How to Optimize Colloidal Silica Synthesis for Uniform Particle Distribution
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Colloidal Silica Synthesis Background and Objectives
Colloidal silica, a dispersion of nanoscale silica particles in a liquid medium, has evolved significantly since its initial development in the early 20th century. The synthesis methods have progressed from simple acid-base reactions to sophisticated controlled processes that enable precise manipulation of particle size, distribution, and surface properties. The evolution of colloidal silica technology has been driven by increasing demands for materials with specific performance characteristics across various industries.
The Stöber process, developed in 1968, marked a significant milestone in colloidal silica synthesis, enabling the production of monodisperse spherical silica particles. Since then, numerous modifications and alternative approaches have emerged, including sol-gel methods, microemulsion techniques, and hydrothermal processes. Recent advancements have focused on achieving greater control over particle morphology, size distribution, and surface functionality.
Current technological trends in colloidal silica synthesis include the development of green chemistry approaches that minimize environmental impact, continuous flow processes for industrial-scale production, and hybrid methods that incorporate organic components for enhanced functionality. The integration of computational modeling and artificial intelligence has also begun to play a crucial role in optimizing synthesis parameters and predicting particle behavior.
The primary objective of optimizing colloidal silica synthesis for uniform particle distribution is to develop reproducible, scalable methods that yield particles with precisely controlled size, narrow size distribution, and consistent surface properties. This optimization aims to address the persistent challenges of aggregation, polydispersity, and batch-to-batch variability that have limited the broader application of colloidal silica in high-performance materials.
Achieving uniform particle distribution is critical for numerous applications, including advanced coatings, catalysts, drug delivery systems, and electronic materials. Each application domain imposes specific requirements on particle characteristics, necessitating tailored synthesis approaches. For instance, optical applications demand exceptional uniformity to minimize light scattering, while biomedical applications require biocompatibility and controlled degradation profiles.
The technical goals of this research include developing robust synthesis protocols that can be readily scaled from laboratory to industrial production while maintaining particle uniformity, identifying key process parameters that influence particle distribution, and establishing quantitative relationships between synthesis conditions and resultant particle characteristics. Additionally, the research aims to explore novel characterization techniques that provide real-time feedback during synthesis, enabling dynamic process adjustments for optimal outcomes.
The Stöber process, developed in 1968, marked a significant milestone in colloidal silica synthesis, enabling the production of monodisperse spherical silica particles. Since then, numerous modifications and alternative approaches have emerged, including sol-gel methods, microemulsion techniques, and hydrothermal processes. Recent advancements have focused on achieving greater control over particle morphology, size distribution, and surface functionality.
Current technological trends in colloidal silica synthesis include the development of green chemistry approaches that minimize environmental impact, continuous flow processes for industrial-scale production, and hybrid methods that incorporate organic components for enhanced functionality. The integration of computational modeling and artificial intelligence has also begun to play a crucial role in optimizing synthesis parameters and predicting particle behavior.
The primary objective of optimizing colloidal silica synthesis for uniform particle distribution is to develop reproducible, scalable methods that yield particles with precisely controlled size, narrow size distribution, and consistent surface properties. This optimization aims to address the persistent challenges of aggregation, polydispersity, and batch-to-batch variability that have limited the broader application of colloidal silica in high-performance materials.
Achieving uniform particle distribution is critical for numerous applications, including advanced coatings, catalysts, drug delivery systems, and electronic materials. Each application domain imposes specific requirements on particle characteristics, necessitating tailored synthesis approaches. For instance, optical applications demand exceptional uniformity to minimize light scattering, while biomedical applications require biocompatibility and controlled degradation profiles.
The technical goals of this research include developing robust synthesis protocols that can be readily scaled from laboratory to industrial production while maintaining particle uniformity, identifying key process parameters that influence particle distribution, and establishing quantitative relationships between synthesis conditions and resultant particle characteristics. Additionally, the research aims to explore novel characterization techniques that provide real-time feedback during synthesis, enabling dynamic process adjustments for optimal outcomes.
Market Applications and Demand Analysis
Colloidal silica with uniform particle distribution has established itself as a critical material across multiple high-value industries, driving substantial market demand. The global colloidal silica market was valued at approximately 4.1 billion USD in 2022 and is projected to grow at a CAGR of 5.8% through 2030, with applications requiring uniform particle distribution commanding premium pricing.
The semiconductor industry represents one of the largest consumers of high-quality colloidal silica, where it serves as a crucial component in chemical-mechanical planarization (CMP) slurries. The precision required for modern semiconductor manufacturing processes necessitates exceptionally uniform silica particles to achieve nanometer-level surface flatness without introducing defects. As chip manufacturers continue to reduce node sizes below 5nm, the demand for ultra-uniform colloidal silica continues to intensify.
In the coatings and surface treatment sector, colloidal silica with uniform particle distribution enables the development of scratch-resistant coatings, anti-reflective films, and specialized functional surfaces. The automotive industry increasingly incorporates these coatings for improved durability and appearance retention, while the construction sector utilizes them for self-cleaning surfaces and enhanced weatherability of building materials.
The pharmaceutical and biomedical fields represent rapidly expanding markets for uniform colloidal silica. Drug delivery systems benefit from precisely controlled silica nanoparticles as carriers for targeted therapeutics, while diagnostic applications leverage their optical properties for enhanced detection sensitivity. The biocompatibility of silica makes it particularly valuable for in vivo applications, driving research investment and commercial development.
Environmental applications constitute another growth segment, with water treatment processes utilizing colloidal silica for flocculation and filtration. The effectiveness of these applications correlates directly with particle uniformity, as it ensures consistent performance and predictable outcomes in purification systems.
Market analysis reveals regional variations in demand patterns. Asia-Pacific dominates consumption due to its robust semiconductor manufacturing base, while North America and Europe lead in high-specification applications for pharmaceutical and advanced materials sectors. Emerging economies are showing accelerated adoption rates as their industrial bases mature and environmental regulations tighten.
Customer requirements increasingly emphasize not only particle size uniformity but also surface chemistry consistency, stability in various pH environments, and compatibility with other formulation components. This trend has prompted manufacturers to develop customized colloidal silica products tailored to specific application requirements, creating premium market segments with higher profit margins.
The semiconductor industry represents one of the largest consumers of high-quality colloidal silica, where it serves as a crucial component in chemical-mechanical planarization (CMP) slurries. The precision required for modern semiconductor manufacturing processes necessitates exceptionally uniform silica particles to achieve nanometer-level surface flatness without introducing defects. As chip manufacturers continue to reduce node sizes below 5nm, the demand for ultra-uniform colloidal silica continues to intensify.
In the coatings and surface treatment sector, colloidal silica with uniform particle distribution enables the development of scratch-resistant coatings, anti-reflective films, and specialized functional surfaces. The automotive industry increasingly incorporates these coatings for improved durability and appearance retention, while the construction sector utilizes them for self-cleaning surfaces and enhanced weatherability of building materials.
The pharmaceutical and biomedical fields represent rapidly expanding markets for uniform colloidal silica. Drug delivery systems benefit from precisely controlled silica nanoparticles as carriers for targeted therapeutics, while diagnostic applications leverage their optical properties for enhanced detection sensitivity. The biocompatibility of silica makes it particularly valuable for in vivo applications, driving research investment and commercial development.
Environmental applications constitute another growth segment, with water treatment processes utilizing colloidal silica for flocculation and filtration. The effectiveness of these applications correlates directly with particle uniformity, as it ensures consistent performance and predictable outcomes in purification systems.
Market analysis reveals regional variations in demand patterns. Asia-Pacific dominates consumption due to its robust semiconductor manufacturing base, while North America and Europe lead in high-specification applications for pharmaceutical and advanced materials sectors. Emerging economies are showing accelerated adoption rates as their industrial bases mature and environmental regulations tighten.
Customer requirements increasingly emphasize not only particle size uniformity but also surface chemistry consistency, stability in various pH environments, and compatibility with other formulation components. This trend has prompted manufacturers to develop customized colloidal silica products tailored to specific application requirements, creating premium market segments with higher profit margins.
Current Synthesis Challenges and Technical Limitations
Despite significant advancements in colloidal silica synthesis over the past decades, several persistent challenges continue to impede the consistent production of uniformly distributed silica particles. The Stöber process, while widely adopted, suffers from batch-to-batch variability that compromises reproducibility in industrial settings. This variability stems primarily from sensitivity to minor fluctuations in reaction parameters such as temperature, pH, and reagent purity, which can dramatically alter nucleation and growth kinetics.
Temperature control represents a critical limitation, as even slight deviations (±1°C) during synthesis can lead to heterogeneous nucleation rates, resulting in polydisperse particle populations. Similarly, pH stability throughout the reaction remains difficult to maintain, particularly in scaled-up processes where localized concentration gradients become more pronounced, leading to regions with varying reaction rates within the same batch.
Reagent purity and concentration consistency pose additional challenges, especially when transitioning from laboratory to industrial scale. Commercial-grade precursors often contain impurities that act as heterogeneous nucleation sites, disrupting the controlled formation of uniform particles. The hydrolysis rate of silicon alkoxides (typically TEOS) is notoriously difficult to control precisely, leading to competing nucleation and growth processes that broaden particle size distribution.
Mixing efficiency presents a significant technical limitation, particularly in larger reaction vessels. Inadequate mixing creates concentration gradients that result in different reaction environments throughout the solution, yielding particles with varying sizes and morphologies. Current reactor designs struggle to achieve the homogeneous conditions necessary for uniform particle formation while maintaining scalability.
Aging and ripening processes following initial synthesis introduce further complications. Ostwald ripening, where smaller particles dissolve and redeposit onto larger ones, can significantly alter the final particle size distribution if not carefully controlled. Current methodologies lack precise control mechanisms to manage this phenomenon, especially during scale-up operations.
Surface modification and stabilization of colloidal silica present additional challenges. Achieving consistent surface chemistry across all particles is difficult, leading to variations in colloidal stability and aggregation behavior. Existing stabilization techniques often compromise the narrow size distribution initially achieved during synthesis.
Characterization limitations further complicate optimization efforts. Real-time monitoring of particle formation and growth remains technically challenging, forcing researchers to rely on post-synthesis analysis that provides limited insight into the dynamic processes occurring during synthesis. This knowledge gap hinders the development of truly predictive models for optimizing synthesis parameters.
Temperature control represents a critical limitation, as even slight deviations (±1°C) during synthesis can lead to heterogeneous nucleation rates, resulting in polydisperse particle populations. Similarly, pH stability throughout the reaction remains difficult to maintain, particularly in scaled-up processes where localized concentration gradients become more pronounced, leading to regions with varying reaction rates within the same batch.
Reagent purity and concentration consistency pose additional challenges, especially when transitioning from laboratory to industrial scale. Commercial-grade precursors often contain impurities that act as heterogeneous nucleation sites, disrupting the controlled formation of uniform particles. The hydrolysis rate of silicon alkoxides (typically TEOS) is notoriously difficult to control precisely, leading to competing nucleation and growth processes that broaden particle size distribution.
Mixing efficiency presents a significant technical limitation, particularly in larger reaction vessels. Inadequate mixing creates concentration gradients that result in different reaction environments throughout the solution, yielding particles with varying sizes and morphologies. Current reactor designs struggle to achieve the homogeneous conditions necessary for uniform particle formation while maintaining scalability.
Aging and ripening processes following initial synthesis introduce further complications. Ostwald ripening, where smaller particles dissolve and redeposit onto larger ones, can significantly alter the final particle size distribution if not carefully controlled. Current methodologies lack precise control mechanisms to manage this phenomenon, especially during scale-up operations.
Surface modification and stabilization of colloidal silica present additional challenges. Achieving consistent surface chemistry across all particles is difficult, leading to variations in colloidal stability and aggregation behavior. Existing stabilization techniques often compromise the narrow size distribution initially achieved during synthesis.
Characterization limitations further complicate optimization efforts. Real-time monitoring of particle formation and growth remains technically challenging, forcing researchers to rely on post-synthesis analysis that provides limited insight into the dynamic processes occurring during synthesis. This knowledge gap hinders the development of truly predictive models for optimizing synthesis parameters.
Established Protocols for Uniform Particle Distribution
01 Methods for achieving uniform particle size distribution in colloidal silica
Various methods can be employed to achieve uniform particle size distribution in colloidal silica. These include controlled synthesis processes, precise pH control during formation, and specialized dispersion techniques. Uniform particle distribution is critical for consistent performance in applications requiring predictable rheological properties and stability. Advanced processing techniques help minimize aggregation and ensure homogeneous dispersion throughout the colloidal system.- Methods for achieving uniform colloidal silica particle distribution: Various methods can be employed to achieve uniform distribution of colloidal silica particles in suspensions or composites. These methods include controlled synthesis parameters, specialized mixing techniques, and surface modification of particles to prevent agglomeration. Uniform distribution is critical for ensuring consistent properties throughout the material and optimizing performance in applications such as coatings, films, and composite materials.
- Stabilization techniques for colloidal silica suspensions: Stabilization techniques are essential for maintaining uniform particle distribution in colloidal silica suspensions. These include pH adjustment, addition of dispersants, electrostatic stabilization, and steric stabilization using polymeric additives. These approaches prevent particle agglomeration by creating repulsive forces between particles, thereby ensuring long-term stability and uniform distribution of the colloidal silica particles in various applications.
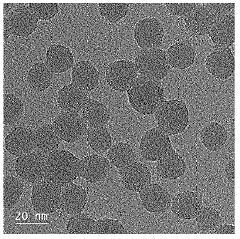
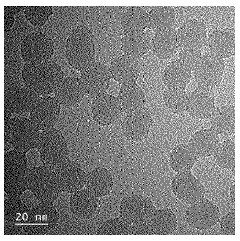
- Characterization and measurement of particle distribution uniformity: Various analytical techniques are used to characterize and measure the uniformity of colloidal silica particle distribution. These include dynamic light scattering, electron microscopy, laser diffraction, and image analysis methods. These techniques provide quantitative data on particle size distribution, spatial arrangement, and degree of agglomeration, which are crucial for quality control and optimization of colloidal silica-based products.
- Surface modification of colloidal silica for improved distribution: Surface modification of colloidal silica particles can significantly improve their distribution uniformity in various matrices. Modification techniques include silanization, polymer grafting, and functionalization with organic groups. These treatments alter the surface properties of the particles, enhancing their compatibility with the surrounding medium and preventing agglomeration, resulting in more uniform particle distribution in composites, coatings, and other applications.
- Applications requiring uniform colloidal silica distribution: Uniform distribution of colloidal silica particles is critical in various applications including optical coatings, semiconductor polishing, paper manufacturing, and reinforcement of polymers and elastomers. The even distribution of particles ensures consistent performance properties such as transparency, mechanical strength, and surface characteristics. Advanced manufacturing processes have been developed to maintain this uniformity across different application domains.
02 Surface modification techniques for colloidal silica uniformity
Surface modification of colloidal silica particles can significantly improve distribution uniformity. By applying functional groups or coatings to silica particles, surface interactions can be controlled to prevent agglomeration. These modifications alter the electrostatic or steric properties of the particles, enhancing stability in various media. Modified particles maintain better separation distances from each other, resulting in more uniform dispersions across different application environments.Expand Specific Solutions03 Stabilization mechanisms for colloidal silica suspensions
Various stabilization mechanisms can be employed to maintain uniform distribution of colloidal silica particles in suspension. These include electrostatic stabilization through surface charge manipulation, steric stabilization using polymeric additives, and electrosteric stabilization combining both approaches. Properly stabilized colloidal silica systems resist flocculation and sedimentation, maintaining their uniform particle distribution during storage and application, which is crucial for consistent performance in industrial processes.Expand Specific Solutions04 Characterization and measurement of colloidal silica uniformity
Advanced analytical techniques are essential for characterizing and measuring the uniformity of colloidal silica particle distributions. Methods such as dynamic light scattering, electron microscopy, and particle size analyzers provide quantitative data on size distribution and dispersion quality. These measurements help in quality control and optimization of manufacturing processes to achieve desired uniformity specifications. Regular monitoring ensures consistency across production batches and helps identify factors affecting particle distribution.Expand Specific Solutions05 Applications requiring precise colloidal silica distribution control
Numerous applications demand precise control over colloidal silica particle distribution uniformity. In semiconductor processing, uniform silica particles are crucial for chemical-mechanical planarization. In coatings and films, even distribution ensures consistent optical and mechanical properties. Other applications include catalysts, where uniform distribution affects reaction efficiency, and composite materials, where particle dispersion influences structural integrity. The specific requirements for uniformity vary by application, driving specialized formulation approaches.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The colloidal silica synthesis market is currently in a growth phase, with increasing demand across electronics, catalysts, and chemical applications driving market expansion. The global market size is estimated to exceed $1.5 billion, growing at approximately 6-8% annually. Technologically, the field shows varying maturity levels, with established players like Evonik, W.R. Grace, and Wacker Chemie offering standardized solutions, while companies such as Fuso Chemical and Nissan Chemical lead in developing advanced synthesis methods for uniform particle distribution. Japanese firms (AGC, Nippon Shokubai) demonstrate particular strength in high-precision applications, while emerging players from China (Suzhou Nanodispersions, Jiangsu Xinsheng) are rapidly advancing their capabilities. The competitive landscape reveals a bifurcation between specialty manufacturers focusing on high-performance applications and larger chemical conglomerates leveraging economies of scale.
Fuso Chemical Co., Ltd.
Technical Solution: Fuso Chemical has developed the "Controlled Ion Exchange" method for synthesizing colloidal silica with uniform particle distribution. Their approach begins with sodium silicate as a precursor and employs a proprietary ion-exchange process to remove sodium ions while precisely controlling the polymerization of silicic acid. The company utilizes specialized ion-exchange resins with tailored pore structures that regulate the release rate of silicic acid monomers, ensuring consistent supersaturation levels throughout the reaction. Fuso's technology incorporates a multi-stage growth process where seed particles are systematically enlarged through controlled addition of silicic acid, maintaining narrow size distribution. Their process includes real-time monitoring of particle growth using dynamic light scattering and automated feedback systems that adjust reaction parameters to maintain optimal growth conditions. The company has also developed specialized stabilization techniques using both inorganic and organic surface modifiers that prevent agglomeration through electrostatic repulsion and steric hindrance. Fuso's method produces spherical silica particles with size ranges from 10-100 nm and coefficient of variation below 15%.
Strengths: Cost-effective process using inexpensive sodium silicate as starting material, environmentally friendly water-based synthesis, and excellent control over particle surface charge. Weaknesses: More limited size range compared to some competitors, challenges in removing all sodium ions which can affect performance in certain applications, and somewhat broader size distribution compared to alkoxide-based methods.
Evonik Operations GmbH
Technical Solution: Evonik has developed a proprietary controlled hydrolysis process for colloidal silica synthesis that utilizes precise pH control and temperature regulation to achieve uniform particle distribution. Their AEROSIL® technology employs flame hydrolysis of silicon tetrachloride in a hydrogen-oxygen flame, allowing for precise control of particle size distribution within the 5-50 nm range. The company has further refined this process by implementing in-line monitoring systems that use dynamic light scattering to provide real-time feedback on particle size distribution during synthesis. This enables automated adjustments to reaction parameters, maintaining consistency across production batches. Evonik has also pioneered surface modification techniques that prevent agglomeration through steric stabilization, using proprietary silane coupling agents that create a uniform shell around each silica particle, maintaining dispersion stability even at high solid content (up to 40% w/w).
Strengths: Exceptional control over particle size distribution with standard deviation <5%, scalable production capabilities from lab to industrial scale, and advanced surface modification expertise. Weaknesses: Higher production costs compared to traditional methods, requires specialized equipment for flame hydrolysis, and process is energy-intensive with associated environmental considerations.
Key Patents and Innovations in Synthesis Control
Method for controling uniformity of colloidal silica particle size
PatentInactiveUS6906109B2
Innovation
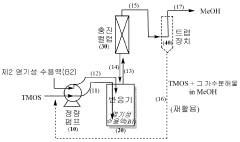
- A system and method involving preformed colloidal silica particles, alkali metal silicate, and cation exchange resin, where the feed rate of silica is controlled to be below the nucleation rate, allowing for uniform particle growth and low sodium content, using potassium silicate with low sodium concentrations and ultrafiltration to maintain purity.
Method for preparing high-concentration colloidal silica
PatentWO2023229271A1
Innovation
- A method involving direct synthesis of high-concentration colloidal silica by reacting tetraalkyl orthosilicate precursors with a basic aqueous solution at controlled pH, eliminating the need for distillation concentration and excess water, thereby reducing reaction time and space while maintaining particle monodispersity.
Scale-up Considerations for Industrial Production
Scaling up colloidal silica synthesis from laboratory to industrial production presents significant engineering challenges that must be addressed to maintain uniform particle distribution. The transition requires careful consideration of reactor design, as industrial-scale vessels introduce different mixing dynamics compared to laboratory equipment. Larger reactors often create zones with varying temperature and concentration gradients, which can lead to inconsistent nucleation and growth rates across the batch, ultimately affecting particle uniformity.
Temperature control becomes increasingly critical at industrial scale. While laboratory syntheses can maintain precise temperature conditions in small volumes, industrial reactors require sophisticated heating and cooling systems to ensure temperature homogeneity throughout the reaction medium. Implementation of advanced temperature monitoring networks and responsive control systems is essential to prevent localized overheating or cooling that would compromise particle uniformity.
Mixing efficiency represents another fundamental consideration in scale-up operations. Industrial production typically employs mechanical agitation systems that must be carefully designed to provide consistent shear rates throughout the reaction volume. Computational fluid dynamics modeling can optimize impeller design and placement to minimize dead zones and ensure uniform distribution of reactants, particularly during the critical nucleation phase of silica particle formation.
Process automation and monitoring capabilities become indispensable at industrial scale. Real-time monitoring of key parameters such as pH, temperature, and reactant concentrations allows for dynamic adjustments during synthesis. Advanced process analytical technology (PAT) tools, including in-line particle size analyzers and spectroscopic methods, enable continuous quality verification without disrupting production, facilitating immediate corrective actions when deviations occur.
Economic considerations must balance quality requirements with production efficiency. Batch-to-batch consistency demands rigorous standardization of raw materials, which may necessitate more stringent supplier qualifications and incoming material testing protocols. Additionally, waste management strategies must be developed to handle larger volumes of byproducts and process waters, potentially incorporating recovery systems for valuable reagents to improve cost-effectiveness while maintaining environmental compliance.
Finally, quality control protocols must be adapted for industrial production volumes. Statistical sampling methods become necessary, requiring careful design to ensure representative assessment of particle uniformity across production batches. Implementation of quality-by-design principles during scale-up can identify critical process parameters that most significantly impact particle distribution, allowing for focused control strategies that maintain product quality while maximizing production efficiency.
Temperature control becomes increasingly critical at industrial scale. While laboratory syntheses can maintain precise temperature conditions in small volumes, industrial reactors require sophisticated heating and cooling systems to ensure temperature homogeneity throughout the reaction medium. Implementation of advanced temperature monitoring networks and responsive control systems is essential to prevent localized overheating or cooling that would compromise particle uniformity.
Mixing efficiency represents another fundamental consideration in scale-up operations. Industrial production typically employs mechanical agitation systems that must be carefully designed to provide consistent shear rates throughout the reaction volume. Computational fluid dynamics modeling can optimize impeller design and placement to minimize dead zones and ensure uniform distribution of reactants, particularly during the critical nucleation phase of silica particle formation.
Process automation and monitoring capabilities become indispensable at industrial scale. Real-time monitoring of key parameters such as pH, temperature, and reactant concentrations allows for dynamic adjustments during synthesis. Advanced process analytical technology (PAT) tools, including in-line particle size analyzers and spectroscopic methods, enable continuous quality verification without disrupting production, facilitating immediate corrective actions when deviations occur.
Economic considerations must balance quality requirements with production efficiency. Batch-to-batch consistency demands rigorous standardization of raw materials, which may necessitate more stringent supplier qualifications and incoming material testing protocols. Additionally, waste management strategies must be developed to handle larger volumes of byproducts and process waters, potentially incorporating recovery systems for valuable reagents to improve cost-effectiveness while maintaining environmental compliance.
Finally, quality control protocols must be adapted for industrial production volumes. Statistical sampling methods become necessary, requiring careful design to ensure representative assessment of particle uniformity across production batches. Implementation of quality-by-design principles during scale-up can identify critical process parameters that most significantly impact particle distribution, allowing for focused control strategies that maintain product quality while maximizing production efficiency.
Environmental Impact and Sustainability Factors
The synthesis of colloidal silica inherently involves various environmental considerations that must be addressed to ensure sustainable production practices. Traditional synthesis methods often utilize hazardous chemicals such as tetraethyl orthosilicate (TEOS) and ammonia, which can contribute to air pollution and pose health risks to workers. The disposal of waste products from these processes, particularly acidic or basic solutions used in pH control, may lead to soil and water contamination if not properly managed.
Energy consumption represents another significant environmental concern in colloidal silica production. Conventional synthesis methods typically require elevated temperatures and extended reaction times, resulting in substantial energy expenditure and associated carbon emissions. This energy-intensive nature contradicts modern sustainability goals and increases the carbon footprint of the final product.
Water usage in colloidal silica synthesis presents additional sustainability challenges. The process demands large volumes of ultrapure water for both synthesis and purification stages. In regions facing water scarcity, this intensive consumption pattern raises important questions about resource allocation and conservation priorities.
Recent advancements in green chemistry approaches offer promising alternatives for more sustainable colloidal silica production. Bio-based precursors derived from agricultural waste can replace traditional silicon sources, significantly reducing reliance on petrochemical feedstocks. Additionally, room-temperature synthesis methods utilizing milder reagents have demonstrated potential for producing uniform silica particles while minimizing energy requirements and hazardous waste generation.
Lifecycle assessment studies indicate that optimizing reaction parameters not only improves particle uniformity but can simultaneously reduce environmental impact. For instance, precise control of reaction kinetics through careful temperature management and reagent addition rates can minimize byproduct formation while achieving desired particle size distributions, thereby reducing waste and resource consumption.
Circular economy principles are increasingly being applied to colloidal silica production. Recovery and recycling of solvents and unreacted precursors can substantially decrease waste generation and raw material consumption. Furthermore, the development of closed-loop systems for water recycling within the production process represents a significant opportunity for enhancing sustainability in water-intensive operations.
Regulatory frameworks worldwide are evolving to address environmental concerns in nanomaterial production. Manufacturers optimizing colloidal silica synthesis must consider these emerging regulations, which increasingly emphasize reduced environmental footprint, worker safety, and responsible end-of-life management for nanomaterials.
Energy consumption represents another significant environmental concern in colloidal silica production. Conventional synthesis methods typically require elevated temperatures and extended reaction times, resulting in substantial energy expenditure and associated carbon emissions. This energy-intensive nature contradicts modern sustainability goals and increases the carbon footprint of the final product.
Water usage in colloidal silica synthesis presents additional sustainability challenges. The process demands large volumes of ultrapure water for both synthesis and purification stages. In regions facing water scarcity, this intensive consumption pattern raises important questions about resource allocation and conservation priorities.
Recent advancements in green chemistry approaches offer promising alternatives for more sustainable colloidal silica production. Bio-based precursors derived from agricultural waste can replace traditional silicon sources, significantly reducing reliance on petrochemical feedstocks. Additionally, room-temperature synthesis methods utilizing milder reagents have demonstrated potential for producing uniform silica particles while minimizing energy requirements and hazardous waste generation.
Lifecycle assessment studies indicate that optimizing reaction parameters not only improves particle uniformity but can simultaneously reduce environmental impact. For instance, precise control of reaction kinetics through careful temperature management and reagent addition rates can minimize byproduct formation while achieving desired particle size distributions, thereby reducing waste and resource consumption.
Circular economy principles are increasingly being applied to colloidal silica production. Recovery and recycling of solvents and unreacted precursors can substantially decrease waste generation and raw material consumption. Furthermore, the development of closed-loop systems for water recycling within the production process represents a significant opportunity for enhancing sustainability in water-intensive operations.
Regulatory frameworks worldwide are evolving to address environmental concerns in nanomaterial production. Manufacturers optimizing colloidal silica synthesis must consider these emerging regulations, which increasingly emphasize reduced environmental footprint, worker safety, and responsible end-of-life management for nanomaterials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!