Electrolyte Conductivity And Its Role In Product Separation
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Conductivity Background and Objectives
Electrolyte conductivity represents a fundamental property in electrochemistry that has evolved significantly since its initial discovery in the early 19th century. This property refers to the ability of an electrolyte solution to conduct electricity due to the presence of ions that serve as charge carriers. The historical progression of understanding electrolyte conductivity began with Faraday's laws of electrolysis and has advanced through numerous theoretical frameworks including the Debye-Hückel theory and more recent computational models.
The evolution of electrolyte conductivity research has been marked by several pivotal developments. In the 1920s, Peter Debye and Erich Hückel established the theoretical foundation for understanding ion interactions in solution. The 1950s-1970s saw significant advancements in measurement techniques, including the development of high-precision conductivity meters. Recent decades have witnessed the integration of computational methods and molecular dynamics simulations, enabling more accurate predictions of conductivity behavior in complex systems.
Current technological trends in this field include the development of advanced ionic liquids with tailored conductivity properties, the exploration of nano-confined electrolytes, and the investigation of conductivity in extreme conditions. These innovations are driving applications across multiple industries, from energy storage to analytical chemistry and separation technologies.
The fundamental relationship between electrolyte conductivity and separation processes stems from the differential migration of ions under electrical fields, which forms the basis for techniques such as electrodialysis, electrodeionization, and various electrochemical separation methods. This relationship has become increasingly important as industries seek more efficient and environmentally sustainable separation technologies.
The primary objectives of this technical research report are threefold. First, to comprehensively analyze the current state of electrolyte conductivity science as it applies to product separation technologies. Second, to identify key technological barriers limiting the efficiency and applicability of conductivity-based separation methods. Third, to explore emerging innovations and potential breakthrough approaches that could significantly enhance separation processes across industrial applications.
This research aims to bridge fundamental electrochemical principles with practical industrial applications, providing insights that can guide future research and development efforts. By examining both theoretical advancements and practical implementations, we seek to establish a roadmap for optimizing electrolyte conductivity properties to achieve more efficient, selective, and sustainable product separation processes.
The evolution of electrolyte conductivity research has been marked by several pivotal developments. In the 1920s, Peter Debye and Erich Hückel established the theoretical foundation for understanding ion interactions in solution. The 1950s-1970s saw significant advancements in measurement techniques, including the development of high-precision conductivity meters. Recent decades have witnessed the integration of computational methods and molecular dynamics simulations, enabling more accurate predictions of conductivity behavior in complex systems.
Current technological trends in this field include the development of advanced ionic liquids with tailored conductivity properties, the exploration of nano-confined electrolytes, and the investigation of conductivity in extreme conditions. These innovations are driving applications across multiple industries, from energy storage to analytical chemistry and separation technologies.
The fundamental relationship between electrolyte conductivity and separation processes stems from the differential migration of ions under electrical fields, which forms the basis for techniques such as electrodialysis, electrodeionization, and various electrochemical separation methods. This relationship has become increasingly important as industries seek more efficient and environmentally sustainable separation technologies.
The primary objectives of this technical research report are threefold. First, to comprehensively analyze the current state of electrolyte conductivity science as it applies to product separation technologies. Second, to identify key technological barriers limiting the efficiency and applicability of conductivity-based separation methods. Third, to explore emerging innovations and potential breakthrough approaches that could significantly enhance separation processes across industrial applications.
This research aims to bridge fundamental electrochemical principles with practical industrial applications, providing insights that can guide future research and development efforts. By examining both theoretical advancements and practical implementations, we seek to establish a roadmap for optimizing electrolyte conductivity properties to achieve more efficient, selective, and sustainable product separation processes.
Market Analysis for Electrolyte-Based Separation Technologies
The global market for electrolyte-based separation technologies has experienced significant growth in recent years, driven by increasing demands across multiple industries including pharmaceuticals, food and beverage, water treatment, and chemical processing. The market value reached approximately $5.2 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 7.8% through 2028, potentially reaching $8.1 billion by the end of the forecast period.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 38% of the total market share. This dominance is attributed to the critical need for high-purity separation processes in drug development and production. The water treatment sector follows closely at 27%, with growing environmental regulations worldwide driving adoption of advanced separation technologies.
Geographically, North America leads the market with approximately 35% share, followed by Europe (28%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 9.2% annually, primarily due to rapid industrialization in China and India, coupled with increasing investments in pharmaceutical manufacturing and water treatment infrastructure.
Key market drivers include stringent regulatory requirements for product purity across industries, growing demand for sustainable separation processes with reduced environmental impact, and technological advancements improving separation efficiency and reducing operational costs. The pharmaceutical industry's shift toward continuous manufacturing processes has particularly accelerated demand for advanced electrolyte-based separation systems.
Customer demand patterns indicate a growing preference for modular and scalable separation systems that offer flexibility in production capacity. Additionally, there is increasing interest in hybrid separation technologies that combine electrolyte conductivity principles with other separation mechanisms to achieve higher selectivity and efficiency.
Market challenges include high initial capital investment requirements, technical complexity requiring specialized operational expertise, and competition from alternative separation technologies such as membrane-based systems. The economic sensitivity of certain end-user industries, particularly in developing regions, also presents adoption barriers due to cost considerations.
Emerging market opportunities exist in specialized applications such as rare earth element separation, battery material recycling, and high-value biopharmaceutical purification processes. The integration of digital monitoring and control systems with electrolyte-based separation technologies represents another growth avenue, enabling process optimization and predictive maintenance capabilities.
Pharmaceutical and biotechnology sectors represent the largest market segment, accounting for nearly 38% of the total market share. This dominance is attributed to the critical need for high-purity separation processes in drug development and production. The water treatment sector follows closely at 27%, with growing environmental regulations worldwide driving adoption of advanced separation technologies.
Geographically, North America leads the market with approximately 35% share, followed by Europe (28%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 9.2% annually, primarily due to rapid industrialization in China and India, coupled with increasing investments in pharmaceutical manufacturing and water treatment infrastructure.
Key market drivers include stringent regulatory requirements for product purity across industries, growing demand for sustainable separation processes with reduced environmental impact, and technological advancements improving separation efficiency and reducing operational costs. The pharmaceutical industry's shift toward continuous manufacturing processes has particularly accelerated demand for advanced electrolyte-based separation systems.
Customer demand patterns indicate a growing preference for modular and scalable separation systems that offer flexibility in production capacity. Additionally, there is increasing interest in hybrid separation technologies that combine electrolyte conductivity principles with other separation mechanisms to achieve higher selectivity and efficiency.
Market challenges include high initial capital investment requirements, technical complexity requiring specialized operational expertise, and competition from alternative separation technologies such as membrane-based systems. The economic sensitivity of certain end-user industries, particularly in developing regions, also presents adoption barriers due to cost considerations.
Emerging market opportunities exist in specialized applications such as rare earth element separation, battery material recycling, and high-value biopharmaceutical purification processes. The integration of digital monitoring and control systems with electrolyte-based separation technologies represents another growth avenue, enabling process optimization and predictive maintenance capabilities.
Current Challenges in Electrolyte Conductivity Applications
Despite significant advancements in electrolyte conductivity applications for product separation, several critical challenges continue to impede optimal performance and widespread industrial adoption. One of the most persistent issues is the trade-off between selectivity and throughput in separation processes. As conductivity increases to improve separation efficiency, energy consumption often rises exponentially, creating economic barriers for large-scale implementation.
Temperature sensitivity presents another significant challenge, as most electrolyte systems exhibit dramatic conductivity variations with temperature fluctuations. This necessitates precise temperature control systems that add complexity and cost to separation units, particularly problematic in environments where temperature stability cannot be guaranteed.
Electrode fouling and membrane degradation remain unresolved issues in continuous operation scenarios. The accumulation of organic and inorganic deposits on electrode surfaces progressively reduces conductivity and separation efficiency, while membrane materials exposed to varying electrolyte conditions experience accelerated degradation, shortening operational lifespans and increasing maintenance requirements.
The scalability of laboratory-proven technologies to industrial applications encounters significant engineering hurdles. Maintaining uniform electric fields and consistent electrolyte properties across large separation units has proven technically challenging, resulting in performance discrepancies between small-scale demonstrations and industrial implementations.
Concentration polarization effects create localized conductivity gradients that diminish separation efficiency over time. These boundary layer phenomena are particularly problematic in high-throughput systems where rapid ion transport is essential for effective separation.
Environmental and safety concerns also present significant challenges, especially regarding the disposal or recycling of spent electrolytes. Many high-performance electrolyte systems contain components that pose environmental risks, necessitating additional treatment processes that impact overall system economics.
The integration of electrolyte conductivity-based separation with existing industrial processes presents compatibility challenges. Retrofitting conventional separation trains with electrolyte-based technologies often requires substantial process modifications and capital investment, creating adoption barriers despite potential performance benefits.
Recent research has highlighted the need for more sophisticated modeling approaches that can accurately predict electrolyte behavior under dynamic operating conditions. Current models often fail to capture the complex interplay between conductivity, ion mobility, and separation efficiency across varying process parameters.
Temperature sensitivity presents another significant challenge, as most electrolyte systems exhibit dramatic conductivity variations with temperature fluctuations. This necessitates precise temperature control systems that add complexity and cost to separation units, particularly problematic in environments where temperature stability cannot be guaranteed.
Electrode fouling and membrane degradation remain unresolved issues in continuous operation scenarios. The accumulation of organic and inorganic deposits on electrode surfaces progressively reduces conductivity and separation efficiency, while membrane materials exposed to varying electrolyte conditions experience accelerated degradation, shortening operational lifespans and increasing maintenance requirements.
The scalability of laboratory-proven technologies to industrial applications encounters significant engineering hurdles. Maintaining uniform electric fields and consistent electrolyte properties across large separation units has proven technically challenging, resulting in performance discrepancies between small-scale demonstrations and industrial implementations.
Concentration polarization effects create localized conductivity gradients that diminish separation efficiency over time. These boundary layer phenomena are particularly problematic in high-throughput systems where rapid ion transport is essential for effective separation.
Environmental and safety concerns also present significant challenges, especially regarding the disposal or recycling of spent electrolytes. Many high-performance electrolyte systems contain components that pose environmental risks, necessitating additional treatment processes that impact overall system economics.
The integration of electrolyte conductivity-based separation with existing industrial processes presents compatibility challenges. Retrofitting conventional separation trains with electrolyte-based technologies often requires substantial process modifications and capital investment, creating adoption barriers despite potential performance benefits.
Recent research has highlighted the need for more sophisticated modeling approaches that can accurately predict electrolyte behavior under dynamic operating conditions. Current models often fail to capture the complex interplay between conductivity, ion mobility, and separation efficiency across varying process parameters.
Current Electrolyte Conductivity Separation Solutions
01 Electrolyte additives for enhanced conductivity
Various additives can be incorporated into electrolytes to enhance their ionic conductivity. These additives include salts, ionic liquids, and conductive polymers that increase the concentration of charge carriers or facilitate their movement through the electrolyte medium. The improved conductivity leads to better performance in applications such as batteries, fuel cells, and electrochemical devices.- Electrolyte additives for enhanced conductivity: Various additives can be incorporated into electrolytes to enhance their ionic conductivity. These additives include salts, ionic liquids, and conductive polymers that increase the concentration of charge carriers or facilitate their movement through the electrolyte medium. The improved conductivity leads to better performance in applications such as batteries, fuel cells, and electrochemical sensors.
- Polymer-based electrolyte systems: Polymer-based electrolytes offer advantages in terms of mechanical stability and safety compared to liquid electrolytes. These systems incorporate conductive polymers or polymer matrices infused with ionic compounds to achieve sufficient conductivity. Modifications to the polymer structure, such as cross-linking or the addition of plasticizers, can be employed to optimize the balance between mechanical properties and ionic conductivity.
- Temperature effects on electrolyte conductivity: The conductivity of electrolytes is significantly influenced by temperature. Higher temperatures generally increase ionic mobility and conductivity, while lower temperatures can lead to decreased performance. Various formulations and additives are developed to maintain adequate conductivity across a wide temperature range, which is crucial for applications operating in diverse environmental conditions.
- Solid-state electrolyte technologies: Solid-state electrolytes represent an advanced approach to achieving high conductivity without the safety risks associated with liquid electrolytes. These materials include ceramic electrolytes, glass electrolytes, and composite systems that combine different types of solid conductors. Research focuses on improving room-temperature conductivity and interfacial contact with electrodes to enable practical applications in energy storage devices.
- Measurement and characterization techniques: Accurate measurement and characterization of electrolyte conductivity are essential for research and quality control. Various techniques are employed, including impedance spectroscopy, four-probe methods, and specialized conductivity cells. These measurements help in understanding the fundamental mechanisms of ionic transport and in optimizing electrolyte formulations for specific applications.
02 Polymer-based electrolyte systems
Polymer-based electrolytes offer advantages in terms of mechanical stability and safety compared to liquid electrolytes. These systems incorporate conductive polymers or polymer matrices infused with ionic compounds to achieve suitable conductivity levels. Modifications to polymer structure, cross-linking density, and the addition of plasticizers can significantly impact the ionic conductivity of these electrolyte systems.Expand Specific Solutions03 Temperature effects on electrolyte conductivity
The conductivity of electrolytes is highly temperature-dependent, with most systems showing increased conductivity at elevated temperatures due to enhanced ion mobility. Specialized electrolyte formulations can be designed to maintain adequate conductivity across wide temperature ranges, which is crucial for applications in extreme environments. Understanding these temperature-conductivity relationships is essential for optimizing electrolyte performance in various devices.Expand Specific Solutions04 Solid-state electrolyte conductivity enhancement
Solid-state electrolytes offer safety advantages but typically suffer from lower conductivity compared to liquid systems. Various approaches to enhance solid electrolyte conductivity include doping with aliovalent ions, creating engineered interfaces, controlling grain boundary properties, and developing composite structures. These techniques aim to create conduction pathways that facilitate ion transport while maintaining the mechanical and safety benefits of solid systems.Expand Specific Solutions05 Measurement and characterization of electrolyte conductivity
Accurate measurement and characterization of electrolyte conductivity are essential for research and quality control. Various techniques including impedance spectroscopy, four-probe methods, and specialized conductivity cells are employed to determine ionic conductivity under different conditions. These measurements help in understanding the fundamental transport mechanisms and in optimizing electrolyte formulations for specific applications.Expand Specific Solutions
Leading Companies and Research Institutions in Electrolyte Technology
Electrolyte conductivity plays a critical role in product separation technologies, with the market currently in a growth phase driven by increasing demand for energy storage solutions and advanced separation processes. The global market size is expanding rapidly, projected to reach significant value by 2030. From a technological maturity perspective, established players like LG Energy Solution, BASF, and Panasonic have developed commercial applications with proven performance, while research institutions such as California Institute of Technology and Wisconsin Alumni Research Foundation continue to advance fundamental understanding. Emerging companies like Dioxycle and Xiamen Hithium are introducing innovative approaches to electrolyte conductivity optimization. The competitive landscape shows a mix of large chemical and electronics corporations alongside specialized material science companies, with increasing focus on sustainable and high-efficiency electrolyte systems for next-generation separation technologies.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced electrolyte formulations that enhance ionic conductivity through the incorporation of fluorinated solvents and lithium salts. Their proprietary electrolyte systems utilize a combination of linear and cyclic carbonates with optimized salt concentrations to achieve superior ionic transport properties. The company has pioneered the use of electrolyte additives that form stable solid electrolyte interphase (SEI) layers, which significantly improve the separation of electrode materials while maintaining high lithium-ion conductivity. Their recent innovations include temperature-resistant electrolyte formulations that maintain consistent conductivity across a wide operating range (-30°C to 60°C), enabling more efficient product separation in energy storage applications. LG has also developed dual-salt electrolyte systems that demonstrate enhanced aluminum corrosion resistance while maintaining high ionic conductivity.
Strengths: Superior ionic conductivity across wide temperature ranges; excellent SEI formation properties leading to better electrode separation; advanced manufacturing capabilities for consistent quality. Weaknesses: Higher production costs compared to standard electrolyte formulations; some proprietary additives have limited availability; potential long-term stability issues in extreme environmental conditions.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed a multi-component electrolyte system that optimizes conductivity while enhancing product separation efficiency in battery applications. Their technology employs a combination of ethylene carbonate, diethyl carbonate, and proprietary additives that create a stable interface between electrodes and electrolyte. Panasonic's approach focuses on controlling the solvation structure of lithium ions to facilitate faster ion transport while maintaining separation integrity. Their electrolyte formulations incorporate flame-retardant additives that do not compromise ionic conductivity, addressing safety concerns in energy storage applications. The company has also pioneered the use of ionic liquid-based electrolytes with high thermal stability and wide electrochemical windows, enabling more efficient separation processes in specialized applications. Panasonic's recent innovations include electrolyte systems with self-healing properties that can restore conductivity pathways after mechanical stress or thermal cycling.
Strengths: Excellent balance between conductivity and safety features; proven track record in commercial battery applications; robust manufacturing processes ensuring consistent quality. Weaknesses: Higher cost compared to conventional electrolyte systems; some formulations show performance degradation at extreme temperatures; intellectual property restrictions limiting wider adoption.
Key Patents and Research in Electrolyte Conductivity Enhancement
Product separation from electrolyte salts maintained in molten state
PatentInactiveUS4564689A
Innovation
- The use of low-melting salts, such as lithium acetate and potassium acetate, allows for the separation of γ-vinyl-γ-butyrolactone from reaction mixtures maintained in a fluid state, enabling the solvent and product to be evaporated and separated while the salt residue remains molten, facilitating its return to the reaction zone.
Electrolytic perovskites
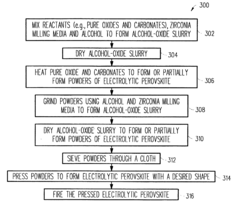
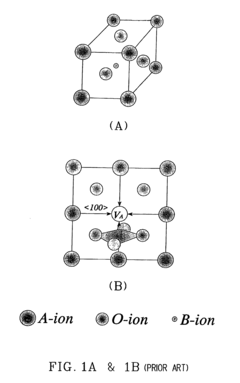
PatentInactiveUS20040062968A1
Innovation
- Development of electrolytic perovskites with specific compositions such as Li1/8Na3/8La1/4Zr1/4O3 and Li1/8K1/2La1/8NbO3 that exhibit high Li+, H+, Cu+, Ag+, Na+, or Mg2+ conductivity (>10^-5 S/cm) in the 0-400°C temperature range, enabling the creation of solid proton conductors for use in fuel cells and other devices.
Environmental Impact of Electrolyte Separation Processes
Electrolyte separation processes, while offering significant advantages in product separation and purification, carry substantial environmental implications that warrant careful consideration. The environmental footprint of these technologies extends across multiple dimensions, from resource consumption to waste generation and energy utilization.
Water usage represents a primary environmental concern, as electrolyte-based separation typically requires substantial volumes of high-purity water for solution preparation and system maintenance. In regions facing water scarcity, this dependency creates additional pressure on already strained resources. Furthermore, the chemicals employed as electrolytes—often including acids, bases, and various salts—may introduce toxicity risks if discharged without proper treatment.
Energy consumption constitutes another significant environmental factor. Electrolyte separation processes frequently demand considerable electrical input to maintain ionic gradients and drive separation mechanisms. This energy requirement translates directly into carbon emissions when sourced from fossil fuel-based electricity generation, contributing to climate change impacts. Recent industry analyses suggest that electrolyte separation can account for 15-30% of total energy consumption in certain chemical manufacturing operations.
Waste management challenges also emerge from these processes. Spent electrolyte solutions often contain contaminants that require specialized treatment before disposal. The regeneration of electrolytes, while reducing waste volume, typically involves additional chemical processes with their own environmental implications. Moreover, membrane fouling and degradation in electrolyte-based systems generate solid waste requiring appropriate disposal protocols.
The environmental profile varies significantly across different electrolyte separation technologies. Membrane-based systems generally demonstrate lower environmental impacts compared to traditional chemical precipitation methods, primarily due to reduced chemical consumption and waste generation. Emerging technologies utilizing biodegradable or recoverable electrolytes show particular promise in minimizing environmental footprints.
Life cycle assessments of electrolyte separation processes reveal that environmental impacts extend beyond operational considerations to include raw material extraction, equipment manufacturing, and end-of-life disposal. This comprehensive perspective highlights opportunities for environmental optimization throughout the technology lifecycle, from material selection to process design and waste management strategies.
Regulatory frameworks increasingly recognize these environmental dimensions, with stricter standards emerging for wastewater discharge, chemical handling, and energy efficiency in industrial separation processes. Forward-thinking organizations are proactively addressing these challenges through closed-loop systems, energy recovery mechanisms, and alternative electrolyte formulations with reduced environmental hazards.
Water usage represents a primary environmental concern, as electrolyte-based separation typically requires substantial volumes of high-purity water for solution preparation and system maintenance. In regions facing water scarcity, this dependency creates additional pressure on already strained resources. Furthermore, the chemicals employed as electrolytes—often including acids, bases, and various salts—may introduce toxicity risks if discharged without proper treatment.
Energy consumption constitutes another significant environmental factor. Electrolyte separation processes frequently demand considerable electrical input to maintain ionic gradients and drive separation mechanisms. This energy requirement translates directly into carbon emissions when sourced from fossil fuel-based electricity generation, contributing to climate change impacts. Recent industry analyses suggest that electrolyte separation can account for 15-30% of total energy consumption in certain chemical manufacturing operations.
Waste management challenges also emerge from these processes. Spent electrolyte solutions often contain contaminants that require specialized treatment before disposal. The regeneration of electrolytes, while reducing waste volume, typically involves additional chemical processes with their own environmental implications. Moreover, membrane fouling and degradation in electrolyte-based systems generate solid waste requiring appropriate disposal protocols.
The environmental profile varies significantly across different electrolyte separation technologies. Membrane-based systems generally demonstrate lower environmental impacts compared to traditional chemical precipitation methods, primarily due to reduced chemical consumption and waste generation. Emerging technologies utilizing biodegradable or recoverable electrolytes show particular promise in minimizing environmental footprints.
Life cycle assessments of electrolyte separation processes reveal that environmental impacts extend beyond operational considerations to include raw material extraction, equipment manufacturing, and end-of-life disposal. This comprehensive perspective highlights opportunities for environmental optimization throughout the technology lifecycle, from material selection to process design and waste management strategies.
Regulatory frameworks increasingly recognize these environmental dimensions, with stricter standards emerging for wastewater discharge, chemical handling, and energy efficiency in industrial separation processes. Forward-thinking organizations are proactively addressing these challenges through closed-loop systems, energy recovery mechanisms, and alternative electrolyte formulations with reduced environmental hazards.
Regulatory Framework for Industrial Electrolyte Applications
The regulatory landscape governing electrolyte applications in industrial settings has evolved significantly over the past decade, reflecting growing concerns about environmental impact, worker safety, and product quality. At the international level, organizations such as the International Organization for Standardization (ISO) have established comprehensive standards for electrolyte conductivity measurements and applications in separation processes, with ISO 7888 specifically addressing conductivity measurement protocols.
In the United States, the Environmental Protection Agency (EPA) regulates industrial electrolyte applications through the Clean Water Act and the Resource Conservation and Recovery Act, imposing strict guidelines on discharge limits for electrolyte-containing solutions. The Food and Drug Administration (FDA) maintains additional oversight for electrolyte applications in pharmaceutical and food processing industries, where product separation processes must comply with Current Good Manufacturing Practice (cGMP) regulations.
The European Union has implemented the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, which requires comprehensive safety assessments for electrolytes used in industrial separation processes. Additionally, the EU's Restriction of Hazardous Substances (RoHS) directive limits the use of certain hazardous substances in electrical and electronic equipment, affecting electrolyte formulations used in electrochemical separation technologies.
Emerging economies, particularly China and India, have recently strengthened their regulatory frameworks. China's Ministry of Ecology and Environment has introduced the Measures for Environmental Management of New Chemical Substances, which includes specific provisions for electrolytes used in industrial applications. Similarly, India's Central Pollution Control Board has established guidelines for effluent discharge standards that directly impact electrolyte-based separation processes.
Industry-specific regulations also play a crucial role in shaping electrolyte applications. The semiconductor industry follows the SEMI standards, which include detailed specifications for ultrapure water and chemical purity requirements essential for electrolyte-based separation processes. The pharmaceutical industry adheres to International Conference on Harmonisation (ICH) guidelines, which establish parameters for electrolyte conductivity in various separation techniques.
Compliance with these regulatory frameworks necessitates rigorous documentation, regular testing, and continuous monitoring of electrolyte conductivity parameters. Many jurisdictions require periodic reporting of conductivity measurements and verification that separation processes maintain specified electrolyte concentration ranges. Non-compliance can result in substantial penalties, production shutdowns, and potential market access restrictions.
As sustainability concerns grow, regulatory bodies are increasingly focusing on the environmental footprint of electrolyte-based separation processes, driving the development of greener alternatives and more efficient recovery systems. This regulatory trend is expected to accelerate, potentially transforming industrial practices in electrolyte management and product separation technologies.
In the United States, the Environmental Protection Agency (EPA) regulates industrial electrolyte applications through the Clean Water Act and the Resource Conservation and Recovery Act, imposing strict guidelines on discharge limits for electrolyte-containing solutions. The Food and Drug Administration (FDA) maintains additional oversight for electrolyte applications in pharmaceutical and food processing industries, where product separation processes must comply with Current Good Manufacturing Practice (cGMP) regulations.
The European Union has implemented the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation, which requires comprehensive safety assessments for electrolytes used in industrial separation processes. Additionally, the EU's Restriction of Hazardous Substances (RoHS) directive limits the use of certain hazardous substances in electrical and electronic equipment, affecting electrolyte formulations used in electrochemical separation technologies.
Emerging economies, particularly China and India, have recently strengthened their regulatory frameworks. China's Ministry of Ecology and Environment has introduced the Measures for Environmental Management of New Chemical Substances, which includes specific provisions for electrolytes used in industrial applications. Similarly, India's Central Pollution Control Board has established guidelines for effluent discharge standards that directly impact electrolyte-based separation processes.
Industry-specific regulations also play a crucial role in shaping electrolyte applications. The semiconductor industry follows the SEMI standards, which include detailed specifications for ultrapure water and chemical purity requirements essential for electrolyte-based separation processes. The pharmaceutical industry adheres to International Conference on Harmonisation (ICH) guidelines, which establish parameters for electrolyte conductivity in various separation techniques.
Compliance with these regulatory frameworks necessitates rigorous documentation, regular testing, and continuous monitoring of electrolyte conductivity parameters. Many jurisdictions require periodic reporting of conductivity measurements and verification that separation processes maintain specified electrolyte concentration ranges. Non-compliance can result in substantial penalties, production shutdowns, and potential market access restrictions.
As sustainability concerns grow, regulatory bodies are increasingly focusing on the environmental footprint of electrolyte-based separation processes, driving the development of greener alternatives and more efficient recovery systems. This regulatory trend is expected to accelerate, potentially transforming industrial practices in electrolyte management and product separation technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!