Stability Testing And Deactivation Modes For Photocatalysts
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalyst Stability Background and Objectives
Photocatalysis has emerged as a promising technology for environmental remediation and renewable energy applications since the groundbreaking discovery of water splitting on TiO2 electrodes by Fujishima and Honda in 1972. Over the past five decades, significant research efforts have focused on developing highly efficient photocatalytic materials capable of harnessing solar energy to drive chemical reactions. However, the practical implementation of photocatalytic technologies has been hindered by a critical yet often overlooked aspect: catalyst stability.
The evolution of photocatalyst research has predominantly emphasized activity enhancement rather than stability improvement. This imbalance has created a significant gap between laboratory demonstrations and real-world applications. While numerous photocatalysts show impressive initial performance under controlled laboratory conditions, their activity often deteriorates rapidly under prolonged operation or realistic environmental conditions, rendering them impractical for commercial deployment.
Understanding photocatalyst stability represents a multifaceted challenge that encompasses physical, chemical, and operational dimensions. Physical stability concerns the structural integrity of the catalyst under reaction conditions, while chemical stability relates to resistance against corrosion, poisoning, and other chemical degradation mechanisms. Operational stability addresses the maintenance of catalytic performance over extended periods under varying conditions.
The deactivation modes of photocatalysts are diverse and complex, ranging from photocorrosion and surface poisoning to thermal degradation and mechanical attrition. These mechanisms often operate simultaneously and synergistically, complicating efforts to develop comprehensive stability testing protocols. Furthermore, the relationship between catalyst composition, structure, and stability remains poorly understood, impeding rational design approaches for stable photocatalysts.
This technical research report aims to systematically examine the current understanding of photocatalyst stability testing methodologies and deactivation mechanisms. By establishing a comprehensive framework for evaluating photocatalyst stability, we seek to bridge the gap between laboratory research and practical applications. The objectives include identifying standardized protocols for stability assessment, elucidating fundamental deactivation pathways, and proposing design strategies for developing inherently stable photocatalytic materials.
Additionally, this report will explore the correlation between photocatalyst stability and activity, challenging the conventional wisdom that often treats these properties as independent or even contradictory. By integrating stability considerations into the early stages of photocatalyst design, we aim to accelerate the development of materials that maintain high performance under realistic operating conditions for extended periods, ultimately enabling the widespread implementation of photocatalytic technologies.
The evolution of photocatalyst research has predominantly emphasized activity enhancement rather than stability improvement. This imbalance has created a significant gap between laboratory demonstrations and real-world applications. While numerous photocatalysts show impressive initial performance under controlled laboratory conditions, their activity often deteriorates rapidly under prolonged operation or realistic environmental conditions, rendering them impractical for commercial deployment.
Understanding photocatalyst stability represents a multifaceted challenge that encompasses physical, chemical, and operational dimensions. Physical stability concerns the structural integrity of the catalyst under reaction conditions, while chemical stability relates to resistance against corrosion, poisoning, and other chemical degradation mechanisms. Operational stability addresses the maintenance of catalytic performance over extended periods under varying conditions.
The deactivation modes of photocatalysts are diverse and complex, ranging from photocorrosion and surface poisoning to thermal degradation and mechanical attrition. These mechanisms often operate simultaneously and synergistically, complicating efforts to develop comprehensive stability testing protocols. Furthermore, the relationship between catalyst composition, structure, and stability remains poorly understood, impeding rational design approaches for stable photocatalysts.
This technical research report aims to systematically examine the current understanding of photocatalyst stability testing methodologies and deactivation mechanisms. By establishing a comprehensive framework for evaluating photocatalyst stability, we seek to bridge the gap between laboratory research and practical applications. The objectives include identifying standardized protocols for stability assessment, elucidating fundamental deactivation pathways, and proposing design strategies for developing inherently stable photocatalytic materials.
Additionally, this report will explore the correlation between photocatalyst stability and activity, challenging the conventional wisdom that often treats these properties as independent or even contradictory. By integrating stability considerations into the early stages of photocatalyst design, we aim to accelerate the development of materials that maintain high performance under realistic operating conditions for extended periods, ultimately enabling the widespread implementation of photocatalytic technologies.
Market Analysis for Stable Photocatalytic Materials
The global market for stable photocatalytic materials is experiencing significant growth, driven by increasing environmental concerns and the push for sustainable technologies. Currently valued at approximately 2.5 billion USD, this market is projected to reach 5.7 billion USD by 2028, representing a compound annual growth rate of 12.8%. This growth trajectory is primarily fueled by applications in water treatment, air purification, self-cleaning surfaces, and renewable energy production.
Water treatment represents the largest application segment, accounting for nearly 35% of the market share. The increasing scarcity of clean water resources globally has intensified the demand for advanced water purification technologies, with photocatalysis emerging as a cost-effective and environmentally friendly solution. Particularly in developing regions facing severe water pollution challenges, the adoption of photocatalytic water treatment systems is accelerating.
Air purification applications follow closely, comprising approximately 28% of the market. With rising concerns about indoor and outdoor air quality, especially in urban environments and industrial zones, photocatalytic air purifiers are gaining traction. The COVID-19 pandemic has further accelerated this trend, as consumers and businesses seek technologies that can effectively eliminate airborne pathogens.
Regionally, Asia-Pacific dominates the market with a 42% share, led by China, Japan, and South Korea. These countries have implemented stringent environmental regulations and invested heavily in research and development of advanced materials. North America and Europe collectively account for 48% of the market, with growing adoption in both industrial and consumer applications.
A critical market driver is the increasing focus on stability and longevity of photocatalysts. End-users are demonstrating willingness to pay premium prices for materials that maintain consistent performance over extended periods without significant deactivation. This preference is reflected in the price premium of 15-30% commanded by highly stable photocatalytic materials compared to their conventional counterparts.
Industry surveys indicate that consumers and industrial users prioritize stability as the second most important factor after efficiency when selecting photocatalytic products. This market preference is driving manufacturers to invest in advanced stability testing protocols and innovative formulations that resist common deactivation mechanisms such as photocorrosion, poisoning, and thermal degradation.
The competitive landscape is characterized by a mix of established chemical companies and specialized materials science startups. Major players are increasingly focusing on developing proprietary stabilization technologies as a key differentiator in this growing market.
Water treatment represents the largest application segment, accounting for nearly 35% of the market share. The increasing scarcity of clean water resources globally has intensified the demand for advanced water purification technologies, with photocatalysis emerging as a cost-effective and environmentally friendly solution. Particularly in developing regions facing severe water pollution challenges, the adoption of photocatalytic water treatment systems is accelerating.
Air purification applications follow closely, comprising approximately 28% of the market. With rising concerns about indoor and outdoor air quality, especially in urban environments and industrial zones, photocatalytic air purifiers are gaining traction. The COVID-19 pandemic has further accelerated this trend, as consumers and businesses seek technologies that can effectively eliminate airborne pathogens.
Regionally, Asia-Pacific dominates the market with a 42% share, led by China, Japan, and South Korea. These countries have implemented stringent environmental regulations and invested heavily in research and development of advanced materials. North America and Europe collectively account for 48% of the market, with growing adoption in both industrial and consumer applications.
A critical market driver is the increasing focus on stability and longevity of photocatalysts. End-users are demonstrating willingness to pay premium prices for materials that maintain consistent performance over extended periods without significant deactivation. This preference is reflected in the price premium of 15-30% commanded by highly stable photocatalytic materials compared to their conventional counterparts.
Industry surveys indicate that consumers and industrial users prioritize stability as the second most important factor after efficiency when selecting photocatalytic products. This market preference is driving manufacturers to invest in advanced stability testing protocols and innovative formulations that resist common deactivation mechanisms such as photocorrosion, poisoning, and thermal degradation.
The competitive landscape is characterized by a mix of established chemical companies and specialized materials science startups. Major players are increasingly focusing on developing proprietary stabilization technologies as a key differentiator in this growing market.
Current Challenges in Photocatalyst Longevity
Despite significant advancements in photocatalyst development, longevity remains a critical challenge that hinders widespread commercial application. Photocatalysts frequently experience performance degradation over time, with many systems showing substantial activity loss after just hours or days of operation. This instability presents a fundamental barrier to industrial adoption, as commercial viability typically requires operational lifetimes of months or years.
The primary deactivation mechanisms affecting photocatalyst longevity include photocorrosion, where the catalyst material itself undergoes oxidation or reduction reactions; thermal degradation during operation; poisoning by reaction intermediates or contaminants; and physical changes such as particle agglomeration or surface area reduction. For metal oxide photocatalysts like TiO2, surface hydroxyl group depletion represents another significant degradation pathway.
Current stability testing protocols lack standardization, making cross-comparison between different research studies challenging. Most academic research focuses on short-term activity measurements (typically hours), while industrial applications require stability verification over thousands of hours. This disconnect creates significant uncertainty when translating laboratory results to practical applications.
Environmental factors substantially impact photocatalyst stability. Variations in pH, temperature, light intensity, and the presence of common water constituents (such as inorganic ions, natural organic matter, and microorganisms) can dramatically accelerate deactivation processes. These real-world conditions are rarely incorporated into laboratory testing protocols, leading to overly optimistic stability projections.
Regeneration strategies represent another underdeveloped area. While some photocatalysts can be partially regenerated through thermal treatment, UV exposure, or chemical washing, these processes often fail to fully restore initial activity and may themselves contribute to long-term structural changes that permanently reduce performance.
The economic implications of photocatalyst instability are substantial. Frequent replacement or regeneration requirements significantly increase operational costs and environmental footprint, offsetting the sustainability benefits these materials aim to provide. Life cycle assessments indicate that catalyst longevity often represents the determining factor in whether photocatalytic technologies offer net environmental benefits compared to conventional alternatives.
Addressing these challenges requires developing accelerated aging protocols that reliably predict long-term stability, standardized testing methodologies that enable meaningful comparisons between different catalyst systems, and fundamental mechanistic studies that elucidate deactivation pathways at the molecular level. Without these advances, photocatalyst longevity will continue to represent a critical barrier to widespread implementation.
The primary deactivation mechanisms affecting photocatalyst longevity include photocorrosion, where the catalyst material itself undergoes oxidation or reduction reactions; thermal degradation during operation; poisoning by reaction intermediates or contaminants; and physical changes such as particle agglomeration or surface area reduction. For metal oxide photocatalysts like TiO2, surface hydroxyl group depletion represents another significant degradation pathway.
Current stability testing protocols lack standardization, making cross-comparison between different research studies challenging. Most academic research focuses on short-term activity measurements (typically hours), while industrial applications require stability verification over thousands of hours. This disconnect creates significant uncertainty when translating laboratory results to practical applications.
Environmental factors substantially impact photocatalyst stability. Variations in pH, temperature, light intensity, and the presence of common water constituents (such as inorganic ions, natural organic matter, and microorganisms) can dramatically accelerate deactivation processes. These real-world conditions are rarely incorporated into laboratory testing protocols, leading to overly optimistic stability projections.
Regeneration strategies represent another underdeveloped area. While some photocatalysts can be partially regenerated through thermal treatment, UV exposure, or chemical washing, these processes often fail to fully restore initial activity and may themselves contribute to long-term structural changes that permanently reduce performance.
The economic implications of photocatalyst instability are substantial. Frequent replacement or regeneration requirements significantly increase operational costs and environmental footprint, offsetting the sustainability benefits these materials aim to provide. Life cycle assessments indicate that catalyst longevity often represents the determining factor in whether photocatalytic technologies offer net environmental benefits compared to conventional alternatives.
Addressing these challenges requires developing accelerated aging protocols that reliably predict long-term stability, standardized testing methodologies that enable meaningful comparisons between different catalyst systems, and fundamental mechanistic studies that elucidate deactivation pathways at the molecular level. Without these advances, photocatalyst longevity will continue to represent a critical barrier to widespread implementation.
Established Stability Testing Methodologies
01 Metal oxide stabilization techniques for photocatalysts
Various metal oxide stabilization techniques can be employed to enhance the stability of photocatalysts. These include doping with transition metals, creating core-shell structures, and surface modification with protective layers. These approaches help prevent photocorrosion, maintain catalytic activity over extended periods, and improve resistance to harsh reaction conditions. Stabilized metal oxide photocatalysts show enhanced durability in applications such as water treatment and air purification.- Metal oxide stabilization techniques: Various methods can be employed to enhance the stability of metal oxide photocatalysts, which are prone to degradation under prolonged exposure to light and environmental conditions. These techniques include doping with transition metals, surface modification with protective coatings, and controlled crystallization processes. These approaches help maintain photocatalytic activity over extended periods by preventing phase transformation, particle agglomeration, and surface deactivation, resulting in more durable photocatalytic systems for environmental and energy applications.
- Composite photocatalyst structures: Composite structures combining multiple photocatalytic materials can significantly improve stability compared to single-component systems. These composites often feature core-shell architectures, heterojunctions between semiconductors with different band gaps, or immobilization on stable support materials. The synergistic effects between components not only enhance photocatalytic efficiency but also protect active sites from deactivation, reduce electron-hole recombination rates, and provide mechanical stability, resulting in photocatalysts with extended operational lifetimes.
- Encapsulation and protective coating strategies: Encapsulation and protective coating strategies involve surrounding photocatalytic materials with protective layers that shield them from degradation while maintaining their activity. These approaches include polymer encapsulation, silica coating, and formation of core-shell structures. The protective layers prevent leaching of active components, reduce exposure to deactivating substances, and provide physical barriers against mechanical stress, while still allowing light penetration and reactant diffusion to the catalytic sites.
- pH and thermal stability enhancement: Improving the pH and thermal stability of photocatalysts is crucial for their application in various environmental conditions. This can be achieved through surface modification with pH-resistant functional groups, incorporation of thermally stable components, and development of specialized synthesis methods. These approaches enable photocatalysts to maintain their activity across wider pH ranges and temperature conditions, making them suitable for diverse applications including wastewater treatment, air purification, and chemical synthesis under challenging operational environments.
- Novel stabilization additives and dopants: The incorporation of specific additives and dopants can significantly enhance photocatalyst stability. These include rare earth elements, noble metals, non-metal dopants, and specialized organic compounds that can be integrated into the photocatalyst structure. These additives work by trapping free radicals, preventing crystal phase transformations, modifying electronic structures, and creating oxygen vacancies that improve charge separation. The strategic selection of these stabilizing agents can lead to photocatalysts with improved resistance to photocorrosion and chemical degradation.
02 Polymer-supported photocatalyst systems
Incorporating photocatalysts into polymer matrices or supports significantly improves their stability and reusability. The polymer matrix protects the active catalytic sites from degradation while allowing sufficient light penetration and reactant diffusion. These composite materials combine the photocatalytic activity of the catalyst with the mechanical strength and processability of polymers, resulting in more durable photocatalytic systems that can withstand repeated use cycles without significant loss of activity.Expand Specific Solutions03 Carbon-based materials for photocatalyst stabilization
Carbon-based materials such as graphene, carbon nanotubes, and carbon quantum dots can be used to stabilize photocatalysts. These carbon materials provide excellent electron transport properties, prevent aggregation of catalyst particles, and enhance light absorption. The resulting carbon-photocatalyst composites demonstrate improved stability under continuous irradiation and harsh reaction conditions, making them suitable for long-term photocatalytic applications in environmental remediation and energy conversion.Expand Specific Solutions04 Encapsulation and coating strategies for photocatalyst protection
Encapsulation and coating strategies involve surrounding photocatalyst particles with protective layers that shield them from degradation while maintaining their photocatalytic activity. These protective layers can be made from silica, alumina, or other inorganic materials that are transparent to light but prevent direct contact with corrosive media. This approach significantly extends the operational lifetime of photocatalysts in various applications, including water splitting, organic synthesis, and environmental remediation.Expand Specific Solutions05 pH-resistant and thermally stable photocatalyst formulations
Developing photocatalysts that maintain stability across a wide pH range and at elevated temperatures is crucial for industrial applications. These formulations often incorporate specific crystal structures, surface modifications, or composite materials that resist dissolution, phase transformation, or sintering under extreme conditions. pH-resistant and thermally stable photocatalysts can operate effectively in diverse environments, from acidic industrial wastewater to high-temperature solar thermal applications, without significant degradation of their catalytic performance.Expand Specific Solutions
Leading Researchers and Industrial Players
The photocatalyst stability testing and deactivation modes market is currently in a growth phase, with increasing research focus on improving catalyst longevity for sustainable applications. The global market is estimated at approximately $3.5-4 billion, driven by environmental regulations and clean energy initiatives. Leading companies demonstrate varying levels of technical maturity: TOTO Ltd. and Merck Patent GmbH have established advanced stability testing protocols; Dow Technology and JSR Corp. focus on novel deactivation prevention methods; while academic institutions like North China Electric Power University collaborate with State Grid Corp. to develop grid-integrated photocatalytic systems. Chinese corporations including Huawei and OPPO are increasingly investing in photocatalytic applications for consumer electronics, indicating market expansion beyond traditional environmental applications.
TOTO Ltd.
Technical Solution: TOTO has developed advanced photocatalyst stability testing protocols focusing on their proprietary HYDROTECT technology, which utilizes titanium dioxide photocatalysts. Their approach involves accelerated aging tests under various environmental conditions including UV exposure, temperature cycling, and chemical exposure to simulate real-world degradation. TOTO employs a comprehensive testing framework that measures photocatalytic activity retention over time through methylene blue degradation rates and contact angle measurements. Their stability enhancement techniques include metal ion doping (particularly with platinum and silver) to prevent electron-hole recombination and surface modification with silicon compounds to protect against deactivation from organic contaminants. TOTO has established standardized protocols for quantifying photocatalyst longevity in both indoor and outdoor applications, with particular emphasis on maintaining performance under low-light conditions.
Strengths: Exceptional long-term stability in real-world applications with documented performance retention exceeding 10 years in building materials. Superior resistance to common deactivation factors like organic fouling. Weaknesses: Higher production costs due to noble metal doping techniques. Performance degradation in high-humidity environments remains a challenge despite mitigation efforts.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp (Sinopec) has developed specialized photocatalyst stability testing protocols focused on petrochemical applications, particularly for wastewater treatment and air purification systems. Their approach involves comprehensive stability assessment under harsh chemical environments, including exposure to various organic solvents, sulfur compounds, and heavy metals commonly found in industrial settings. Sinopec employs a multi-stage testing framework that evaluates both short-term deactivation (through pulse poisoning tests) and long-term degradation (through continuous flow reactors operating for 3000+ hours). Their research has identified catalyst deactivation mechanisms specific to hydrocarbon-rich environments, including coking, metal deposition, and structural collapse due to exothermic reactions. To address these challenges, Sinopec has developed composite photocatalysts with hierarchical pore structures that resist fouling and facilitate regeneration. Their latest innovation involves integrating photocatalysts with traditional hydroprocessing catalysts to create dual-function systems with enhanced stability in petroleum refining applications.
Strengths: Exceptional resistance to chemical poisoning in complex industrial environments containing multiple contaminants. Effective regeneration protocols that can restore >90% of initial activity after deactivation. Weaknesses: Lower initial photocatalytic activity compared to specialized environmental catalysts. Limited performance under visible light, requiring higher energy UV sources for activation.
Key Deactivation Mechanisms Analysis
Deactivation resistant photocatalysts
PatentActiveUS8513157B2
Innovation
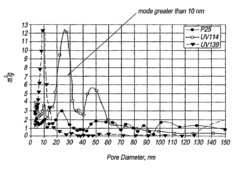
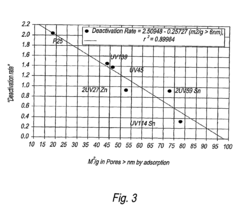
- A porous photocatalyst with a specific pore structure, comprising cylindrical pores with a majority of the surface area in pores 5 nm or larger, which reduces deactivation by minimizing blockage from deposits and maintaining activity over time.
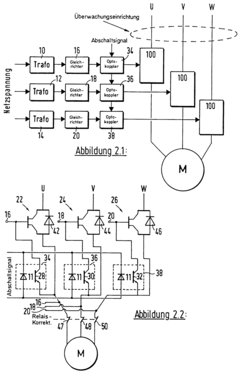
Method for monitoring a stationary condition or rotational speed during set-up of a drive system, in particular a fast response servo control system, and power relays for use with such a method
PatentInactiveEP0770877A1
Innovation
- The solution involves analyzing and comparing motor currents and voltages to detect dynamic standstill and set-up speed, using electronic components like IGBTs and semiconductor relays for fast and safe monitoring, allowing for continuous signal evaluation and rapid shutdown.
Environmental Impact of Photocatalyst Degradation
The environmental implications of photocatalyst degradation extend far beyond mere efficiency concerns, representing a critical aspect of sustainability in advanced oxidation processes. As photocatalysts deteriorate, they may release potentially harmful components into water bodies, soil, or air, depending on their application environment. Titanium dioxide (TiO₂), the most widely used photocatalyst, generally exhibits low toxicity in its intact form; however, its nanoparticle derivatives and degradation products warrant careful monitoring due to potential bioaccumulation effects.
Degradation byproducts from doped photocatalysts present particular environmental concerns. Metal-doped catalysts containing elements such as silver, copper, or platinum can release ionic species during deactivation that may contribute to heavy metal contamination in treated water systems. Similarly, non-metal doped catalysts containing nitrogen, sulfur, or carbon compounds may form secondary pollutants through partial oxidation processes when catalyst integrity fails.
The environmental fate of deactivated photocatalysts depends significantly on their immobilization method. Fixed-bed systems generally contain degradation products more effectively than slurry reactors, where nanoparticle leaching presents a more substantial risk. Research indicates that up to 15% of catalyst material may be lost to the environment during extended operation in poorly designed systems, creating potential ecological exposure pathways.
Lifecycle assessment studies reveal that environmental impacts vary considerably across different photocatalyst formulations. While pristine TiO₂ demonstrates relatively benign environmental characteristics, composite photocatalysts incorporating graphene, quantum dots, or rare earth elements may present more complex environmental footprints upon degradation. The ecological persistence of these materials ranges from weeks to decades depending on environmental conditions and material composition.
Regulatory frameworks addressing photocatalyst end-of-life management remain underdeveloped globally, with significant regional variations in monitoring requirements and disposal protocols. The European Union's REACH regulations provide the most comprehensive approach to date, requiring thorough characterization of nanomaterial environmental impacts, though specific provisions for photocatalyst degradation products remain limited.
Emerging research suggests promising approaches to mitigate environmental impacts through green synthesis methods and biodegradable support materials. Encapsulation technologies using natural polymers have demonstrated up to 80% reduction in nanoparticle leaching during catalyst deactivation phases, while maintaining acceptable photocatalytic performance. These developments represent critical advances toward environmentally responsible implementation of photocatalytic technologies across industrial and environmental remediation applications.
Degradation byproducts from doped photocatalysts present particular environmental concerns. Metal-doped catalysts containing elements such as silver, copper, or platinum can release ionic species during deactivation that may contribute to heavy metal contamination in treated water systems. Similarly, non-metal doped catalysts containing nitrogen, sulfur, or carbon compounds may form secondary pollutants through partial oxidation processes when catalyst integrity fails.
The environmental fate of deactivated photocatalysts depends significantly on their immobilization method. Fixed-bed systems generally contain degradation products more effectively than slurry reactors, where nanoparticle leaching presents a more substantial risk. Research indicates that up to 15% of catalyst material may be lost to the environment during extended operation in poorly designed systems, creating potential ecological exposure pathways.
Lifecycle assessment studies reveal that environmental impacts vary considerably across different photocatalyst formulations. While pristine TiO₂ demonstrates relatively benign environmental characteristics, composite photocatalysts incorporating graphene, quantum dots, or rare earth elements may present more complex environmental footprints upon degradation. The ecological persistence of these materials ranges from weeks to decades depending on environmental conditions and material composition.
Regulatory frameworks addressing photocatalyst end-of-life management remain underdeveloped globally, with significant regional variations in monitoring requirements and disposal protocols. The European Union's REACH regulations provide the most comprehensive approach to date, requiring thorough characterization of nanomaterial environmental impacts, though specific provisions for photocatalyst degradation products remain limited.
Emerging research suggests promising approaches to mitigate environmental impacts through green synthesis methods and biodegradable support materials. Encapsulation technologies using natural polymers have demonstrated up to 80% reduction in nanoparticle leaching during catalyst deactivation phases, while maintaining acceptable photocatalytic performance. These developments represent critical advances toward environmentally responsible implementation of photocatalytic technologies across industrial and environmental remediation applications.
Standardization Efforts in Stability Assessment
The standardization of stability assessment protocols for photocatalysts represents a critical frontier in advancing photocatalytic technologies toward commercial viability. Currently, the field suffers from significant inconsistencies in testing methodologies, making cross-study comparisons challenging and hindering technological progress. Several international organizations have recognized this gap and initiated efforts to establish standardized protocols for evaluating photocatalyst stability.
The International Organization for Standardization (ISO) has developed preliminary guidelines through its Technical Committee 229, focusing on nanomaterials used in photocatalysis. These guidelines propose standardized testing conditions including light source specifications, temperature controls, and sample preparation methods. However, these standards primarily address performance metrics rather than long-term stability assessment.
IUPAC (International Union of Pure and Applied Chemistry) has established a working group dedicated to photocatalyst characterization, which recently published recommendations for reporting stability data. These recommendations emphasize the importance of extended testing periods, standardized aging protocols, and comprehensive reporting of deactivation parameters.
The Joint Research Centre of the European Commission has launched an initiative to harmonize photocatalyst testing methodologies across EU member states. Their framework includes specific protocols for assessing stability under various environmental conditions, with particular attention to water treatment and air purification applications.
In the United States, NIST (National Institute of Standards and Technology) has developed reference materials and measurement protocols specifically designed for benchmarking photocatalyst stability. These include standardized test reactors and analytical methods for quantifying performance degradation over time.
Industry consortia have also contributed significantly to standardization efforts. The Photocatalysis Industry Association of Japan has established widely-adopted protocols for commercial photocatalytic materials, particularly focusing on stability under real-world operating conditions.
Despite these advances, significant challenges remain in achieving global consensus. Different application domains (water treatment, hydrogen production, CO2 reduction) require tailored stability metrics, complicating the development of universal standards. Additionally, accelerated aging tests that reliably predict long-term stability remain elusive for many photocatalytic systems.
Moving forward, the integration of artificial intelligence and high-throughput testing methodologies offers promising avenues for developing more comprehensive stability assessment frameworks. These approaches could potentially address the complex, multifactorial nature of photocatalyst deactivation while streamlining the standardization process across diverse material systems and applications.
The International Organization for Standardization (ISO) has developed preliminary guidelines through its Technical Committee 229, focusing on nanomaterials used in photocatalysis. These guidelines propose standardized testing conditions including light source specifications, temperature controls, and sample preparation methods. However, these standards primarily address performance metrics rather than long-term stability assessment.
IUPAC (International Union of Pure and Applied Chemistry) has established a working group dedicated to photocatalyst characterization, which recently published recommendations for reporting stability data. These recommendations emphasize the importance of extended testing periods, standardized aging protocols, and comprehensive reporting of deactivation parameters.
The Joint Research Centre of the European Commission has launched an initiative to harmonize photocatalyst testing methodologies across EU member states. Their framework includes specific protocols for assessing stability under various environmental conditions, with particular attention to water treatment and air purification applications.
In the United States, NIST (National Institute of Standards and Technology) has developed reference materials and measurement protocols specifically designed for benchmarking photocatalyst stability. These include standardized test reactors and analytical methods for quantifying performance degradation over time.
Industry consortia have also contributed significantly to standardization efforts. The Photocatalysis Industry Association of Japan has established widely-adopted protocols for commercial photocatalytic materials, particularly focusing on stability under real-world operating conditions.
Despite these advances, significant challenges remain in achieving global consensus. Different application domains (water treatment, hydrogen production, CO2 reduction) require tailored stability metrics, complicating the development of universal standards. Additionally, accelerated aging tests that reliably predict long-term stability remain elusive for many photocatalytic systems.
Moving forward, the integration of artificial intelligence and high-throughput testing methodologies offers promising avenues for developing more comprehensive stability assessment frameworks. These approaches could potentially address the complex, multifactorial nature of photocatalyst deactivation while streamlining the standardization process across diverse material systems and applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!