Photocatalyst Poisoning Resistance In Real-World Air Streams
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalyst Poisoning Mechanisms and Research Objectives
Photocatalyst poisoning represents a significant challenge in the application of photocatalytic technology for air purification systems. This phenomenon occurs when certain compounds present in real-world air streams interact with the catalyst surface, leading to deactivation or reduced efficiency of the photocatalyst. Understanding these poisoning mechanisms is crucial for developing more resilient photocatalytic materials and systems.
The primary poisoning mechanisms in photocatalytic systems include competitive adsorption, where pollutants compete for active sites on the catalyst surface; chemical poisoning, involving the formation of stable complexes between catalyst and contaminants; and physical blockage, where particulate matter physically covers active sites. Silicon-containing compounds, sulfur compounds, and volatile organic compounds (VOCs) are among the most common poisoning agents in ambient air conditions.
Historical development of photocatalyst research has evolved from basic TiO2-based systems in controlled laboratory environments to more complex composite materials designed specifically to address poisoning issues. The trajectory shows increasing focus on understanding real-world performance limitations rather than idealized laboratory conditions, marking a significant shift in research priorities over the past decade.
Current research indicates that catalyst poisoning in real-world applications can reduce efficiency by 30-70% compared to laboratory performance, depending on air stream composition and environmental conditions. This substantial performance gap highlights the critical importance of addressing poisoning resistance in practical applications.
The primary research objectives in this field include developing comprehensive models of poisoning mechanisms under various environmental conditions, creating standardized testing protocols that accurately simulate real-world conditions, and engineering novel catalyst formulations with enhanced poisoning resistance properties. Additionally, there is growing interest in regeneration techniques that can restore catalyst activity after poisoning events.
Recent technological breakthroughs have focused on surface modification strategies, including the incorporation of protective layers, development of self-cleaning mechanisms, and creation of sacrificial components that preferentially attract poisoning agents away from active catalytic sites. These innovations represent promising directions for enhancing long-term catalyst stability.
The ultimate goal of this research direction is to develop photocatalysts that maintain at least 80% of their initial activity after 5,000 hours of operation in diverse real-world environments, including high humidity conditions, environments with variable VOC concentrations, and areas with significant levels of airborne particulates and potential poisoning agents.
The primary poisoning mechanisms in photocatalytic systems include competitive adsorption, where pollutants compete for active sites on the catalyst surface; chemical poisoning, involving the formation of stable complexes between catalyst and contaminants; and physical blockage, where particulate matter physically covers active sites. Silicon-containing compounds, sulfur compounds, and volatile organic compounds (VOCs) are among the most common poisoning agents in ambient air conditions.
Historical development of photocatalyst research has evolved from basic TiO2-based systems in controlled laboratory environments to more complex composite materials designed specifically to address poisoning issues. The trajectory shows increasing focus on understanding real-world performance limitations rather than idealized laboratory conditions, marking a significant shift in research priorities over the past decade.
Current research indicates that catalyst poisoning in real-world applications can reduce efficiency by 30-70% compared to laboratory performance, depending on air stream composition and environmental conditions. This substantial performance gap highlights the critical importance of addressing poisoning resistance in practical applications.
The primary research objectives in this field include developing comprehensive models of poisoning mechanisms under various environmental conditions, creating standardized testing protocols that accurately simulate real-world conditions, and engineering novel catalyst formulations with enhanced poisoning resistance properties. Additionally, there is growing interest in regeneration techniques that can restore catalyst activity after poisoning events.
Recent technological breakthroughs have focused on surface modification strategies, including the incorporation of protective layers, development of self-cleaning mechanisms, and creation of sacrificial components that preferentially attract poisoning agents away from active catalytic sites. These innovations represent promising directions for enhancing long-term catalyst stability.
The ultimate goal of this research direction is to develop photocatalysts that maintain at least 80% of their initial activity after 5,000 hours of operation in diverse real-world environments, including high humidity conditions, environments with variable VOC concentrations, and areas with significant levels of airborne particulates and potential poisoning agents.
Market Analysis for Air Purification Technologies
The global air purification market has experienced significant growth in recent years, driven by increasing awareness of indoor air quality and its impact on health. As of 2023, the market was valued at approximately $13.6 billion and is projected to reach $22.8 billion by 2028, representing a compound annual growth rate (CAGR) of 10.9%. This growth trajectory is particularly relevant for photocatalytic air purification technologies, which represent an innovative segment within the broader market.
The demand for advanced air purification solutions with photocatalyst technology is being fueled by several key factors. Rising pollution levels in urban environments, growing health consciousness among consumers, and stringent air quality regulations across various regions are primary drivers. Additionally, the COVID-19 pandemic has substantially accelerated market growth, heightening awareness about airborne pathogens and creating unprecedented demand for effective air purification systems.
Residential applications currently dominate the market share for photocatalytic air purifiers, accounting for approximately 45% of total sales. However, commercial and industrial sectors are showing the fastest growth rates, with healthcare facilities, educational institutions, and manufacturing plants increasingly adopting these technologies to ensure safer environments.
Geographically, North America and Europe lead in market value, collectively representing about 60% of global sales. However, the Asia-Pacific region is emerging as the fastest-growing market, with China, Japan, and South Korea at the forefront of both consumption and innovation in photocatalytic technologies. This regional growth is attributed to severe air pollution challenges, rapid industrialization, and increasing disposable income among consumers.
A critical market trend is the growing demand for photocatalysts with enhanced poisoning resistance in real-world conditions. Traditional photocatalysts often suffer from performance degradation when exposed to complex air streams containing multiple pollutants, particularly VOCs and particulate matter that can deactivate catalytic surfaces. This has created a significant market opportunity for advanced materials that maintain efficacy over extended periods without frequent replacement or regeneration.
Consumer preferences are increasingly shifting toward multifunctional air purification systems that combine photocatalytic technology with other filtration methods. Products offering comprehensive solutions for various pollutants, including particulates, VOCs, bacteria, and viruses, command premium pricing and are gaining market share. The average consumer willingness to pay for high-performance photocatalytic air purifiers has increased by approximately 30% since 2020, reflecting growing recognition of their value proposition.
The demand for advanced air purification solutions with photocatalyst technology is being fueled by several key factors. Rising pollution levels in urban environments, growing health consciousness among consumers, and stringent air quality regulations across various regions are primary drivers. Additionally, the COVID-19 pandemic has substantially accelerated market growth, heightening awareness about airborne pathogens and creating unprecedented demand for effective air purification systems.
Residential applications currently dominate the market share for photocatalytic air purifiers, accounting for approximately 45% of total sales. However, commercial and industrial sectors are showing the fastest growth rates, with healthcare facilities, educational institutions, and manufacturing plants increasingly adopting these technologies to ensure safer environments.
Geographically, North America and Europe lead in market value, collectively representing about 60% of global sales. However, the Asia-Pacific region is emerging as the fastest-growing market, with China, Japan, and South Korea at the forefront of both consumption and innovation in photocatalytic technologies. This regional growth is attributed to severe air pollution challenges, rapid industrialization, and increasing disposable income among consumers.
A critical market trend is the growing demand for photocatalysts with enhanced poisoning resistance in real-world conditions. Traditional photocatalysts often suffer from performance degradation when exposed to complex air streams containing multiple pollutants, particularly VOCs and particulate matter that can deactivate catalytic surfaces. This has created a significant market opportunity for advanced materials that maintain efficacy over extended periods without frequent replacement or regeneration.
Consumer preferences are increasingly shifting toward multifunctional air purification systems that combine photocatalytic technology with other filtration methods. Products offering comprehensive solutions for various pollutants, including particulates, VOCs, bacteria, and viruses, command premium pricing and are gaining market share. The average consumer willingness to pay for high-performance photocatalytic air purifiers has increased by approximately 30% since 2020, reflecting growing recognition of their value proposition.
Current Challenges in Photocatalyst Durability
Despite significant advancements in photocatalytic technology for air purification, photocatalyst durability remains a critical challenge in real-world applications. The primary issue facing photocatalysts in actual air streams is poisoning, which occurs when certain compounds irreversibly bind to active sites, progressively reducing catalytic efficiency. This phenomenon is particularly problematic in indoor environments where complex mixtures of volatile organic compounds (VOCs), siloxanes, and other contaminants are present at varying concentrations.
Catalyst deactivation mechanisms in real-world conditions are multifaceted. Chemical poisoning occurs when sulfur compounds, phosphorus derivatives, and heavy metals form strong bonds with catalyst surfaces, permanently blocking active sites. Physical deactivation results from particulate matter accumulation, creating diffusion barriers that prevent pollutants from reaching catalytic surfaces. Additionally, competitive adsorption between different airborne compounds can significantly reduce the efficiency of target pollutant degradation.
Temperature and humidity fluctuations in real environments further complicate photocatalyst performance. Most photocatalysts demonstrate optimal activity within narrow humidity ranges, with performance declining significantly at extreme humidity levels. This variability presents substantial challenges for maintaining consistent purification efficiency across diverse geographical locations and seasonal conditions.
Current commercial photocatalysts, primarily TiO2-based, exhibit limited resistance to poisoning in complex air streams. Research indicates that after 3-6 months of continuous operation in typical indoor environments, photocatalytic efficiency can decrease by 30-50%, necessitating replacement or regeneration. This degradation rate accelerates in industrial settings where higher concentrations of potential catalyst poisons are present.
Regeneration techniques for poisoned photocatalysts remain limited and often energy-intensive. Thermal regeneration requires heating to temperatures exceeding 300°C, which is impractical for most commercial air purification systems. Chemical washing procedures using acids or bases can partially restore activity but may compromise the structural integrity of the catalyst support materials.
The economic implications of photocatalyst poisoning are substantial. The frequent replacement of catalytic materials increases operational costs, while inconsistent purification efficiency compromises air quality standards. For commercial applications, this translates to higher maintenance requirements and reduced consumer confidence in photocatalytic air purification technologies.
Recent research has identified several promising approaches to enhance poisoning resistance, including the development of core-shell structures that physically shield active sites, incorporation of sacrificial materials that preferentially bind to poisons, and the creation of self-regenerating photocatalytic systems. However, these solutions remain largely in laboratory testing phases, with significant challenges in scaling to commercial applications.
Catalyst deactivation mechanisms in real-world conditions are multifaceted. Chemical poisoning occurs when sulfur compounds, phosphorus derivatives, and heavy metals form strong bonds with catalyst surfaces, permanently blocking active sites. Physical deactivation results from particulate matter accumulation, creating diffusion barriers that prevent pollutants from reaching catalytic surfaces. Additionally, competitive adsorption between different airborne compounds can significantly reduce the efficiency of target pollutant degradation.
Temperature and humidity fluctuations in real environments further complicate photocatalyst performance. Most photocatalysts demonstrate optimal activity within narrow humidity ranges, with performance declining significantly at extreme humidity levels. This variability presents substantial challenges for maintaining consistent purification efficiency across diverse geographical locations and seasonal conditions.
Current commercial photocatalysts, primarily TiO2-based, exhibit limited resistance to poisoning in complex air streams. Research indicates that after 3-6 months of continuous operation in typical indoor environments, photocatalytic efficiency can decrease by 30-50%, necessitating replacement or regeneration. This degradation rate accelerates in industrial settings where higher concentrations of potential catalyst poisons are present.
Regeneration techniques for poisoned photocatalysts remain limited and often energy-intensive. Thermal regeneration requires heating to temperatures exceeding 300°C, which is impractical for most commercial air purification systems. Chemical washing procedures using acids or bases can partially restore activity but may compromise the structural integrity of the catalyst support materials.
The economic implications of photocatalyst poisoning are substantial. The frequent replacement of catalytic materials increases operational costs, while inconsistent purification efficiency compromises air quality standards. For commercial applications, this translates to higher maintenance requirements and reduced consumer confidence in photocatalytic air purification technologies.
Recent research has identified several promising approaches to enhance poisoning resistance, including the development of core-shell structures that physically shield active sites, incorporation of sacrificial materials that preferentially bind to poisons, and the creation of self-regenerating photocatalytic systems. However, these solutions remain largely in laboratory testing phases, with significant challenges in scaling to commercial applications.
Existing Anti-Poisoning Strategies
01 Metal doping for enhanced poisoning resistance
Incorporating metal dopants into photocatalyst structures can significantly enhance their resistance to poisoning. Metals such as platinum, palladium, silver, and transition metals can modify the electronic structure of the photocatalyst, creating alternative reaction pathways that bypass poisoning mechanisms. These dopants can also facilitate the desorption of potential poisoning species from active sites, maintaining catalytic activity even in the presence of poisoning agents.- Metal doping for enhanced poisoning resistance: Incorporating metal dopants into photocatalyst structures can significantly enhance their resistance to poisoning. Metals such as platinum, palladium, silver, and transition metals can modify the electronic structure of the photocatalyst, creating alternative reaction pathways that bypass poisoning mechanisms. These dopants can also facilitate the desorption of potential poisoning species from active sites, maintaining catalytic activity over longer periods even in the presence of poisoning agents.
- Core-shell and protective layer structures: Developing core-shell structures or applying protective layers on photocatalysts can physically shield active sites from poisoning agents. These protective layers, often composed of porous materials or selective membranes, allow desired reactants to reach the catalyst while blocking potential poisons. The shell or protective layer can be designed with specific porosity, hydrophobicity, or chemical affinity to selectively filter molecules approaching the catalytic surface, thereby extending the operational lifetime of the photocatalyst in contaminated environments.
- Surface modification techniques: Surface modification of photocatalysts through functionalization with organic groups, polymers, or specific ligands can enhance resistance to poisoning. These modifications can alter the surface charge, hydrophobicity, or steric environment around active sites, preventing the adsorption or binding of poisoning species. Additionally, self-cleaning surface treatments can be applied to photocatalysts, enabling them to periodically shed accumulated poisons through light-activated processes, maintaining catalytic efficiency over extended periods of operation.
- Regeneration and self-healing mechanisms: Incorporating regeneration and self-healing mechanisms into photocatalyst systems can overcome poisoning effects. These mechanisms may include thermal regeneration processes, photoinduced desorption of poisons, or sacrificial reagents that preferentially react with poisoning species. Some advanced photocatalysts are designed with dual-function capabilities that can convert poisoning compounds into harmless or even beneficial substances during operation, effectively maintaining catalytic activity in challenging environments.
- Composite and heterojunction photocatalysts: Developing composite or heterojunction photocatalysts by combining multiple semiconductor materials can enhance poisoning resistance. These structures create synergistic effects that maintain catalytic activity even when certain active sites become poisoned. The heterojunction interfaces facilitate charge separation and provide alternative reaction pathways, reducing the impact of poisoning on overall catalytic performance. Additionally, the diverse surface chemistry of composite catalysts means that if one component becomes poisoned, others can continue to function, ensuring sustained catalytic activity.
02 Core-shell and composite structures for poison resistance
Developing core-shell and composite photocatalyst structures provides effective protection against poisoning. By encapsulating the active photocatalytic material within a protective shell or combining it with supporting materials, these structures create physical barriers that prevent poisoning agents from reaching active sites. Additionally, the synergistic effects between components in composite structures can enhance overall catalytic performance while maintaining resistance to deactivation by poisoning species.Expand Specific Solutions03 Surface modification techniques
Surface modification of photocatalysts through functionalization, coating, or treatment processes can significantly improve poisoning resistance. These techniques alter the surface properties to reduce the adsorption of poisoning species while maintaining or enhancing photocatalytic activity. Methods include applying hydrophobic coatings, creating self-cleaning surfaces, and introducing specific functional groups that repel common poisoning agents while preserving access to active sites for desired reactants.Expand Specific Solutions04 Regeneration and self-cleaning mechanisms
Incorporating regeneration and self-cleaning mechanisms into photocatalyst designs enables sustained performance even after exposure to poisoning agents. These approaches include photoinduced decomposition of adsorbed poisons, thermal regeneration processes, and the integration of secondary catalytic functions that continuously remove potential poisoning species. Such mechanisms allow for the automatic restoration of catalytic activity without requiring external intervention or replacement of the catalyst.Expand Specific Solutions05 Novel material compositions with inherent poison resistance
Development of novel photocatalyst compositions with inherently high resistance to poisoning represents a fundamental approach to addressing this challenge. These materials include mixed metal oxides, non-oxide semiconductors, and hybrid organic-inorganic structures with unique electronic properties that minimize binding with common poisoning species. The carefully engineered electronic structure and surface chemistry of these materials allow them to maintain photocatalytic activity even in environments containing potential catalyst poisons.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The photocatalyst poisoning resistance market in real-world air streams is currently in a growth phase, with increasing demand for durable air purification solutions across healthcare, commercial, and residential sectors. The global market is estimated to reach $12-15 billion by 2025, driven by rising air quality concerns and stringent environmental regulations. Technologically, the field shows varying maturity levels, with companies like Synexis LLC pioneering with their Dry Hydrogen Peroxide technology, while established players such as Carrier Corp., TOTO Ltd., and DuPont are advancing commercial applications. IBM and GlobalFoundries contribute semiconductor expertise crucial for next-generation photocatalysts. Academic-industry collaborations involving MIT, Nanjing University, and AIST are accelerating innovation in poison-resistant formulations, particularly addressing challenges in high-contaminant environments where traditional photocatalysts suffer performance degradation.
TOTO Ltd.
Technical Solution: TOTO has developed advanced photocatalytic technology utilizing titanium dioxide (TiO2) with specific modifications to enhance poisoning resistance in real-world air streams. Their HYDROTECT technology incorporates a specialized coating that combines TiO2 with other metal oxides and dopants to create a more robust photocatalyst surface. The company has engineered their photocatalysts with hierarchical nanostructures that provide multiple active sites, allowing continued functionality even when some sites become poisoned. TOTO's approach includes the development of self-cleaning mechanisms where water (either ambient humidity or periodic washing) helps remove accumulated pollutants and poisons from the catalyst surface, effectively regenerating the photocatalytic activity. Their technology also incorporates visible light activation through nitrogen or carbon doping of TiO2, reducing dependence on UV light and enabling indoor applications where poisoning compounds are often concentrated.
Strengths: Superior durability in real-world conditions with proven long-term performance in various environments including high humidity and pollutant-rich atmospheres. Their self-cleaning mechanism significantly extends catalyst lifetime. Weaknesses: Higher manufacturing costs compared to standard photocatalysts, and performance may still degrade in environments with high concentrations of sulfur compounds or silicone-based contaminants.
Advanced Industrial Science & Technology
Technical Solution: AIST has pioneered composite photocatalyst systems specifically designed to resist poisoning in complex air streams. Their approach involves creating core-shell nanostructures where the active photocatalyst (typically modified TiO2) is protected by a porous outer layer that acts as a selective membrane, allowing target pollutants to reach the catalyst while blocking common poisoning agents. AIST researchers have developed a novel method of incorporating sacrificial materials within the photocatalyst matrix that preferentially bind with poisoning compounds, preserving the main catalytic sites. Their technology includes the integration of secondary catalytic pathways that can be activated when primary pathways become compromised, ensuring continued functionality even in challenging environments. AIST has also implemented advanced surface modification techniques using atomic layer deposition to create precisely engineered surfaces with enhanced resistance to specific poisoning compounds commonly found in industrial and urban air streams.
Strengths: Exceptional selectivity in complex air mixtures and significantly extended operational lifetime in industrial environments with multiple potential poisoning compounds. Their modular design allows customization for specific air quality challenges. Weaknesses: Complex manufacturing process increases production costs, and the technology requires periodic regeneration treatments in extremely contaminated environments.
Key Patents in Photocatalyst Protection Technologies
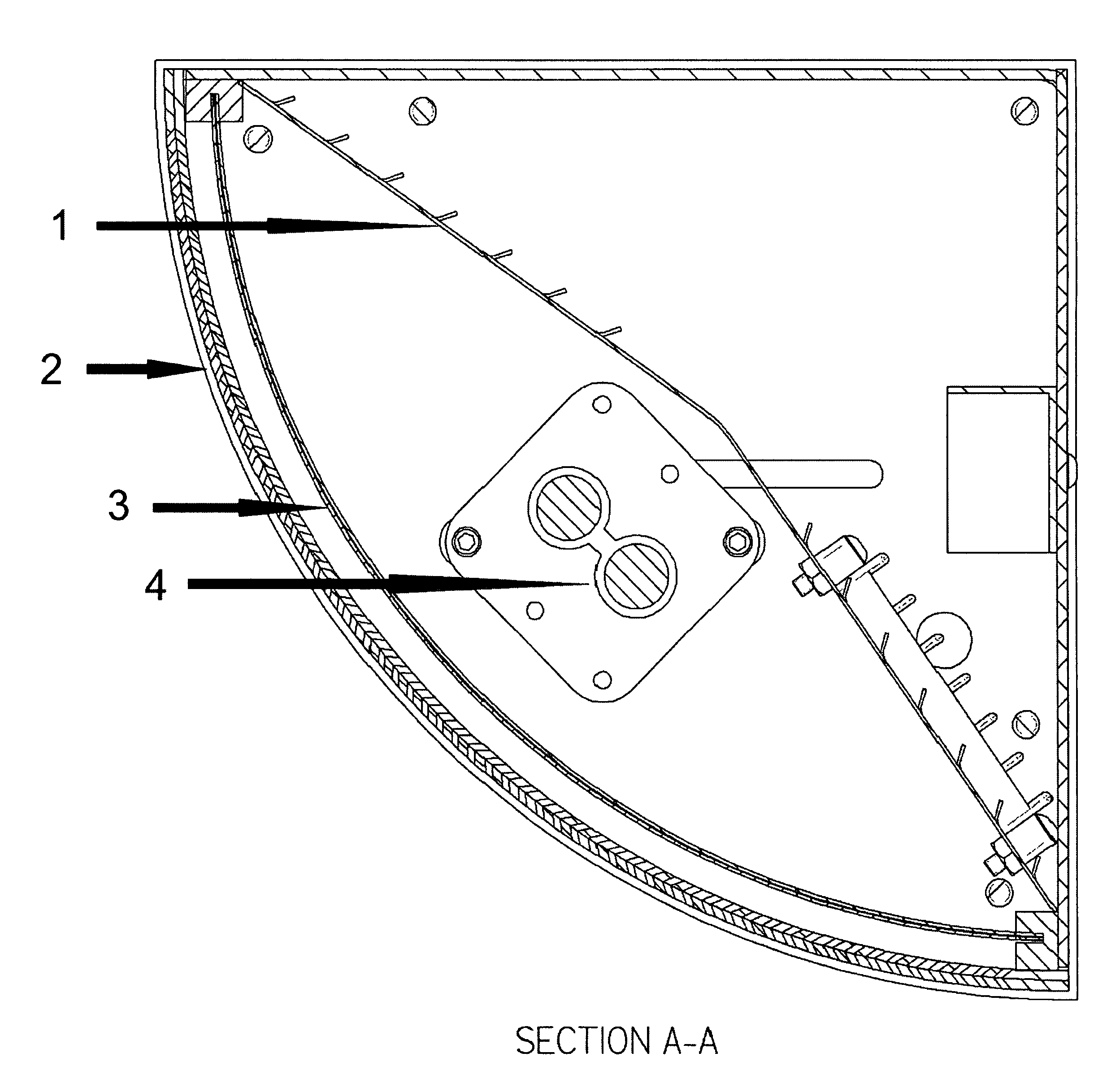
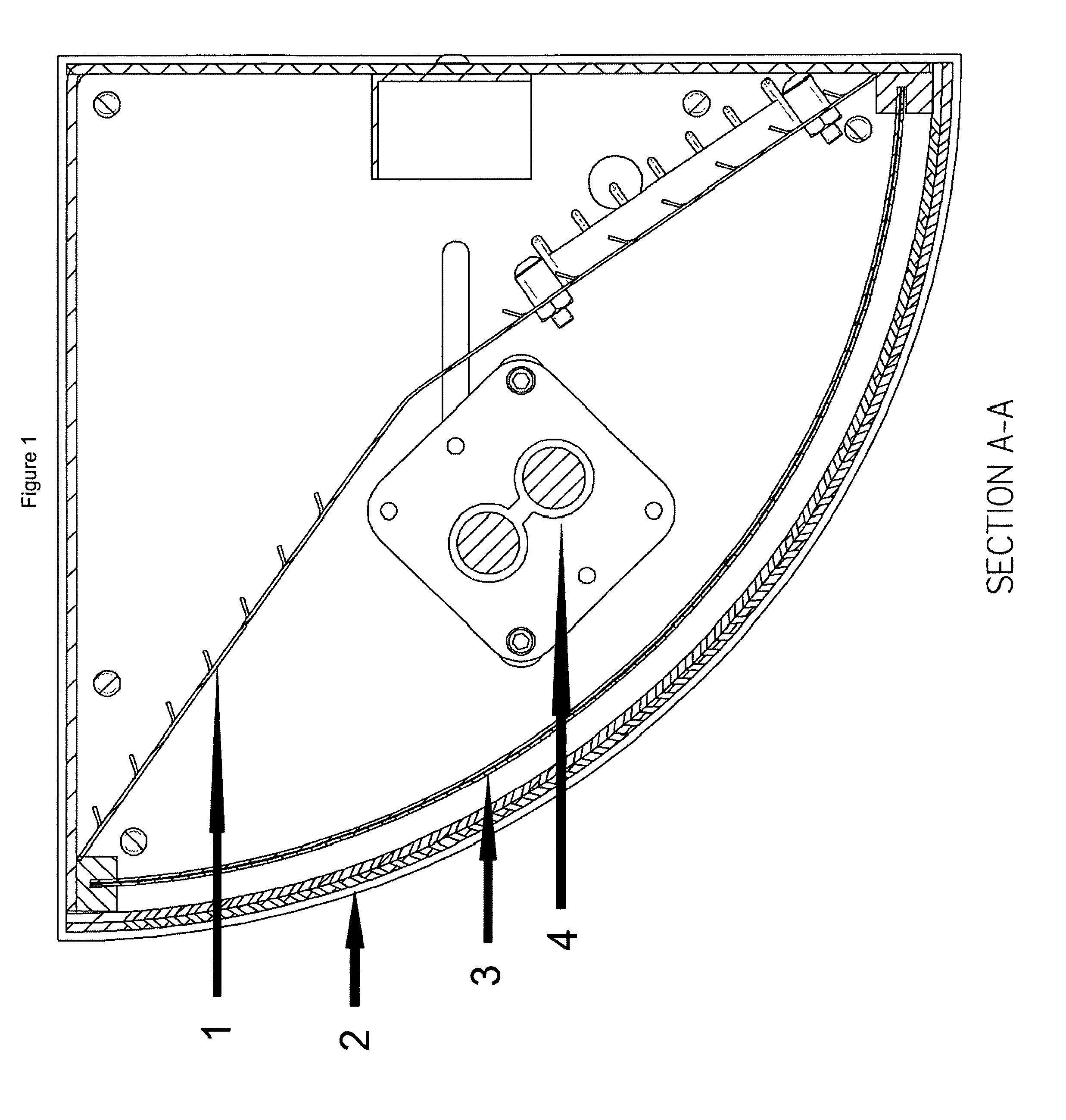
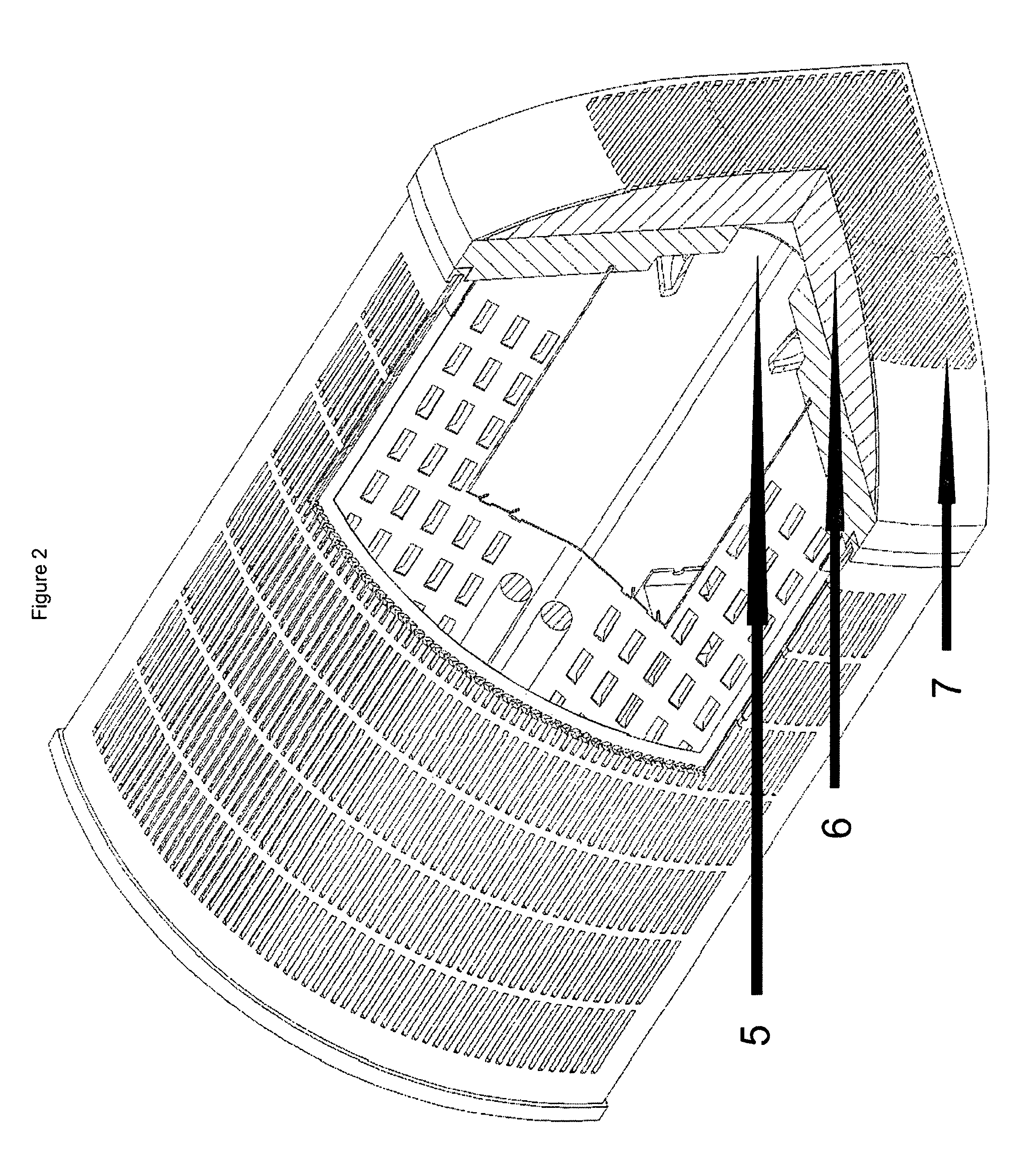
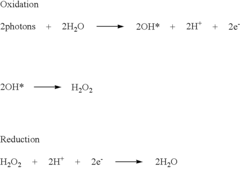
Purified hydrogen peroxide gas generation methods and devices
PatentWO2015171633A1
Innovation
- A device comprising an enclosure, air distribution mechanism, and an air-permeable substrate with a catalyst, exposed to ultraviolet light, which produces non-hydrated PHPG by separating hydroxyl radicals from free electrons, minimizing decomposition, and directing the gas out of the enclosure for environmental release.
Purified hydrogen peroxide gas microbial control methods and devices
PatentActiveUS8168122B2
Innovation
- A method involving a photocatalytic cell that preferentially produces PHPG by exposing a metal or metal oxide catalyst to ultraviolet light in humid air, generating hydrogen peroxide gas that is substantially free of hydration, ozone, plasma species, and organic species, and directing it into the environment for disinfection and remediation.
Environmental Impact Assessment
The environmental impact of photocatalyst systems in air purification extends far beyond their immediate air cleaning capabilities. When assessing photocatalysts with enhanced poisoning resistance for real-world air streams, several critical environmental considerations emerge that affect their sustainability profile and long-term ecological footprint.
Photocatalytic air purification technologies offer significant environmental benefits through their ability to decompose volatile organic compounds (VOCs), nitrogen oxides (NOx), and other harmful pollutants without generating secondary waste streams. Poison-resistant photocatalysts particularly excel in maintaining these benefits over extended operational periods in complex air environments, reducing the frequency of replacement and associated material consumption.
The manufacturing processes for advanced poison-resistant photocatalysts typically involve specialized materials and potentially energy-intensive synthesis methods. Life cycle assessments indicate that while initial production may have higher environmental impacts than conventional catalysts, these are often offset by longer service lifetimes and reduced maintenance requirements. The environmental payback period for these systems typically ranges from 6-18 months depending on application conditions.
Energy consumption represents another crucial environmental consideration. Poison-resistant photocatalysts generally maintain their efficiency longer in challenging conditions, resulting in more consistent energy utilization throughout their operational life. This translates to approximately 15-30% lower lifetime energy consumption compared to conventional catalysts that experience performance degradation when exposed to poisoning agents.
Water usage impacts vary significantly based on catalyst formulation. Some poison-resistant designs incorporate hydrophobic elements that reduce water adsorption and subsequent treatment requirements. However, certain manufacturing processes for specialized catalysts may require substantial water inputs, creating potential trade-offs that must be carefully evaluated in water-stressed regions.
End-of-life management presents both challenges and opportunities. Many poison-resistant photocatalysts contain precious metals or rare earth elements that warrant recovery and recycling. Developing closed-loop systems for these materials is essential to minimize resource depletion and waste generation. Current recycling technologies can recover approximately 70-85% of these valuable components, though optimization efforts continue.
The broader environmental implications extend to reduced pressure on conventional air treatment systems, decreased reliance on chemical interventions, and potential improvements in indoor and outdoor air quality. These cumulative benefits contribute to more sustainable built environments and reduced public health burdens associated with air pollution exposure.
Photocatalytic air purification technologies offer significant environmental benefits through their ability to decompose volatile organic compounds (VOCs), nitrogen oxides (NOx), and other harmful pollutants without generating secondary waste streams. Poison-resistant photocatalysts particularly excel in maintaining these benefits over extended operational periods in complex air environments, reducing the frequency of replacement and associated material consumption.
The manufacturing processes for advanced poison-resistant photocatalysts typically involve specialized materials and potentially energy-intensive synthesis methods. Life cycle assessments indicate that while initial production may have higher environmental impacts than conventional catalysts, these are often offset by longer service lifetimes and reduced maintenance requirements. The environmental payback period for these systems typically ranges from 6-18 months depending on application conditions.
Energy consumption represents another crucial environmental consideration. Poison-resistant photocatalysts generally maintain their efficiency longer in challenging conditions, resulting in more consistent energy utilization throughout their operational life. This translates to approximately 15-30% lower lifetime energy consumption compared to conventional catalysts that experience performance degradation when exposed to poisoning agents.
Water usage impacts vary significantly based on catalyst formulation. Some poison-resistant designs incorporate hydrophobic elements that reduce water adsorption and subsequent treatment requirements. However, certain manufacturing processes for specialized catalysts may require substantial water inputs, creating potential trade-offs that must be carefully evaluated in water-stressed regions.
End-of-life management presents both challenges and opportunities. Many poison-resistant photocatalysts contain precious metals or rare earth elements that warrant recovery and recycling. Developing closed-loop systems for these materials is essential to minimize resource depletion and waste generation. Current recycling technologies can recover approximately 70-85% of these valuable components, though optimization efforts continue.
The broader environmental implications extend to reduced pressure on conventional air treatment systems, decreased reliance on chemical interventions, and potential improvements in indoor and outdoor air quality. These cumulative benefits contribute to more sustainable built environments and reduced public health burdens associated with air pollution exposure.
Standardization and Testing Protocols
The development of standardized testing protocols for photocatalyst poisoning resistance represents a critical challenge in advancing air purification technologies. Currently, the field suffers from fragmented methodologies that impede meaningful comparison between different catalyst systems and their real-world performance. Establishing uniform testing conditions that accurately simulate diverse environmental contaminants is essential for reliable assessment of catalyst longevity and effectiveness.
International standards organizations, including ISO and ASTM, have begun addressing this gap through initiatives like ISO 22197 series, which provides frameworks for evaluating photocatalytic performance. However, these standards require significant expansion to specifically address poisoning resistance in complex air streams containing multiple contaminants at varying concentrations and humidity levels.
A comprehensive standardization approach must incorporate accelerated aging protocols that can reliably predict long-term performance without requiring prohibitively lengthy testing periods. Such protocols should include exposure to common catalyst poisons such as VOCs, sulfur compounds, siloxanes, and particulate matter at concentrations representative of target application environments, from industrial settings to residential indoor air.
Testing methodologies should evaluate performance across multiple cycles of exposure and regeneration, measuring not only initial degradation but recovery potential and cumulative permanent damage. Quantitative metrics must be established for poison resistance, including threshold poison concentrations, performance degradation rates, and recovery efficiency parameters.
Round-robin testing across multiple laboratories has emerged as a valuable approach to validate protocol reliability and reproducibility. Recent collaborative efforts between academic institutions and industry partners have demonstrated that interlaboratory variations can be minimized through detailed procedural specifications and reference materials.
Analytical techniques for characterizing catalyst surfaces before, during, and after poison exposure should be standardized as part of these protocols. Techniques including XPS, FTIR, and chemisorption measurements provide crucial insights into poisoning mechanisms but require consistent application methodologies to yield comparable results across research groups.
Economic considerations must also be integrated into testing frameworks, balancing the need for comprehensive evaluation against practical testing costs. Tiered testing approaches, beginning with screening protocols followed by more intensive evaluation for promising candidates, offer a pragmatic solution to this challenge.
The development of digital repositories for test results would significantly advance the field by enabling meta-analyses across multiple studies and accelerating the identification of structural and compositional features that enhance poison resistance in real-world conditions.
International standards organizations, including ISO and ASTM, have begun addressing this gap through initiatives like ISO 22197 series, which provides frameworks for evaluating photocatalytic performance. However, these standards require significant expansion to specifically address poisoning resistance in complex air streams containing multiple contaminants at varying concentrations and humidity levels.
A comprehensive standardization approach must incorporate accelerated aging protocols that can reliably predict long-term performance without requiring prohibitively lengthy testing periods. Such protocols should include exposure to common catalyst poisons such as VOCs, sulfur compounds, siloxanes, and particulate matter at concentrations representative of target application environments, from industrial settings to residential indoor air.
Testing methodologies should evaluate performance across multiple cycles of exposure and regeneration, measuring not only initial degradation but recovery potential and cumulative permanent damage. Quantitative metrics must be established for poison resistance, including threshold poison concentrations, performance degradation rates, and recovery efficiency parameters.
Round-robin testing across multiple laboratories has emerged as a valuable approach to validate protocol reliability and reproducibility. Recent collaborative efforts between academic institutions and industry partners have demonstrated that interlaboratory variations can be minimized through detailed procedural specifications and reference materials.
Analytical techniques for characterizing catalyst surfaces before, during, and after poison exposure should be standardized as part of these protocols. Techniques including XPS, FTIR, and chemisorption measurements provide crucial insights into poisoning mechanisms but require consistent application methodologies to yield comparable results across research groups.
Economic considerations must also be integrated into testing frameworks, balancing the need for comprehensive evaluation against practical testing costs. Tiered testing approaches, beginning with screening protocols followed by more intensive evaluation for promising candidates, offer a pragmatic solution to this challenge.
The development of digital repositories for test results would significantly advance the field by enabling meta-analyses across multiple studies and accelerating the identification of structural and compositional features that enhance poison resistance in real-world conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!