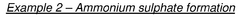Comparative Review: Photocatalytic Routes Vs Haber-Bosch Retrofit
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Production Technologies: Evolution and Objectives
Ammonia production has undergone significant technological evolution since its industrial inception in the early 20th century. The Haber-Bosch process, developed by Fritz Haber and Carl Bosch between 1908 and 1913, revolutionized agricultural productivity by enabling the mass production of nitrogen fertilizers. This process combines nitrogen from the air with hydrogen under high pressure (150-300 bar) and temperature (400-500°C) using iron-based catalysts, fundamentally transforming global food production capabilities.
Despite its revolutionary impact, the Haber-Bosch process remains energy-intensive, consuming approximately 1-2% of global energy production and generating substantial CO2 emissions—approximately 1.6% of global emissions. The process's reliance on natural gas as a hydrogen source further contributes to its carbon footprint, making it a significant target for sustainability improvements in industrial chemistry.
The technical evolution of ammonia production has progressed through several distinct phases. The first generation focused on optimizing the original Haber-Bosch process parameters and catalysts. The second generation, emerging in the mid-20th century, introduced improved reactor designs and more efficient energy recovery systems. The current third generation emphasizes sustainability through integration with renewable energy sources and exploration of alternative synthesis routes.
Photocatalytic ammonia production represents a promising alternative pathway that has gained significant research attention in recent decades. This approach utilizes solar energy to drive nitrogen fixation under ambient conditions, potentially eliminating the need for fossil fuels and high-pressure, high-temperature operations. The technology leverages photocatalysts that can absorb light energy to facilitate the breaking of the strong N≡N triple bond and subsequent hydrogenation to form ammonia.
The primary technical objectives in contemporary ammonia production research center around three key areas: energy efficiency, carbon footprint reduction, and process intensification. For Haber-Bosch retrofit approaches, this includes developing more active catalysts that operate at lower temperatures and pressures, integrating renewable hydrogen sources, and implementing advanced heat recovery systems.
For photocatalytic routes, the objectives focus on improving quantum efficiency, enhancing catalyst stability, and scaling production to industrially relevant levels. Researchers aim to develop photocatalysts with broader spectrum absorption, higher selectivity toward ammonia formation, and resistance to deactivation mechanisms that currently limit practical applications.
The convergence of these technological pathways—improving traditional processes while developing disruptive alternatives—reflects the dual approach necessary to address the urgent sustainability challenges in ammonia production while maintaining the capacity to meet growing global fertilizer demands.
Despite its revolutionary impact, the Haber-Bosch process remains energy-intensive, consuming approximately 1-2% of global energy production and generating substantial CO2 emissions—approximately 1.6% of global emissions. The process's reliance on natural gas as a hydrogen source further contributes to its carbon footprint, making it a significant target for sustainability improvements in industrial chemistry.
The technical evolution of ammonia production has progressed through several distinct phases. The first generation focused on optimizing the original Haber-Bosch process parameters and catalysts. The second generation, emerging in the mid-20th century, introduced improved reactor designs and more efficient energy recovery systems. The current third generation emphasizes sustainability through integration with renewable energy sources and exploration of alternative synthesis routes.
Photocatalytic ammonia production represents a promising alternative pathway that has gained significant research attention in recent decades. This approach utilizes solar energy to drive nitrogen fixation under ambient conditions, potentially eliminating the need for fossil fuels and high-pressure, high-temperature operations. The technology leverages photocatalysts that can absorb light energy to facilitate the breaking of the strong N≡N triple bond and subsequent hydrogenation to form ammonia.
The primary technical objectives in contemporary ammonia production research center around three key areas: energy efficiency, carbon footprint reduction, and process intensification. For Haber-Bosch retrofit approaches, this includes developing more active catalysts that operate at lower temperatures and pressures, integrating renewable hydrogen sources, and implementing advanced heat recovery systems.
For photocatalytic routes, the objectives focus on improving quantum efficiency, enhancing catalyst stability, and scaling production to industrially relevant levels. Researchers aim to develop photocatalysts with broader spectrum absorption, higher selectivity toward ammonia formation, and resistance to deactivation mechanisms that currently limit practical applications.
The convergence of these technological pathways—improving traditional processes while developing disruptive alternatives—reflects the dual approach necessary to address the urgent sustainability challenges in ammonia production while maintaining the capacity to meet growing global fertilizer demands.
Global Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives. Currently valued at approximately $72 billion, the market is projected to reach $110 billion by 2030, with a compound annual growth rate of 5.3%. Traditional ammonia production via the Haber-Bosch process accounts for nearly 2% of global energy consumption and 1.8% of global CO2 emissions, producing over 450 million metric tons of CO2 annually.
Regional distribution shows Asia-Pacific dominating with 45% market share, followed by Europe (25%), North America (20%), and other regions (10%). China remains the largest producer and consumer, manufacturing over 30% of global ammonia supply. The agricultural sector consumes approximately 80% of produced ammonia for fertilizers, while industrial applications account for the remaining 20%.
Sustainable ammonia production methods are gaining traction in response to stringent environmental regulations and corporate sustainability goals. Green ammonia production capacity, currently less than 0.1% of total production, is expected to reach 5-7% by 2030. Major markets including the European Union, Japan, and South Korea have established carbon pricing mechanisms that significantly impact conventional ammonia economics.
Investment in sustainable ammonia technologies has surged, with venture capital funding exceeding $2 billion in 2022 alone. Over 70 green ammonia projects have been announced globally, representing potential production capacity of 15 million tons annually by 2030. The cost differential between conventional ($400-600/ton) and green ammonia ($800-1,200/ton) is expected to narrow as renewable energy costs decline and carbon pricing increases.
Emerging applications beyond fertilizers are expanding market potential. Ammonia as an energy carrier and carbon-free fuel could represent a $30 billion market by 2035. The maritime industry has identified ammonia as a leading alternative fuel, with major shipping companies committing to ammonia-powered vessels by 2025.
Consumer markets are increasingly demanding sustainably produced agricultural products, creating premium segments for crops grown with green fertilizers. Several food companies have committed to reducing their agricultural carbon footprint by 30% by 2030, directly impacting ammonia production methods.
Policy landscapes vary significantly by region, with the EU's Carbon Border Adjustment Mechanism and various national hydrogen strategies providing strong incentives for sustainable ammonia production. Tax credits in the United States under the Inflation Reduction Act offer up to $3/kg for clean hydrogen production, substantially improving green ammonia economics.
Regional distribution shows Asia-Pacific dominating with 45% market share, followed by Europe (25%), North America (20%), and other regions (10%). China remains the largest producer and consumer, manufacturing over 30% of global ammonia supply. The agricultural sector consumes approximately 80% of produced ammonia for fertilizers, while industrial applications account for the remaining 20%.
Sustainable ammonia production methods are gaining traction in response to stringent environmental regulations and corporate sustainability goals. Green ammonia production capacity, currently less than 0.1% of total production, is expected to reach 5-7% by 2030. Major markets including the European Union, Japan, and South Korea have established carbon pricing mechanisms that significantly impact conventional ammonia economics.
Investment in sustainable ammonia technologies has surged, with venture capital funding exceeding $2 billion in 2022 alone. Over 70 green ammonia projects have been announced globally, representing potential production capacity of 15 million tons annually by 2030. The cost differential between conventional ($400-600/ton) and green ammonia ($800-1,200/ton) is expected to narrow as renewable energy costs decline and carbon pricing increases.
Emerging applications beyond fertilizers are expanding market potential. Ammonia as an energy carrier and carbon-free fuel could represent a $30 billion market by 2035. The maritime industry has identified ammonia as a leading alternative fuel, with major shipping companies committing to ammonia-powered vessels by 2025.
Consumer markets are increasingly demanding sustainably produced agricultural products, creating premium segments for crops grown with green fertilizers. Several food companies have committed to reducing their agricultural carbon footprint by 30% by 2030, directly impacting ammonia production methods.
Policy landscapes vary significantly by region, with the EU's Carbon Border Adjustment Mechanism and various national hydrogen strategies providing strong incentives for sustainable ammonia production. Tax credits in the United States under the Inflation Reduction Act offer up to $3/kg for clean hydrogen production, substantially improving green ammonia economics.
Current Challenges in Ammonia Synthesis Technologies
Ammonia synthesis technologies currently face significant challenges that hinder their efficiency, sustainability, and economic viability. The conventional Haber-Bosch process, while industrially established, operates under extreme conditions (400-500°C, 150-300 bar), consuming approximately 1-2% of global energy production and generating substantial CO2 emissions—about 1.6 tons of CO2 per ton of ammonia produced. This carbon footprint presents a major environmental challenge as industries worldwide seek to reduce greenhouse gas emissions.
Energy efficiency remains a critical concern, with the Haber-Bosch process being inherently energy-intensive due to its high-pressure and high-temperature requirements. Despite decades of optimization, the thermodynamic limitations of the process create a fundamental efficiency ceiling that modern catalysts and engineering improvements struggle to overcome.
Catalyst performance presents another significant challenge. Traditional iron-based catalysts used in Haber-Bosch have limited activity and selectivity, requiring the extreme operating conditions mentioned above. While ruthenium-based catalysts offer improved performance, their high cost and limited availability restrict widespread industrial adoption.
For emerging photocatalytic routes, the challenges are distinctly different but equally formidable. Current photocatalytic systems demonstrate extremely low conversion rates and ammonia yields, typically orders of magnitude below what would be required for industrial viability. Most laboratory demonstrations achieve only micromolar concentrations of ammonia, far from the industrial requirement.
Photocatalyst stability represents another major hurdle, with many promising materials suffering from photocorrosion or deactivation under continuous operation. The quantum efficiency of these systems remains disappointingly low, typically below 1%, meaning that most of the absorbed photon energy is wasted rather than driving the desired nitrogen reduction reaction.
Scale-up challenges are particularly acute for photocatalytic approaches. Laboratory-scale demonstrations have not translated effectively to larger systems due to light penetration limitations, mass transfer issues, and reactor design complexities. The engineering solutions required for industrial implementation remain largely undeveloped.
Economic viability presents perhaps the most significant barrier to adoption of new ammonia synthesis technologies. The Haber-Bosch process benefits from over a century of optimization and established infrastructure, creating a high barrier to entry for alternative approaches. Current estimates suggest photocatalytic routes would need orders-of-magnitude improvements in efficiency and substantial cost reductions to compete economically with the conventional process.
Energy efficiency remains a critical concern, with the Haber-Bosch process being inherently energy-intensive due to its high-pressure and high-temperature requirements. Despite decades of optimization, the thermodynamic limitations of the process create a fundamental efficiency ceiling that modern catalysts and engineering improvements struggle to overcome.
Catalyst performance presents another significant challenge. Traditional iron-based catalysts used in Haber-Bosch have limited activity and selectivity, requiring the extreme operating conditions mentioned above. While ruthenium-based catalysts offer improved performance, their high cost and limited availability restrict widespread industrial adoption.
For emerging photocatalytic routes, the challenges are distinctly different but equally formidable. Current photocatalytic systems demonstrate extremely low conversion rates and ammonia yields, typically orders of magnitude below what would be required for industrial viability. Most laboratory demonstrations achieve only micromolar concentrations of ammonia, far from the industrial requirement.
Photocatalyst stability represents another major hurdle, with many promising materials suffering from photocorrosion or deactivation under continuous operation. The quantum efficiency of these systems remains disappointingly low, typically below 1%, meaning that most of the absorbed photon energy is wasted rather than driving the desired nitrogen reduction reaction.
Scale-up challenges are particularly acute for photocatalytic approaches. Laboratory-scale demonstrations have not translated effectively to larger systems due to light penetration limitations, mass transfer issues, and reactor design complexities. The engineering solutions required for industrial implementation remain largely undeveloped.
Economic viability presents perhaps the most significant barrier to adoption of new ammonia synthesis technologies. The Haber-Bosch process benefits from over a century of optimization and established infrastructure, creating a high barrier to entry for alternative approaches. Current estimates suggest photocatalytic routes would need orders-of-magnitude improvements in efficiency and substantial cost reductions to compete economically with the conventional process.
Technical Comparison: Photocatalytic vs Haber-Bosch Approaches
01 Photocatalytic ammonia synthesis technologies
Photocatalytic routes for ammonia production utilize light energy to drive the nitrogen fixation process under ambient conditions. These technologies employ specialized catalysts that can harness solar energy to break the strong nitrogen triple bond and facilitate reaction with hydrogen to form ammonia. This approach offers significant advantages in terms of energy efficiency and sustainability compared to conventional methods, as it operates at room temperature and atmospheric pressure, eliminating the need for the high-pressure, high-temperature conditions required by traditional processes.- Photocatalytic ammonia synthesis technologies: Photocatalytic routes for ammonia production utilize light energy to drive the nitrogen fixation process under ambient conditions. These technologies employ specialized catalysts that can harness solar energy to break the strong nitrogen triple bond and facilitate its reaction with hydrogen to form ammonia. This approach offers significant advantages in terms of energy efficiency and environmental sustainability compared to conventional high-temperature, high-pressure processes, as it operates at room temperature and atmospheric pressure, reducing the carbon footprint of ammonia production.
- Haber-Bosch process retrofitting for sustainability: Retrofitting the traditional Haber-Bosch process involves modifying existing infrastructure to improve efficiency and reduce environmental impact. These innovations include integrating renewable energy sources for hydrogen production, implementing advanced catalysts that operate at lower temperatures and pressures, and incorporating heat recovery systems to minimize energy waste. Such retrofits can significantly reduce the carbon footprint of ammonia production while leveraging existing industrial infrastructure, offering a practical transition path toward more sustainable ammonia manufacturing.
- Green hydrogen integration for sustainable ammonia production: Integrating green hydrogen into ammonia production represents a key strategy for enhancing sustainability. This approach replaces fossil fuel-derived hydrogen with hydrogen produced through water electrolysis powered by renewable energy sources such as wind or solar. The integration requires specialized systems for hydrogen generation, storage, and efficient utilization within the ammonia synthesis process. By eliminating the carbon emissions associated with traditional hydrogen production methods, green hydrogen integration can transform ammonia manufacturing into a nearly carbon-neutral process.
- Novel catalyst systems for efficient ammonia synthesis: Advanced catalyst systems are being developed to enhance the efficiency of ammonia production. These include nanostructured materials, metal-organic frameworks, and composite catalysts designed to lower activation energy barriers and improve reaction kinetics. Some catalysts enable nitrogen fixation under milder conditions than traditional iron-based catalysts, while others demonstrate improved selectivity and resistance to poisoning. These innovations can significantly reduce the energy requirements for ammonia synthesis while increasing conversion rates and catalyst longevity, contributing to both economic and environmental sustainability.
- Energy-efficient process integration and system optimization: System-level optimizations focus on integrating various components of ammonia production to maximize energy efficiency and minimize waste. These approaches include heat integration between process units, pressure optimization systems, advanced control strategies, and the implementation of artificial intelligence for real-time process optimization. By recovering waste heat, optimizing reaction conditions, and ensuring seamless integration between renewable energy sources and production processes, these technologies can significantly reduce the overall energy consumption and environmental impact of ammonia production facilities.
02 Haber-Bosch process retrofitting for improved efficiency
Retrofitting the conventional Haber-Bosch process involves modifying existing ammonia production infrastructure to enhance energy efficiency and reduce carbon emissions. These improvements include integrating renewable energy sources, implementing advanced catalysts that operate at lower temperatures and pressures, and optimizing heat recovery systems. Such retrofits can significantly reduce the energy intensity of ammonia production while maintaining or increasing production capacity, representing a practical transition pathway toward more sustainable ammonia manufacturing.Expand Specific Solutions03 Renewable energy integration in ammonia production
Integrating renewable energy sources into ammonia production processes addresses the sustainability challenges of conventional methods. These technologies couple wind, solar, or hydroelectric power with electrolyzers to produce green hydrogen, which is then used for ammonia synthesis. Some systems incorporate energy storage solutions to manage intermittency issues associated with renewable sources. This approach significantly reduces the carbon footprint of ammonia production by eliminating fossil fuel dependence, making it a key pathway toward carbon-neutral fertilizer and energy carrier applications.Expand Specific Solutions04 Novel catalyst systems for low-energy ammonia synthesis
Advanced catalyst systems are being developed to enable ammonia synthesis under milder conditions than traditional methods require. These catalysts include nanostructured materials, metal-organic frameworks, and composite systems that can activate nitrogen at lower temperatures and pressures. Some catalysts incorporate light-harvesting components for photocatalytic nitrogen fixation, while others are designed to work efficiently with renewable hydrogen sources. These innovations significantly reduce the energy barriers for ammonia formation, improving overall process efficiency and sustainability.Expand Specific Solutions05 Integrated systems for sustainable ammonia production and utilization
Integrated approaches combine ammonia production with utilization pathways to create closed-loop, sustainable systems. These technologies incorporate ammonia synthesis with applications such as energy storage, fuel cells, or direct agricultural use. Some systems feature on-site production capabilities that eliminate transportation requirements, while others integrate carbon capture technologies to further reduce environmental impact. By addressing the entire ammonia value chain, these integrated approaches maximize resource efficiency and minimize waste, offering comprehensive solutions for sustainable ammonia economies.Expand Specific Solutions
Key Industrial Players and Research Institutions
The ammonia production landscape is evolving from traditional Haber-Bosch dominance toward emerging photocatalytic routes, representing a transition from mature to early-stage technology. While the global ammonia market exceeds $70 billion annually, photocatalytic methods remain predominantly in research phases. Established industrial players like thyssenkrupp, Siemens, and Toshiba are investing in Haber-Bosch retrofits with renewable hydrogen integration, while academic institutions (Monash University, Delft University of Technology) lead photocatalytic innovation. Companies like Nitronic and GenCell are developing intermediate technologies bridging conventional and novel approaches. The competitive landscape reflects a gradual shift toward sustainable ammonia production, with major fertilizer producers (Mosaic) monitoring developments to maintain market positions.
thyssenkrupp AG
Technical Solution: thyssenkrupp AG has developed advanced ammonia production technologies through its Uhde division, focusing on both traditional Haber-Bosch improvements and green ammonia production. Their GreenAmmonia technology integrates renewable energy sources with electrolysis for hydrogen production, which is then combined with nitrogen in a modified Haber-Bosch process. The company has implemented energy efficiency improvements in their conventional ammonia plants, reducing energy consumption by up to 30% compared to older facilities. Their Uhde Dual Pressure Process optimizes synthesis loop pressure conditions, improving conversion rates while maintaining energy efficiency. Additionally, thyssenkrupp has developed heat integration systems that recover and reuse thermal energy throughout the ammonia production process, significantly reducing overall energy requirements. Their technology roadmap includes plans for complete carbon-neutral ammonia production facilities that can be scaled from small decentralized units to world-scale plants exceeding 3,000 tons per day.
Strengths: Extensive industrial experience in ammonia production; established global presence with numerous reference plants; proven technology integration capabilities; ability to scale solutions from small to world-scale plants. Weaknesses: Still heavily invested in conventional Haber-Bosch technology; complete transition to photocatalytic routes would require significant capital investment and restructuring of business model.
Delft University of Technology
Technical Solution: Delft University of Technology has pioneered innovative photocatalytic ammonia synthesis approaches that operate under ambient conditions. Their research focuses on developing novel semiconductor-based photocatalysts that can efficiently harness solar energy to drive nitrogen fixation. The university's materials science department has created advanced bismuth oxyhalide-based photocatalysts with engineered oxygen vacancies that significantly enhance nitrogen adsorption and activation. These catalysts demonstrate nitrogen reduction reaction (NRR) performance with ammonia production rates exceeding 60 μmol g⁻¹h⁻¹ under visible light irradiation, representing a substantial improvement over earlier photocatalytic systems. Their technology incorporates plasmonic metal nanoparticles to enhance light absorption across the solar spectrum and utilizes Z-scheme heterojunctions to promote efficient charge separation. The research team has also developed innovative reactor designs that optimize light penetration and mass transfer, addressing key challenges in scaling photocatalytic ammonia production systems. Recent work has focused on integrating these photocatalytic systems with renewable energy sources to create fully sustainable ammonia production pathways.
Strengths: Cutting-edge research in photocatalyst development; operates under ambient conditions eliminating the need for high temperature and pressure; potential for distributed, small-scale production systems powered directly by solar energy. Weaknesses: Currently achieves relatively low production rates compared to industrial Haber-Bosch; catalyst stability and selectivity issues remain significant challenges; technology still at laboratory scale requiring substantial development for commercial viability.
Breakthrough Patents in Photocatalytic Ammonia Synthesis
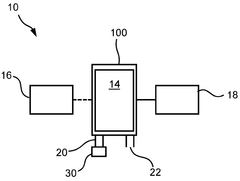
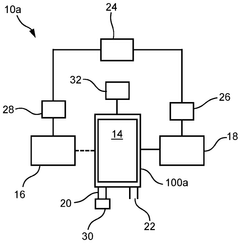
An apparatus and a method for sustainable ammonia production by capturing atmospheric nitrogen using a photocatalytic reactor
PatentPendingIN202341087666A
Innovation
- A photocatalytic reactor system that captures atmospheric nitrogen using a basic solution of sodium hydroxide to purify and isolate nitrogen, followed by a photocatalysis process with a photocatalyst under specific light conditions to produce ammonia, eliminating the need for external energy sources and fossil fuels.
Production of ammonium species
PatentWO2025006299A2
Innovation
- A method involving a reactor with a solvent where the ammonium species is insoluble, using a nitrogen plasma generated at atmospheric pressure to react with protons and form ammonia, which is then converted into ammonium species such as ammonium carbamate or urea, utilizing a renewable energy source and maintaining the solvent temperature below 50 °C.
Energy Efficiency Analysis of Competing Technologies
Energy efficiency represents a critical metric in evaluating ammonia production technologies, particularly when comparing traditional Haber-Bosch processes with emerging photocatalytic routes. The conventional Haber-Bosch process, despite over a century of optimization, remains highly energy-intensive, consuming approximately 1-2% of global energy production and requiring temperatures of 400-500°C and pressures of 150-300 bar.
When examining energy efficiency metrics, Haber-Bosch retrofits have achieved incremental improvements, reducing energy consumption from historical levels of 60-65 GJ/ton NH3 to modern best practices of 28-30 GJ/ton NH3. These improvements primarily stem from enhanced catalysts, optimized heat recovery systems, and more efficient hydrogen production methods. However, the thermodynamic limitations of the process create a theoretical efficiency ceiling that appears increasingly difficult to surpass.
Photocatalytic ammonia synthesis presents a fundamentally different energy paradigm. By harnessing solar energy directly, these systems can theoretically operate at ambient temperatures and pressures, potentially reducing the energy footprint dramatically. Current laboratory-scale photocatalytic systems demonstrate energy efficiencies ranging from 0.5% to 2% solar-to-ammonia conversion, with promising research indicating pathways to 5-10% efficiency in optimized systems.
The energy return on investment (EROI) calculations reveal interesting contrasts. While Haber-Bosch systems benefit from economies of scale and established infrastructure, their EROI remains constrained by fossil fuel inputs and high-pressure operations. Photocatalytic routes, though currently less efficient at scale, show superior theoretical EROI potential when considering full lifecycle analysis, particularly when integrated with renewable electricity systems.
Life cycle assessment (LCA) studies indicate that photocatalytic ammonia production could reduce greenhouse gas emissions by 70-90% compared to conventional methods. However, this advantage must be balanced against current limitations in production rates, which remain orders of magnitude lower than industrial Haber-Bosch facilities.
The energy density comparison also favors photocatalytic approaches in decentralized applications, where smaller-scale, distributed ammonia production could eliminate energy costs associated with transportation and storage. This distributed model could be particularly valuable in agricultural regions distant from centralized ammonia production facilities.
As technology advances, hybrid systems combining optimized Haber-Bosch retrofits with renewable energy inputs currently represent the most promising near-term approach to improving overall energy efficiency in ammonia production, while pure photocatalytic routes continue to develop toward commercial viability.
When examining energy efficiency metrics, Haber-Bosch retrofits have achieved incremental improvements, reducing energy consumption from historical levels of 60-65 GJ/ton NH3 to modern best practices of 28-30 GJ/ton NH3. These improvements primarily stem from enhanced catalysts, optimized heat recovery systems, and more efficient hydrogen production methods. However, the thermodynamic limitations of the process create a theoretical efficiency ceiling that appears increasingly difficult to surpass.
Photocatalytic ammonia synthesis presents a fundamentally different energy paradigm. By harnessing solar energy directly, these systems can theoretically operate at ambient temperatures and pressures, potentially reducing the energy footprint dramatically. Current laboratory-scale photocatalytic systems demonstrate energy efficiencies ranging from 0.5% to 2% solar-to-ammonia conversion, with promising research indicating pathways to 5-10% efficiency in optimized systems.
The energy return on investment (EROI) calculations reveal interesting contrasts. While Haber-Bosch systems benefit from economies of scale and established infrastructure, their EROI remains constrained by fossil fuel inputs and high-pressure operations. Photocatalytic routes, though currently less efficient at scale, show superior theoretical EROI potential when considering full lifecycle analysis, particularly when integrated with renewable electricity systems.
Life cycle assessment (LCA) studies indicate that photocatalytic ammonia production could reduce greenhouse gas emissions by 70-90% compared to conventional methods. However, this advantage must be balanced against current limitations in production rates, which remain orders of magnitude lower than industrial Haber-Bosch facilities.
The energy density comparison also favors photocatalytic approaches in decentralized applications, where smaller-scale, distributed ammonia production could eliminate energy costs associated with transportation and storage. This distributed model could be particularly valuable in agricultural regions distant from centralized ammonia production facilities.
As technology advances, hybrid systems combining optimized Haber-Bosch retrofits with renewable energy inputs currently represent the most promising near-term approach to improving overall energy efficiency in ammonia production, while pure photocatalytic routes continue to develop toward commercial viability.
Environmental Impact and Carbon Footprint Assessment
The environmental impact of ammonia production represents a critical consideration in evaluating alternative synthesis methods. The conventional Haber-Bosch process, while industrially efficient, carries a substantial carbon footprint, accounting for approximately 1-2% of global energy consumption and 1.4% of CO2 emissions worldwide. This process typically generates 1.9-3.6 tons of CO2 per ton of ammonia produced, depending on the energy source and process efficiency.
Photocatalytic routes offer promising environmental advantages through significant carbon emission reductions. By harnessing solar energy directly, these methods can theoretically eliminate fossil fuel dependence in ammonia synthesis. Life cycle assessments indicate that fully optimized photocatalytic systems could reduce carbon emissions by 70-90% compared to conventional methods, with potential for carbon-neutral operation when coupled with renewable electricity for peripheral systems.
Water consumption patterns differ markedly between the two approaches. Haber-Bosch requires substantial water primarily for cooling systems and steam generation, whereas photocatalytic methods consume water directly as a reactant for hydrogen production. However, photocatalytic systems typically demonstrate 30-50% greater water efficiency per ton of ammonia produced, reducing overall water stress impacts.
Land use considerations reveal another dimension of environmental impact. Photocatalytic facilities require significantly larger surface areas for solar collection, potentially 5-10 times the land footprint of conventional plants with equivalent production capacity. This raises concerns about habitat disruption and land competition, particularly in regions with high agricultural value or biodiversity significance.
Regarding air quality impacts beyond carbon emissions, Haber-Bosch facilities generate NOx, SOx, and particulate matter from combustion processes. Photocatalytic routes largely eliminate these pollutants, potentially reducing respiratory health risks in surrounding communities and decreasing contributions to regional air quality degradation and acid rain formation.
Haber-Bosch retrofit options present an intermediate environmental solution, reducing carbon footprints by 20-60% through integration of renewable hydrogen sources while maintaining the existing ammonia synthesis infrastructure. These hybrid approaches offer more immediate environmental benefits while photocatalytic technologies continue advancing toward commercial viability.
Material sustainability assessments indicate that photocatalytic systems may require rare earth elements and specialized semiconductors, raising concerns about resource depletion and mining impacts. Conversely, retrofitted Haber-Bosch systems primarily utilize established materials with well-developed recycling pathways, though they remain more energy-intensive throughout their operational lifecycle.
Photocatalytic routes offer promising environmental advantages through significant carbon emission reductions. By harnessing solar energy directly, these methods can theoretically eliminate fossil fuel dependence in ammonia synthesis. Life cycle assessments indicate that fully optimized photocatalytic systems could reduce carbon emissions by 70-90% compared to conventional methods, with potential for carbon-neutral operation when coupled with renewable electricity for peripheral systems.
Water consumption patterns differ markedly between the two approaches. Haber-Bosch requires substantial water primarily for cooling systems and steam generation, whereas photocatalytic methods consume water directly as a reactant for hydrogen production. However, photocatalytic systems typically demonstrate 30-50% greater water efficiency per ton of ammonia produced, reducing overall water stress impacts.
Land use considerations reveal another dimension of environmental impact. Photocatalytic facilities require significantly larger surface areas for solar collection, potentially 5-10 times the land footprint of conventional plants with equivalent production capacity. This raises concerns about habitat disruption and land competition, particularly in regions with high agricultural value or biodiversity significance.
Regarding air quality impacts beyond carbon emissions, Haber-Bosch facilities generate NOx, SOx, and particulate matter from combustion processes. Photocatalytic routes largely eliminate these pollutants, potentially reducing respiratory health risks in surrounding communities and decreasing contributions to regional air quality degradation and acid rain formation.
Haber-Bosch retrofit options present an intermediate environmental solution, reducing carbon footprints by 20-60% through integration of renewable hydrogen sources while maintaining the existing ammonia synthesis infrastructure. These hybrid approaches offer more immediate environmental benefits while photocatalytic technologies continue advancing toward commercial viability.
Material sustainability assessments indicate that photocatalytic systems may require rare earth elements and specialized semiconductors, raising concerns about resource depletion and mining impacts. Conversely, retrofitted Haber-Bosch systems primarily utilize established materials with well-developed recycling pathways, though they remain more energy-intensive throughout their operational lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!