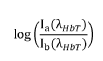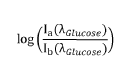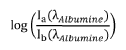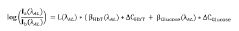Operando Spectroscopies For Tracking N₂ Reduction Intermediates
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
N₂ Reduction Spectroscopy Background and Objectives
Nitrogen reduction to ammonia represents one of the most significant chemical processes in modern industry, with the Haber-Bosch process consuming approximately 1-2% of global energy production. The development of alternative, energy-efficient methods for nitrogen fixation has become increasingly important as we face growing environmental concerns and energy constraints. Operando spectroscopy techniques have emerged as powerful tools for understanding the fundamental mechanisms of N₂ reduction, offering real-time insights into reaction intermediates and pathways.
The evolution of spectroscopic methods for studying N₂ reduction dates back to the 1970s, when researchers first applied infrared spectroscopy to observe surface-bound nitrogen species. Significant advancements occurred in the 1990s with the development of in-situ X-ray absorption spectroscopy (XAS) techniques, allowing scientists to probe catalyst structures under reaction conditions. The past decade has witnessed remarkable progress in operando methods, including advanced vibrational spectroscopies, synchrotron-based techniques, and time-resolved measurements capable of capturing transient intermediates.
Current technological trends point toward multi-modal spectroscopic approaches that combine complementary techniques to provide comprehensive mechanistic understanding. The integration of computational methods with experimental spectroscopy has also accelerated, enabling more accurate interpretation of complex spectral data and prediction of reaction pathways. Additionally, the miniaturization of spectroscopic equipment has facilitated the development of portable systems for real-world applications.
The primary technical objectives for operando spectroscopy in N₂ reduction include identifying and characterizing key reaction intermediates such as N₂H, N₂H₂, and NH species that have been theoretically predicted but remain experimentally elusive. Researchers aim to establish correlations between catalyst structure, electronic properties, and catalytic performance under realistic reaction conditions. Another critical goal involves developing spectroscopic methods with sufficient temporal resolution to capture the kinetics of elementary reaction steps.
Furthermore, the field seeks to bridge the "pressure gap" between ultra-high vacuum studies and practical operating conditions, requiring spectroscopic techniques that function effectively at ambient or elevated pressures. Enhancing spatial resolution to observe site-specific reactivity on heterogeneous catalysts represents another significant challenge, as does the development of standardized protocols for data acquisition and analysis to enable meaningful comparisons across different research groups.
The ultimate objective is to leverage spectroscopic insights to guide rational catalyst design, potentially enabling ambient-condition nitrogen fixation with significantly reduced energy requirements compared to the Haber-Bosch process. This would represent a transformative advancement with profound implications for sustainable ammonia production and related nitrogen-based chemical manufacturing.
The evolution of spectroscopic methods for studying N₂ reduction dates back to the 1970s, when researchers first applied infrared spectroscopy to observe surface-bound nitrogen species. Significant advancements occurred in the 1990s with the development of in-situ X-ray absorption spectroscopy (XAS) techniques, allowing scientists to probe catalyst structures under reaction conditions. The past decade has witnessed remarkable progress in operando methods, including advanced vibrational spectroscopies, synchrotron-based techniques, and time-resolved measurements capable of capturing transient intermediates.
Current technological trends point toward multi-modal spectroscopic approaches that combine complementary techniques to provide comprehensive mechanistic understanding. The integration of computational methods with experimental spectroscopy has also accelerated, enabling more accurate interpretation of complex spectral data and prediction of reaction pathways. Additionally, the miniaturization of spectroscopic equipment has facilitated the development of portable systems for real-world applications.
The primary technical objectives for operando spectroscopy in N₂ reduction include identifying and characterizing key reaction intermediates such as N₂H, N₂H₂, and NH species that have been theoretically predicted but remain experimentally elusive. Researchers aim to establish correlations between catalyst structure, electronic properties, and catalytic performance under realistic reaction conditions. Another critical goal involves developing spectroscopic methods with sufficient temporal resolution to capture the kinetics of elementary reaction steps.
Furthermore, the field seeks to bridge the "pressure gap" between ultra-high vacuum studies and practical operating conditions, requiring spectroscopic techniques that function effectively at ambient or elevated pressures. Enhancing spatial resolution to observe site-specific reactivity on heterogeneous catalysts represents another significant challenge, as does the development of standardized protocols for data acquisition and analysis to enable meaningful comparisons across different research groups.
The ultimate objective is to leverage spectroscopic insights to guide rational catalyst design, potentially enabling ambient-condition nitrogen fixation with significantly reduced energy requirements compared to the Haber-Bosch process. This would represent a transformative advancement with profound implications for sustainable ammonia production and related nitrogen-based chemical manufacturing.
Market Applications for N₂ Reduction Technologies
Nitrogen reduction technologies, particularly those focused on converting atmospheric N₂ into ammonia and other nitrogen compounds, represent a significant market opportunity across multiple sectors. The traditional Haber-Bosch process, while effective, consumes approximately 1-2% of global energy production and generates substantial CO₂ emissions. This creates a compelling market need for alternative N₂ reduction technologies that can operate under milder conditions with reduced environmental impact.
The agricultural sector presents the largest immediate market application, with global ammonia demand for fertilizer production exceeding 150 million tons annually. Electrochemical and photocatalytic N₂ reduction technologies could enable distributed, on-site ammonia production for farmers, reducing transportation costs and emissions associated with centralized production. This decentralized approach is particularly valuable for remote agricultural regions with limited infrastructure.
The pharmaceutical and fine chemicals industries represent another significant market opportunity. Nitrogen-containing compounds are essential building blocks in approximately 80% of pharmaceutical products. Technologies that can selectively reduce N₂ to specific intermediates could revolutionize synthetic pathways for drug manufacturing, potentially reducing production costs and environmental impact while enabling new molecular architectures.
The energy sector is increasingly exploring nitrogen compounds as energy carriers and storage media. Ammonia, with its hydrogen density of 17.6% by weight and established handling infrastructure, is gaining attention as a potential carbon-free fuel and hydrogen carrier. The maritime shipping industry has identified ammonia as a leading candidate for decarbonization, with major engine manufacturers developing ammonia-powered marine engines for commercial deployment by 2025.
Emerging applications in semiconductor manufacturing also present market opportunities. Ultra-pure nitrogen compounds are critical in various fabrication processes, and technologies that can produce these compounds with precise control over impurities could command premium pricing in this high-value market segment.
The defense sector has strategic interest in portable N₂ reduction technologies for field production of propellants and explosives, reducing supply chain vulnerabilities in remote operations. This specialized application represents a smaller but higher-margin market opportunity.
Market growth for N₂ reduction technologies is projected to accelerate as climate regulations tighten globally. The European Union's carbon border adjustment mechanism and similar policies will increasingly penalize carbon-intensive ammonia production, creating favorable economics for alternative technologies. Venture capital investment in this space has grown substantially, with over $300 million invested in startups developing electrochemical and photocatalytic N₂ reduction technologies since 2018.
The agricultural sector presents the largest immediate market application, with global ammonia demand for fertilizer production exceeding 150 million tons annually. Electrochemical and photocatalytic N₂ reduction technologies could enable distributed, on-site ammonia production for farmers, reducing transportation costs and emissions associated with centralized production. This decentralized approach is particularly valuable for remote agricultural regions with limited infrastructure.
The pharmaceutical and fine chemicals industries represent another significant market opportunity. Nitrogen-containing compounds are essential building blocks in approximately 80% of pharmaceutical products. Technologies that can selectively reduce N₂ to specific intermediates could revolutionize synthetic pathways for drug manufacturing, potentially reducing production costs and environmental impact while enabling new molecular architectures.
The energy sector is increasingly exploring nitrogen compounds as energy carriers and storage media. Ammonia, with its hydrogen density of 17.6% by weight and established handling infrastructure, is gaining attention as a potential carbon-free fuel and hydrogen carrier. The maritime shipping industry has identified ammonia as a leading candidate for decarbonization, with major engine manufacturers developing ammonia-powered marine engines for commercial deployment by 2025.
Emerging applications in semiconductor manufacturing also present market opportunities. Ultra-pure nitrogen compounds are critical in various fabrication processes, and technologies that can produce these compounds with precise control over impurities could command premium pricing in this high-value market segment.
The defense sector has strategic interest in portable N₂ reduction technologies for field production of propellants and explosives, reducing supply chain vulnerabilities in remote operations. This specialized application represents a smaller but higher-margin market opportunity.
Market growth for N₂ reduction technologies is projected to accelerate as climate regulations tighten globally. The European Union's carbon border adjustment mechanism and similar policies will increasingly penalize carbon-intensive ammonia production, creating favorable economics for alternative technologies. Venture capital investment in this space has grown substantially, with over $300 million invested in startups developing electrochemical and photocatalytic N₂ reduction technologies since 2018.
Current Operando Spectroscopy Limitations and Challenges
Despite significant advancements in operando spectroscopy techniques for tracking N₂ reduction intermediates, several critical limitations and challenges persist that hinder comprehensive understanding of reaction mechanisms. The temporal resolution of current spectroscopic methods remains insufficient to capture transient intermediates that exist on picosecond or femtosecond timescales, particularly during the initial N≡N bond activation steps. This limitation creates significant blind spots in our mechanistic understanding of the nitrogen reduction reaction (NRR).
Spatial resolution constraints also pose major challenges, as many spectroscopic techniques struggle to distinguish between surface-bound species and those in proximity to catalytic sites. This ambiguity complicates the accurate identification of active sites and reaction pathways, especially in heterogeneous catalysts with multiple potential binding configurations. The inability to precisely locate intermediates spatially limits our capacity to optimize catalyst designs.
Signal sensitivity represents another significant hurdle, particularly when tracking nitrogen intermediates that often exist in extremely low concentrations. The weak signal-to-noise ratio in many operando techniques makes it difficult to differentiate genuine intermediate signals from background noise or spectator species. This challenge is especially pronounced when studying industrial-relevant conditions where multiple competing reactions occur simultaneously.
Environmental constraints further complicate operando studies, as many spectroscopic techniques require specialized conditions incompatible with realistic reaction environments. Ultra-high vacuum requirements for certain electron spectroscopies, for instance, create artificial conditions that may not accurately represent catalytic behavior under practical operating conditions. This "pressure gap" between laboratory and industrial settings remains a persistent challenge.
Data interpretation complexities also present significant obstacles. The spectral signatures of nitrogen intermediates often overlap with those of other species, making unambiguous assignment difficult. Additionally, the lack of comprehensive spectral databases and reference standards for nitrogen-containing intermediates complicates accurate identification and quantification of reaction species.
Technical integration challenges exist when attempting to combine multiple spectroscopic techniques for comprehensive analysis. While multi-modal approaches offer more complete mechanistic insights, synchronizing different techniques with varying temporal and spatial resolutions presents significant engineering and data processing challenges. The complexity increases exponentially when attempting real-time correlation between spectroscopic data and catalytic performance metrics.
Finally, computational limitations affect the interpretation of experimental data, as current theoretical models often struggle to accurately predict spectroscopic signatures of complex nitrogen intermediates. The gap between experimental observations and theoretical predictions complicates the validation of proposed reaction mechanisms and intermediate structures.
Spatial resolution constraints also pose major challenges, as many spectroscopic techniques struggle to distinguish between surface-bound species and those in proximity to catalytic sites. This ambiguity complicates the accurate identification of active sites and reaction pathways, especially in heterogeneous catalysts with multiple potential binding configurations. The inability to precisely locate intermediates spatially limits our capacity to optimize catalyst designs.
Signal sensitivity represents another significant hurdle, particularly when tracking nitrogen intermediates that often exist in extremely low concentrations. The weak signal-to-noise ratio in many operando techniques makes it difficult to differentiate genuine intermediate signals from background noise or spectator species. This challenge is especially pronounced when studying industrial-relevant conditions where multiple competing reactions occur simultaneously.
Environmental constraints further complicate operando studies, as many spectroscopic techniques require specialized conditions incompatible with realistic reaction environments. Ultra-high vacuum requirements for certain electron spectroscopies, for instance, create artificial conditions that may not accurately represent catalytic behavior under practical operating conditions. This "pressure gap" between laboratory and industrial settings remains a persistent challenge.
Data interpretation complexities also present significant obstacles. The spectral signatures of nitrogen intermediates often overlap with those of other species, making unambiguous assignment difficult. Additionally, the lack of comprehensive spectral databases and reference standards for nitrogen-containing intermediates complicates accurate identification and quantification of reaction species.
Technical integration challenges exist when attempting to combine multiple spectroscopic techniques for comprehensive analysis. While multi-modal approaches offer more complete mechanistic insights, synchronizing different techniques with varying temporal and spatial resolutions presents significant engineering and data processing challenges. The complexity increases exponentially when attempting real-time correlation between spectroscopic data and catalytic performance metrics.
Finally, computational limitations affect the interpretation of experimental data, as current theoretical models often struggle to accurately predict spectroscopic signatures of complex nitrogen intermediates. The gap between experimental observations and theoretical predictions complicates the validation of proposed reaction mechanisms and intermediate structures.
State-of-the-Art Operando Spectroscopy Methods
01 In-situ spectroscopic techniques for reaction monitoring
Operando spectroscopy techniques allow for real-time monitoring of chemical reactions and catalytic processes. These methods enable researchers to observe reaction intermediates as they form and transform during the reaction, providing valuable insights into reaction mechanisms. Various spectroscopic methods such as infrared, Raman, and X-ray absorption spectroscopy can be integrated with reaction vessels to track intermediates under actual operating conditions.- In-situ spectroscopic techniques for reaction monitoring: Various spectroscopic techniques can be employed in operando conditions to track reaction intermediates in real-time. These methods allow researchers to observe chemical transformations as they occur without disrupting the reaction environment. By monitoring spectral changes during reactions, scientists can identify transient species and understand reaction mechanisms at a molecular level, providing valuable insights for process optimization and catalyst development.
- Advanced data processing for intermediate identification: Sophisticated data processing algorithms and computational methods are essential for analyzing complex spectroscopic data to identify reaction intermediates. These approaches include machine learning techniques, multivariate analysis, and chemometric methods that can extract meaningful information from large datasets. By applying these advanced processing techniques to operando spectroscopy data, researchers can detect subtle spectral features associated with short-lived intermediates and track their evolution throughout reaction pathways.
- Combined spectroscopic methods for comprehensive intermediate analysis: Integrating multiple spectroscopic techniques provides complementary information about reaction intermediates. By combining methods such as infrared spectroscopy, Raman spectroscopy, X-ray absorption spectroscopy, and mass spectrometry, researchers can obtain a more complete picture of reaction mechanisms. This multi-modal approach allows for the simultaneous tracking of different aspects of intermediate species, including structural changes, electronic properties, and concentration profiles during reactions.
- Specialized cell designs for operando spectroscopy: Custom-designed reaction cells and sample holders enable operando spectroscopic measurements under realistic reaction conditions. These specialized cells maintain appropriate temperature, pressure, and flow conditions while providing optical access for spectroscopic measurements. Advanced cell designs incorporate features such as temperature control, gas flow systems, and electrochemical components to simulate industrial processes while allowing for the continuous monitoring of reaction intermediates.
- Time-resolved spectroscopy for capturing transient intermediates: Time-resolved spectroscopic techniques with high temporal resolution are crucial for capturing short-lived reaction intermediates. These methods include ultrafast laser spectroscopy, stopped-flow techniques, and rapid-scan approaches that can monitor spectral changes on timescales ranging from femtoseconds to seconds. By precisely synchronizing spectroscopic measurements with reaction initiation, researchers can observe the formation and decay of transient species that would otherwise be missed in conventional steady-state measurements.
02 Advanced data processing for intermediate identification
Sophisticated data processing algorithms and computational methods are essential for analyzing the complex spectral data obtained during operando measurements. These techniques help in identifying and tracking reaction intermediates that may be short-lived or present in low concentrations. Machine learning approaches can be employed to extract meaningful patterns from spectroscopic data, enabling more accurate identification of reaction intermediates and pathways.Expand Specific Solutions03 Combined spectroscopic methods for comprehensive analysis
Combining multiple spectroscopic techniques provides complementary information about reaction intermediates. For example, coupling infrared spectroscopy with mass spectrometry or nuclear magnetic resonance allows researchers to simultaneously monitor structural changes and composition during reactions. This multi-modal approach enhances the ability to track intermediates throughout complex reaction networks and provides a more complete understanding of reaction mechanisms.Expand Specific Solutions04 Time-resolved spectroscopy for transient intermediate detection
Time-resolved spectroscopic techniques are crucial for capturing fast-changing reaction intermediates. These methods use ultrafast lasers or synchrotron radiation to probe reactions on timescales ranging from femtoseconds to milliseconds. By synchronizing the spectroscopic measurements with reaction initiation, researchers can observe the formation and decay of transient intermediates that would otherwise be missed by conventional steady-state measurements.Expand Specific Solutions05 Specialized reaction cells for operando measurements
Custom-designed reaction cells and sample environments are essential for operando spectroscopic studies. These specialized cells maintain relevant reaction conditions (temperature, pressure, flow) while allowing spectroscopic access to the sample. Advanced cell designs incorporate windows transparent to the probing radiation and can accommodate catalysts in various forms. Some designs enable simultaneous collection of spectroscopic data and reaction products for comprehensive tracking of intermediates throughout the reaction process.Expand Specific Solutions
Leading Research Groups and Industrial Partners
The N₂ reduction technology landscape is currently in an early development stage, characterized by significant academic-industrial collaboration. The market for operando spectroscopy techniques to track nitrogen reduction intermediates is emerging, with estimated growth potential as sustainable ammonia production becomes increasingly critical for agriculture and energy storage applications. Technical maturity varies across players, with research institutions like University of California, McGill University, and East China Normal University leading fundamental research, while industrial entities including Hitachi, Sony, and Pfizer contribute specialized instrumentation expertise. Companies like Servomex and NTT are advancing sensor technologies, while Merck and Abbott focus on analytical chemistry applications. This competitive landscape reflects a pre-commercialization phase where technological breakthroughs in spectroscopic methods could significantly impact future market positioning.
Teknologian Tutkimuskeskus VTT Oy
Technical Solution: VTT Technical Research Centre of Finland has developed a sophisticated operando spectroscopy system for tracking nitrogen reduction reaction intermediates under realistic operating conditions. Their technical solution integrates multiple spectroscopic techniques including operando Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, and X-ray absorption spectroscopy (XAS) in a single modular platform. VTT's system features a proprietary electrochemical cell design that enables simultaneous gas-phase and surface-sensitive measurements while maintaining precise control over reaction parameters. Their approach incorporates advanced microfluidic sample handling that minimizes contamination risks—a critical factor in accurate NRR intermediate detection. The system employs VTT's specialized catalyst coatings on spectroscopically transparent substrates that enhance both catalytic activity and signal detection. Their methodology includes automated data acquisition synchronized with electrochemical protocols, enabling precise correlation between electrical inputs and spectroscopic responses. Additionally, VTT has developed custom chemometric analysis tools specifically optimized for extracting weak spectral signatures of nitrogen-containing intermediates from complex backgrounds with overlapping features.
Strengths: Exceptional versatility through modular design accommodating multiple spectroscopic techniques; superior contamination control through advanced microfluidics; sophisticated data analysis capabilities specifically optimized for NRR intermediates. Weaknesses: Complex integration of multiple techniques requiring extensive calibration; challenging to maintain optimal performance across all measurement modalities; requires significant expertise to fully utilize all system capabilities.
The Regents of the University of California
Technical Solution: The University of California has developed a multi-modal operando spectroscopy platform for tracking nitrogen reduction reaction intermediates. Their technical approach integrates ambient pressure X-ray photoelectron spectroscopy (AP-XPS) with attenuated total reflection infrared spectroscopy (ATR-IR) in a specialized electrochemical cell design that maintains reaction conditions while allowing spectroscopic access. This system enables simultaneous monitoring of electronic structure changes and vibrational signatures of adsorbed species during N₂ reduction. The UC research teams have implemented machine learning algorithms to deconvolute complex spectral data, allowing identification of transient intermediates that would otherwise be obscured by overlapping signals. Their methodology includes pulse experiments where potential is modulated to trap short-lived intermediates, combined with rapid-scan spectroscopic techniques that achieve millisecond time resolution. Additionally, they've developed specialized catalyst materials with enhanced binding sites for N₂ activation that facilitate intermediate stabilization for improved spectroscopic detection.
Strengths: Exceptional temporal resolution for capturing transient species; sophisticated data analysis capabilities through machine learning integration; complementary techniques providing both electronic and molecular structural information. Weaknesses: Requires synchrotron facilities for some measurements limiting accessibility; complex data interpretation requiring specialized expertise; challenging to scale beyond laboratory research settings.
Key Spectroscopic Innovations for N₂ Reduction Intermediates
Device and method for measuring the concentration of a chemical compound in blood
PatentWO2017109440A1
Innovation
- A device and method employing adjustable supports with light sources emitting wavelengths between 700 nm to 3000 nm for backscatter measurements, eliminating the need for intermediate measurements by using reflectance based on intensity ratios between compounds like glucose and hemoglobin, and incorporating photodiodes and microcontrollers for precise concentration calculation.
Environmental Impact and Sustainability Considerations
The development of operando spectroscopic techniques for tracking N₂ reduction intermediates carries significant environmental implications that extend beyond the immediate scientific advancements. These technologies directly support the evolution of more sustainable ammonia production methods, potentially revolutionizing one of the most energy-intensive industrial processes currently in operation.
The conventional Haber-Bosch process, while revolutionary for global food security, consumes approximately 1-2% of the world's total energy production and generates substantial greenhouse gas emissions. Operando spectroscopy enables the development of catalysts that can function under ambient conditions, potentially reducing the carbon footprint of ammonia production by up to 90% compared to traditional methods.
Water consumption represents another critical environmental consideration. Current industrial ammonia synthesis requires significant water resources for cooling and steam generation. Electrocatalytic nitrogen reduction reactions (NRR) monitored through operando techniques can operate with minimal water requirements, potentially conserving this increasingly scarce resource in regions facing water stress.
The materials utilized in spectroscopic equipment and catalysts also present sustainability challenges. Many advanced catalysts contain rare earth elements or precious metals with problematic supply chains and extraction impacts. Real-time monitoring through operando methods can optimize catalyst performance and longevity, reducing material consumption and associated environmental degradation from mining operations.
Lifecycle assessment studies indicate that implementing operando spectroscopy-guided catalyst development could reduce the environmental impact across multiple categories, including acidification potential, eutrophication, and ecotoxicity. These benefits stem from more precise reaction control and reduced waste generation during both catalyst development and ammonia production processes.
From a circular economy perspective, operando spectroscopic techniques facilitate the design of catalysts with improved recyclability and regeneration capabilities. By understanding degradation mechanisms in real-time, researchers can develop systems that maintain activity through multiple cycles, minimizing waste and resource consumption.
The potential for distributed, small-scale ammonia production enabled by ambient-condition catalysts represents perhaps the most transformative sustainability benefit. This decentralization could dramatically reduce transportation emissions associated with ammonia distribution while enabling renewable energy integration through electrolytic processes powered by intermittent sources like solar and wind.
The conventional Haber-Bosch process, while revolutionary for global food security, consumes approximately 1-2% of the world's total energy production and generates substantial greenhouse gas emissions. Operando spectroscopy enables the development of catalysts that can function under ambient conditions, potentially reducing the carbon footprint of ammonia production by up to 90% compared to traditional methods.
Water consumption represents another critical environmental consideration. Current industrial ammonia synthesis requires significant water resources for cooling and steam generation. Electrocatalytic nitrogen reduction reactions (NRR) monitored through operando techniques can operate with minimal water requirements, potentially conserving this increasingly scarce resource in regions facing water stress.
The materials utilized in spectroscopic equipment and catalysts also present sustainability challenges. Many advanced catalysts contain rare earth elements or precious metals with problematic supply chains and extraction impacts. Real-time monitoring through operando methods can optimize catalyst performance and longevity, reducing material consumption and associated environmental degradation from mining operations.
Lifecycle assessment studies indicate that implementing operando spectroscopy-guided catalyst development could reduce the environmental impact across multiple categories, including acidification potential, eutrophication, and ecotoxicity. These benefits stem from more precise reaction control and reduced waste generation during both catalyst development and ammonia production processes.
From a circular economy perspective, operando spectroscopic techniques facilitate the design of catalysts with improved recyclability and regeneration capabilities. By understanding degradation mechanisms in real-time, researchers can develop systems that maintain activity through multiple cycles, minimizing waste and resource consumption.
The potential for distributed, small-scale ammonia production enabled by ambient-condition catalysts represents perhaps the most transformative sustainability benefit. This decentralization could dramatically reduce transportation emissions associated with ammonia distribution while enabling renewable energy integration through electrolytic processes powered by intermittent sources like solar and wind.
Interdisciplinary Collaboration Opportunities
The advancement of operando spectroscopy techniques for tracking N₂ reduction intermediates presents exceptional opportunities for interdisciplinary collaboration across multiple scientific and engineering domains. Materials scientists, chemists, and physicists can jointly develop novel catalyst materials with optimized electronic structures and surface properties specifically designed for nitrogen reduction reaction (NRR) pathways.
Collaboration between spectroscopy experts and computational scientists enables the development of more sophisticated data analysis algorithms and machine learning models that can interpret complex spectral data in real-time. These partnerships can significantly enhance the identification of fleeting intermediates that have previously eluded detection due to their transient nature and low concentrations.
Engineering disciplines, particularly chemical and electrical engineering, offer crucial expertise in reactor design and electrode fabrication that can be integrated with in-situ monitoring capabilities. Such collaborations facilitate the creation of specialized reaction cells that maintain ideal conditions while allowing for high-quality spectroscopic measurements—a persistent challenge in operando studies.
Environmental scientists and agricultural researchers represent valuable collaborative partners as they provide practical insights into the end applications of NRR technologies. Their involvement ensures that laboratory advances in N₂ reduction catalysis remain aligned with real-world implementation challenges and sustainability goals.
Interdisciplinary teams comprising theoretical chemists and experimental spectroscopists can validate computational models against experimental observations, creating a powerful feedback loop that accelerates catalyst design. This synergy is particularly valuable for understanding the complex electronic transitions and vibrational modes characteristic of nitrogen reduction intermediates.
Industry partnerships with academic research groups offer pathways for scaling promising laboratory techniques to industrial applications. Companies specializing in analytical instrumentation can contribute to developing more sensitive, robust spectroscopic tools specifically optimized for operando NRR studies, potentially leading to commercial innovations in both catalyst and instrumentation sectors.
International research networks connecting laboratories with complementary expertise and equipment can maximize resource utilization and knowledge exchange. Such global collaborations are especially important for addressing the significant technical and economic barriers to sustainable ammonia production that no single research group can overcome independently.
Collaboration between spectroscopy experts and computational scientists enables the development of more sophisticated data analysis algorithms and machine learning models that can interpret complex spectral data in real-time. These partnerships can significantly enhance the identification of fleeting intermediates that have previously eluded detection due to their transient nature and low concentrations.
Engineering disciplines, particularly chemical and electrical engineering, offer crucial expertise in reactor design and electrode fabrication that can be integrated with in-situ monitoring capabilities. Such collaborations facilitate the creation of specialized reaction cells that maintain ideal conditions while allowing for high-quality spectroscopic measurements—a persistent challenge in operando studies.
Environmental scientists and agricultural researchers represent valuable collaborative partners as they provide practical insights into the end applications of NRR technologies. Their involvement ensures that laboratory advances in N₂ reduction catalysis remain aligned with real-world implementation challenges and sustainability goals.
Interdisciplinary teams comprising theoretical chemists and experimental spectroscopists can validate computational models against experimental observations, creating a powerful feedback loop that accelerates catalyst design. This synergy is particularly valuable for understanding the complex electronic transitions and vibrational modes characteristic of nitrogen reduction intermediates.
Industry partnerships with academic research groups offer pathways for scaling promising laboratory techniques to industrial applications. Companies specializing in analytical instrumentation can contribute to developing more sensitive, robust spectroscopic tools specifically optimized for operando NRR studies, potentially leading to commercial innovations in both catalyst and instrumentation sectors.
International research networks connecting laboratories with complementary expertise and equipment can maximize resource utilization and knowledge exchange. Such global collaborations are especially important for addressing the significant technical and economic barriers to sustainable ammonia production that no single research group can overcome independently.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!