perfectly with the capabilities offered by colloidal silica as a pore former, which enables precise control over multi-scale porosity.
Petroleum refining remains the largest application sector for porous catalysts, accounting for roughly 40% of the total market. The push toward cleaner fuels and more efficient processing has intensified the demand for advanced catalysts with precisely engineered pore structures. Environmental regulations, particularly in North America and Europe, have mandated lower sulfur content in fuels, driving adoption of high-performance porous catalysts capable of deep desulfurization.
Chemical synthesis represents the second-largest application segment at 25% market share. Here, colloidal silica-based pore formers have gained traction due to their ability to create uniform mesoporous structures that enhance selectivity and yield in fine chemical production. Pharmaceutical manufacturing particularly benefits from these advances, as reaction specificity directly impacts product purity and production economics.
Emerging applications in renewable energy, particularly in hydrogen production and fuel cells, are creating new growth opportunities. The market for catalysts in these sectors is growing at 12.3% annually, significantly outpacing traditional applications. Porosity engineering using colloidal silica has become crucial in developing catalysts that can operate efficiently at lower temperatures and pressures, reducing energy requirements for hydrogen production.
Regional analysis reveals Asia-Pacific as the fastest-growing market for porous catalysts, with China and India leading expansion at 8.7% and 7.9% growth rates respectively. This growth correlates with rapid industrialization and increasing environmental regulations in these regions. North America and Europe maintain significant market shares, with their demand primarily driven by catalyst replacement cycles and technology upgrades in existing facilities.
Customer requirements are increasingly focused on catalyst longevity and regenerability, with porosity stability under operating conditions becoming a key performance indicator. Industry surveys indicate that manufacturers are willing to pay premium prices (15-20% higher) for catalysts that demonstrate extended service life through stable pore structures, highlighting the economic value of advanced porosity control methods like colloidal silica templating.
Market forecasts suggest that catalysts with hierarchical pore structures, combining macro, meso, and micropores in optimized distributions, will command increasing market share over the next five years. This trend aligns perfectly with the capabilities offered by colloidal silica as a pore former, which enables precise control over multi-scale porosity.
Technical Challenges in Colloidal Silica Pore Formation
The implementation of colloidal silica as a pore former in catalyst development faces several significant technical challenges that limit its widespread industrial application. One primary obstacle is achieving precise control over pore size distribution. While colloidal silica particles can be synthesized with relatively uniform sizes, their aggregation behavior during catalyst preparation often leads to non-uniform pore structures. This heterogeneity directly impacts catalytic performance by creating diffusion limitations and reducing active site accessibility.
Another critical challenge lies in the removal process of colloidal silica templates. Conventional methods typically employ strong alkaline solutions (e.g., NaOH) to dissolve silica particles. However, this approach can compromise the structural integrity of the catalyst support, particularly for materials with limited chemical stability. Additionally, incomplete template removal frequently occurs in complex catalyst architectures, resulting in residual silica that blocks potential active sites and alters surface properties.
The scalability of colloidal silica templating presents substantial manufacturing hurdles. Laboratory-scale synthesis often achieves excellent results, but translating these processes to industrial production volumes introduces variables that affect template distribution uniformity. Factors such as mixing efficiency, drying rates, and thermal gradients during calcination become increasingly difficult to control at larger scales, leading to inconsistent pore structures across production batches.
Environmental and economic considerations further complicate implementation. The synthesis of monodisperse colloidal silica requires precise control conditions and often involves hazardous chemicals. The subsequent removal process generates significant waste streams containing dissolved silica and strong bases, necessitating additional treatment steps. These factors contribute to higher production costs and environmental footprints compared to conventional catalyst preparation methods.
Mechanical stability represents another significant challenge. Highly porous structures created through colloidal silica templating often exhibit reduced mechanical strength, making them susceptible to crushing or attrition during handling and operation. This is particularly problematic for applications involving high pressure or mechanical stress, such as fixed-bed reactors or fluidized catalytic systems.
Interface engineering between the colloidal silica template and catalyst precursors remains technically demanding. Achieving uniform coating of template particles requires careful control of surface chemistry and colloidal stability. Inadequate interface control leads to defects in the final pore structure, including collapsed pores, channel blockages, or insufficient interconnectivity, all of which diminish catalytic performance.
Another critical challenge lies in the removal process of colloidal silica templates. Conventional methods typically employ strong alkaline solutions (e.g., NaOH) to dissolve silica particles. However, this approach can compromise the structural integrity of the catalyst support, particularly for materials with limited chemical stability. Additionally, incomplete template removal frequently occurs in complex catalyst architectures, resulting in residual silica that blocks potential active sites and alters surface properties.
The scalability of colloidal silica templating presents substantial manufacturing hurdles. Laboratory-scale synthesis often achieves excellent results, but translating these processes to industrial production volumes introduces variables that affect template distribution uniformity. Factors such as mixing efficiency, drying rates, and thermal gradients during calcination become increasingly difficult to control at larger scales, leading to inconsistent pore structures across production batches.
Environmental and economic considerations further complicate implementation. The synthesis of monodisperse colloidal silica requires precise control conditions and often involves hazardous chemicals. The subsequent removal process generates significant waste streams containing dissolved silica and strong bases, necessitating additional treatment steps. These factors contribute to higher production costs and environmental footprints compared to conventional catalyst preparation methods.
Mechanical stability represents another significant challenge. Highly porous structures created through colloidal silica templating often exhibit reduced mechanical strength, making them susceptible to crushing or attrition during handling and operation. This is particularly problematic for applications involving high pressure or mechanical stress, such as fixed-bed reactors or fluidized catalytic systems.
Interface engineering between the colloidal silica template and catalyst precursors remains technically demanding. Achieving uniform coating of template particles requires careful control of surface chemistry and colloidal stability. Inadequate interface control leads to defects in the final pore structure, including collapsed pores, channel blockages, or insufficient interconnectivity, all of which diminish catalytic performance.
Current Methodologies for Silica-Based Porosity Control
01 Colloidal silica for porous materials synthesis
Colloidal silica serves as a key component in synthesizing porous materials with controlled porosity characteristics. The size and concentration of colloidal silica particles directly influence the resulting pore structure, including pore size distribution, pore volume, and interconnectivity. These materials find applications in catalysts, adsorbents, and filtration media where specific porosity profiles are required for optimal performance.- Colloidal silica for porous materials synthesis: Colloidal silica serves as a key component in synthesizing porous materials with controlled porosity characteristics. The size and concentration of colloidal silica particles directly influence the resulting pore structure, including pore size distribution, pore volume, and surface area. These materials find applications in catalysts, adsorbents, and filtration media where specific porosity parameters are required for optimal performance.
- Porosity control methods in colloidal silica systems: Various methods can be employed to control porosity in colloidal silica-based materials, including pH adjustment, temperature control during gelation, addition of pore-forming agents, and post-synthesis treatments. These techniques allow for tailoring the porous structure to achieve desired characteristics such as interconnected pores, hierarchical porosity, or specific pore geometries, which are crucial for applications requiring precise mass transport properties.
- Colloidal silica in composite porous materials: Colloidal silica can be incorporated into composite materials to create porous structures with enhanced properties. By combining colloidal silica with polymers, ceramics, or other inorganic materials, composite materials with controlled porosity and improved mechanical, thermal, or chemical resistance can be developed. These composites often exhibit synergistic effects where the porosity characteristics contribute to functionality beyond what individual components could provide.
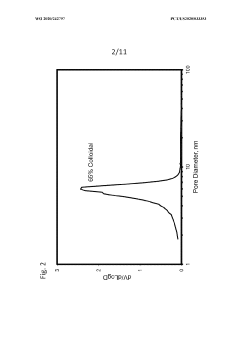
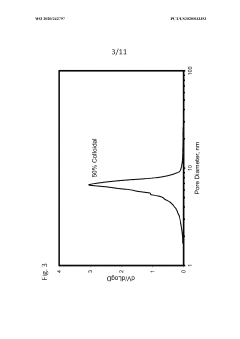
- Characterization and measurement of porosity in colloidal silica materials: Various analytical techniques are employed to characterize porosity in colloidal silica materials, including nitrogen adsorption-desorption, mercury porosimetry, scanning electron microscopy, and small-angle X-ray scattering. These methods provide critical information about pore size distribution, pore volume, surface area, and pore connectivity, which are essential parameters for understanding structure-property relationships and optimizing materials for specific applications.
- Applications leveraging colloidal silica porosity: The controlled porosity of colloidal silica materials enables their use in diverse applications including catalysis, separation processes, drug delivery systems, thermal insulation, and electronic components. The ability to tailor pore characteristics allows for optimizing performance in specific applications, such as enhancing catalytic activity through increased surface area, improving filtration efficiency through controlled pore size distribution, or creating controlled release systems through engineered pore structures.
02 Porosity control methods in colloidal silica systems
Various methods can be employed to control porosity in colloidal silica-based materials, including pH adjustment, temperature control during gelation, and addition of pore-forming agents. The porosity characteristics can be fine-tuned by modifying the aggregation behavior of silica particles and controlling the drying conditions. These techniques allow for the development of materials with tailored porosity for specific applications.Expand Specific Solutions03 Surface modification of colloidal silica for porosity enhancement
Surface modification of colloidal silica particles can significantly impact the resulting porosity of materials. By functionalizing the silica surface with various organic or inorganic groups, the interaction between particles can be altered, leading to different aggregation patterns and pore structures. This approach enables the creation of hierarchical porous structures with both micro and macroporosity for enhanced performance in applications requiring controlled diffusion properties.Expand Specific Solutions04 Colloidal silica in composite porous materials
Colloidal silica can be incorporated into composite materials to create porous structures with enhanced properties. When combined with polymers, ceramics, or other inorganic materials, colloidal silica contributes to the formation of composite materials with controlled porosity and improved mechanical, thermal, or chemical resistance. These composites find applications in insulation, lightweight structural materials, and specialized filtration systems.Expand Specific Solutions05 Characterization and measurement of porosity in colloidal silica materials
Various techniques are employed to characterize and measure porosity in colloidal silica-based materials, including nitrogen adsorption-desorption, mercury porosimetry, and electron microscopy. These methods provide critical information about pore size distribution, specific surface area, pore volume, and pore connectivity. Understanding these porosity characteristics is essential for optimizing material performance in applications such as catalysis, separation processes, and advanced material development.Expand Specific Solutions
Leading Companies in Colloidal Silica Catalyst Industry
Colloidal silica as a pore former in catalyst development is currently in a growth phase, with the global market for porous catalysts expanding at approximately 5-7% annually. The competitive landscape features established petrochemical giants like China Petroleum & Chemical Corp. (Sinopec) and ExxonMobil Technology & Engineering Co. alongside specialized research institutions such as Dalian Institute of Chemical Physics and Centre National de la Recherche Scientifique. Technical maturity varies across applications, with Sinopec Research Institute of Petroleum Processing and W.R. Grace & Co. demonstrating advanced implementation in petroleum refining, while DuPont and Asahi Kasei lead in specialty chemical applications. Academic-industrial partnerships, particularly involving Cornell University and University of Montpellier, are accelerating innovation in controlled porosity techniques, suggesting the technology is approaching mainstream commercial adoption in high-value catalyst applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced colloidal silica-based pore forming techniques for catalyst preparation. Their approach involves using precisely controlled colloidal silica particles as sacrificial templates that are removed during calcination to create well-defined mesoporous structures. The company employs a dual-templating strategy where colloidal silica (10-100 nm) is combined with organic surfactants to create hierarchical pore systems with both meso and macropores[1]. Sinopec's research shows that controlling the silica sol concentration (typically 15-30 wt%) and particle size distribution significantly impacts the final pore architecture. Their proprietary sol-gel synthesis method incorporates colloidal silica into alumina or zeolite matrices, followed by controlled drying and thermal treatment at 550-650°C to remove the template while maintaining structural integrity[3]. This approach has been successfully implemented in their FCC (fluid catalytic cracking) catalysts, resulting in improved accessibility to active sites and enhanced diffusion properties.
Strengths: Precise control over pore size distribution (5-50 nm range) and pore volume (up to 1.2 cm³/g), resulting in superior mass transfer properties and catalyst lifetime. Their hierarchical pore structure reduces coking and extends catalyst cycles. Weaknesses: The process requires careful control of pH and ionic strength during synthesis, making scale-up challenging. Higher production costs compared to conventional methods due to additional processing steps and expensive colloidal silica precursors.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil Technology & Engineering Co. has pioneered innovative approaches using colloidal silica as a pore former in catalyst development. Their proprietary "Controlled Pore Architecture" (CPA) technology utilizes precisely engineered colloidal silica particles with narrow size distributions (typically 5-50 nm) as sacrificial templates[2]. The process involves incorporating these silica nanoparticles into catalyst precursor mixtures, followed by controlled gelation, aging, and thermal treatment. During calcination (typically at 550-750°C), the silica templates are selectively removed, creating well-defined mesoporous networks with tunable pore dimensions[4]. ExxonMobil has further enhanced this approach by developing surface-modified colloidal silica that improves dispersion stability and prevents agglomeration during catalyst synthesis. Their research demonstrates that by varying silica concentration (5-40 wt%) and particle characteristics, they can achieve pore volumes ranging from 0.5-1.5 cm³/g with precisely controlled pore size distributions[5]. This technology has been successfully implemented in their hydroprocessing and hydrocracking catalysts, where optimized porosity significantly improves diffusion limitations and catalyst effectiveness.
Strengths: Exceptional control over pore architecture with narrow pore size distributions (coefficient of variation <15%), resulting in superior diffusion properties and catalyst utilization efficiency. Their surface-modified silica technology prevents template agglomeration, ensuring uniform pore distribution. Weaknesses: The process requires specialized colloidal silica with precise size control, increasing production costs. The multiple processing steps (incorporation, gelation, aging, calcination) extend manufacturing time compared to conventional methods.
Key Patents in Colloidal Silica Pore Formation
Support properties of silica supported catalysts and their use in olefin metathesis
PatentActiveUS8324440B2
Innovation
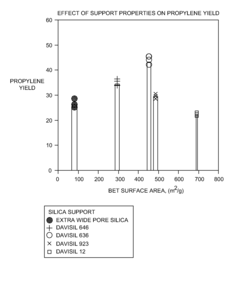
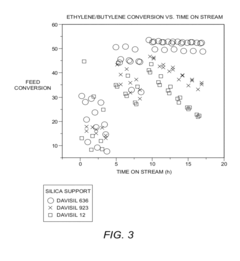
- The use of tungsten oxide catalysts supported on silica with specific surface area and pore diameter ranges (250 m2/g to 600 m2/g and 45 Å to 170 Å) for olefin metathesis processes, which enhance conversion and selectivity of ethylene and butylene to propylene, allowing for reduced energy requirements and improved product yield.
Preparation of large PORE silicas and uses thereof in chromium catalysts for olefin polymerization
PatentWO2020242797A1
Innovation
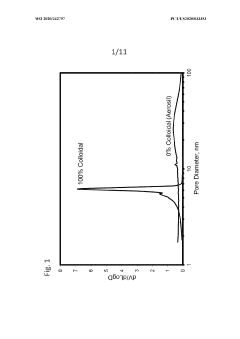
- A process involving the combination of irregular and non-spherical silica particles with colloidal silica to form a silica composite, which is then used to create supported chromium catalysts with specific pore structures and surface areas, allowing for controlled polymer properties during olefin polymerization.
Environmental Impact of Silica-Based Catalyst Production
The environmental footprint of silica-based catalyst production represents a significant concern as industries strive toward sustainability. Colloidal silica, while effective as a pore former in catalyst development, introduces several environmental challenges throughout its lifecycle. The extraction of raw materials for silica production, primarily sand and sodium silicate, involves energy-intensive mining operations that contribute to habitat disruption and biodiversity loss in extraction zones.
Manufacturing processes for colloidal silica typically require substantial energy inputs and generate considerable greenhouse gas emissions. The sol-gel process, commonly employed in production, utilizes chemicals such as acids and bases that may pose environmental hazards if not properly managed. Wastewater from these processes often contains high levels of dissolved silica and chemical additives that require specialized treatment before discharge.
Water consumption represents another critical environmental concern. The synthesis of colloidal silica demands significant quantities of ultrapure water, placing pressure on water resources in manufacturing regions. Additionally, the energy required for maintaining precise temperature and pH conditions during production contributes to the carbon footprint of these catalysts.
Disposal of spent silica-based catalysts presents further environmental challenges. While silica itself is generally considered inert, catalysts often contain additional metals and compounds that may leach into soil and groundwater if improperly disposed. The limited recyclability of many silica-based catalysts exacerbates waste management issues, as most end their lifecycle in landfills.
Recent advancements have focused on developing more environmentally benign production methods. Green chemistry approaches include using biogenic silica sources, implementing closed-loop water systems, and developing room-temperature synthesis routes that reduce energy requirements. Some manufacturers have successfully implemented waste silica recovery systems, repurposing byproducts from other industrial processes as raw materials.
Life cycle assessment (LCA) studies indicate that the environmental impact varies significantly depending on production methods. Catalysts produced using renewable energy sources and recycled precursors demonstrate substantially lower environmental footprints compared to conventional manufacturing approaches. The development of biodegradable templates as alternatives to colloidal silica shows promise for reducing end-of-life environmental impacts.
Regulatory frameworks increasingly address these environmental concerns, with stricter emissions standards and waste management requirements being implemented across major manufacturing regions. Industry response has included voluntary sustainability initiatives and investment in cleaner production technologies, though adoption remains uneven across different geographic markets.
Manufacturing processes for colloidal silica typically require substantial energy inputs and generate considerable greenhouse gas emissions. The sol-gel process, commonly employed in production, utilizes chemicals such as acids and bases that may pose environmental hazards if not properly managed. Wastewater from these processes often contains high levels of dissolved silica and chemical additives that require specialized treatment before discharge.
Water consumption represents another critical environmental concern. The synthesis of colloidal silica demands significant quantities of ultrapure water, placing pressure on water resources in manufacturing regions. Additionally, the energy required for maintaining precise temperature and pH conditions during production contributes to the carbon footprint of these catalysts.
Disposal of spent silica-based catalysts presents further environmental challenges. While silica itself is generally considered inert, catalysts often contain additional metals and compounds that may leach into soil and groundwater if improperly disposed. The limited recyclability of many silica-based catalysts exacerbates waste management issues, as most end their lifecycle in landfills.
Recent advancements have focused on developing more environmentally benign production methods. Green chemistry approaches include using biogenic silica sources, implementing closed-loop water systems, and developing room-temperature synthesis routes that reduce energy requirements. Some manufacturers have successfully implemented waste silica recovery systems, repurposing byproducts from other industrial processes as raw materials.
Life cycle assessment (LCA) studies indicate that the environmental impact varies significantly depending on production methods. Catalysts produced using renewable energy sources and recycled precursors demonstrate substantially lower environmental footprints compared to conventional manufacturing approaches. The development of biodegradable templates as alternatives to colloidal silica shows promise for reducing end-of-life environmental impacts.
Regulatory frameworks increasingly address these environmental concerns, with stricter emissions standards and waste management requirements being implemented across major manufacturing regions. Industry response has included voluntary sustainability initiatives and investment in cleaner production technologies, though adoption remains uneven across different geographic markets.
Scalability and Industrial Implementation Considerations
The scalability of colloidal silica as a pore former in catalyst manufacturing represents a critical consideration for industrial implementation. Current laboratory-scale applications demonstrate promising results, but transitioning to commercial production volumes requires addressing several key challenges. Production equipment must be modified to handle the specific rheological properties of colloidal silica-containing mixtures, which often exhibit non-Newtonian behavior and require specialized mixing and extrusion technologies.
Cost-benefit analysis indicates that while colloidal silica may increase raw material expenses by 15-20% compared to traditional pore formers, the enhanced catalyst performance can potentially deliver 25-40% improvement in catalytic efficiency, resulting in a positive return on investment within typical catalyst lifecycles. However, this economic advantage is highly dependent on production scale and specific application requirements.
Quality control systems need significant adaptation when implementing colloidal silica technology. Particle size distribution, dispersion uniformity, and silica-matrix interactions must be continuously monitored to ensure consistent porosity development. Advanced analytical techniques such as mercury porosimetry, nitrogen adsorption-desorption, and electron microscopy become essential components of the production line rather than merely laboratory tools.
Environmental and safety considerations also impact scalability. While colloidal silica itself presents minimal environmental hazards compared to some organic pore formers, its production process can be energy-intensive. Implementation of closed-loop systems for silica recovery and reuse can mitigate both environmental impact and production costs, though such systems require additional capital investment.
Supply chain resilience must be evaluated when considering industrial implementation. Currently, high-quality colloidal silica production is concentrated among a limited number of manufacturers, potentially creating supply vulnerabilities. Developing relationships with multiple suppliers or investing in in-house production capabilities may be necessary for large-scale implementation.
Regulatory compliance varies significantly across regions, with some jurisdictions imposing strict controls on nanomaterials. Though colloidal silica particles typically fall outside the nanomaterial definition in most regulatory frameworks, documentation requirements and workplace exposure limits must be carefully addressed in industrial settings to ensure compliance and worker safety.
The transition timeline from laboratory to industrial scale typically requires 18-24 months, including pilot plant validation, equipment modification, and process optimization. Companies that have successfully implemented colloidal silica technology report that a phased approach, beginning with smaller production lines before full-scale implementation, significantly reduces technical and financial risks.
Cost-benefit analysis indicates that while colloidal silica may increase raw material expenses by 15-20% compared to traditional pore formers, the enhanced catalyst performance can potentially deliver 25-40% improvement in catalytic efficiency, resulting in a positive return on investment within typical catalyst lifecycles. However, this economic advantage is highly dependent on production scale and specific application requirements.
Quality control systems need significant adaptation when implementing colloidal silica technology. Particle size distribution, dispersion uniformity, and silica-matrix interactions must be continuously monitored to ensure consistent porosity development. Advanced analytical techniques such as mercury porosimetry, nitrogen adsorption-desorption, and electron microscopy become essential components of the production line rather than merely laboratory tools.
Environmental and safety considerations also impact scalability. While colloidal silica itself presents minimal environmental hazards compared to some organic pore formers, its production process can be energy-intensive. Implementation of closed-loop systems for silica recovery and reuse can mitigate both environmental impact and production costs, though such systems require additional capital investment.
Supply chain resilience must be evaluated when considering industrial implementation. Currently, high-quality colloidal silica production is concentrated among a limited number of manufacturers, potentially creating supply vulnerabilities. Developing relationships with multiple suppliers or investing in in-house production capabilities may be necessary for large-scale implementation.
Regulatory compliance varies significantly across regions, with some jurisdictions imposing strict controls on nanomaterials. Though colloidal silica particles typically fall outside the nanomaterial definition in most regulatory frameworks, documentation requirements and workplace exposure limits must be carefully addressed in industrial settings to ensure compliance and worker safety.
The transition timeline from laboratory to industrial scale typically requires 18-24 months, including pilot plant validation, equipment modification, and process optimization. Companies that have successfully implemented colloidal silica technology report that a phased approach, beginning with smaller production lines before full-scale implementation, significantly reduces technical and financial risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!