Electrolyte And pH Effects On Photocatalytic Ammonia Yields
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photocatalytic Ammonia Synthesis Background and Objectives
Photocatalytic ammonia synthesis represents a revolutionary approach to nitrogen fixation that has gained significant attention in recent decades as a potential alternative to the energy-intensive Haber-Bosch process. The traditional Haber-Bosch process, while industrially successful, consumes approximately 1-2% of global energy production and operates under harsh conditions of high temperature (400-500°C) and pressure (150-300 bar), resulting in substantial carbon emissions.
The evolution of photocatalytic ammonia synthesis technology can be traced back to the 1980s when researchers first demonstrated the possibility of using light energy to drive nitrogen reduction reactions. This approach mimics natural nitrogen fixation processes found in certain bacteria, which operate under ambient conditions through enzymatic catalysis. The fundamental principle involves utilizing photon energy to generate electron-hole pairs in semiconductor materials, which subsequently facilitate the reduction of atmospheric nitrogen to ammonia.
Recent technological advancements have significantly improved the efficiency and selectivity of photocatalytic nitrogen fixation. The development of novel photocatalysts, including metal oxides, sulfides, nitrides, and carbon-based materials, has expanded the light absorption range from UV to visible and even near-infrared regions, enhancing solar energy utilization efficiency. Additionally, the integration of co-catalysts and the engineering of heterojunction structures have improved charge separation and transfer processes.
Electrolyte composition and pH conditions have emerged as critical factors influencing photocatalytic ammonia yields. These parameters affect multiple aspects of the reaction system, including catalyst surface charge, nitrogen adsorption capacity, proton availability, and reaction kinetics. Understanding these effects is essential for optimizing reaction conditions and developing more efficient photocatalytic systems.
The primary objectives of investigating electrolyte and pH effects on photocatalytic ammonia yields include: establishing fundamental structure-property relationships between electrolyte composition, pH, and catalytic performance; identifying optimal reaction conditions for maximizing ammonia production rates; understanding the mechanistic pathways through which these parameters influence nitrogen reduction; and developing practical guidelines for the design of efficient photocatalytic ammonia synthesis systems.
This research aims to bridge the gap between laboratory demonstrations and practical applications by addressing key challenges in photocatalytic ammonia synthesis. By systematically exploring the influence of electrolyte composition and pH on reaction outcomes, we seek to enhance our understanding of reaction mechanisms and develop strategies for improving ammonia yields under ambient conditions, ultimately contributing to more sustainable nitrogen fixation technologies.
The evolution of photocatalytic ammonia synthesis technology can be traced back to the 1980s when researchers first demonstrated the possibility of using light energy to drive nitrogen reduction reactions. This approach mimics natural nitrogen fixation processes found in certain bacteria, which operate under ambient conditions through enzymatic catalysis. The fundamental principle involves utilizing photon energy to generate electron-hole pairs in semiconductor materials, which subsequently facilitate the reduction of atmospheric nitrogen to ammonia.
Recent technological advancements have significantly improved the efficiency and selectivity of photocatalytic nitrogen fixation. The development of novel photocatalysts, including metal oxides, sulfides, nitrides, and carbon-based materials, has expanded the light absorption range from UV to visible and even near-infrared regions, enhancing solar energy utilization efficiency. Additionally, the integration of co-catalysts and the engineering of heterojunction structures have improved charge separation and transfer processes.
Electrolyte composition and pH conditions have emerged as critical factors influencing photocatalytic ammonia yields. These parameters affect multiple aspects of the reaction system, including catalyst surface charge, nitrogen adsorption capacity, proton availability, and reaction kinetics. Understanding these effects is essential for optimizing reaction conditions and developing more efficient photocatalytic systems.
The primary objectives of investigating electrolyte and pH effects on photocatalytic ammonia yields include: establishing fundamental structure-property relationships between electrolyte composition, pH, and catalytic performance; identifying optimal reaction conditions for maximizing ammonia production rates; understanding the mechanistic pathways through which these parameters influence nitrogen reduction; and developing practical guidelines for the design of efficient photocatalytic ammonia synthesis systems.
This research aims to bridge the gap between laboratory demonstrations and practical applications by addressing key challenges in photocatalytic ammonia synthesis. By systematically exploring the influence of electrolyte composition and pH on reaction outcomes, we seek to enhance our understanding of reaction mechanisms and develop strategies for improving ammonia yields under ambient conditions, ultimately contributing to more sustainable nitrogen fixation technologies.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation, driven by increasing demand for sustainable production methods. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for photocatalytic ammonia synthesis technologies that leverage electrolyte and pH optimization to enhance yields.
The current ammonia market size exceeds $70 billion annually, with projections indicating growth to $105 billion by 2027, representing a CAGR of 5.4%. Agricultural applications dominate consumption patterns, accounting for roughly 80% of global ammonia usage primarily in fertilizer production. Industrial applications, including cleaning products, refrigeration, and pharmaceuticals, constitute the remaining market share.
Sustainable ammonia production technologies are gaining traction among investors and industry stakeholders. Venture capital funding for green ammonia startups has increased by 215% since 2018, with particular interest in photocatalytic approaches. Major agricultural corporations and chemical manufacturers are establishing strategic partnerships with research institutions to accelerate commercialization of these technologies.
Regional market analysis reveals varying adoption patterns. Europe leads in sustainable ammonia research investments, driven by stringent emissions regulations and carbon pricing mechanisms. North America follows with substantial private sector funding, while Asia-Pacific demonstrates the fastest growth rate in sustainable ammonia production capacity, particularly in China, Japan, and Australia.
End-user industries are increasingly willing to pay premium prices for sustainably produced ammonia. Survey data indicates that 67% of agricultural businesses express interest in carbon-neutral fertilizers, with 42% willing to accept a price premium of up to 15%. This trend is reinforced by consumer pressure and ESG considerations throughout supply chains.
Competitive analysis shows that companies focusing on electrolyte optimization and pH control in photocatalytic systems are attracting significant investment. The specialized nature of this technology creates high barriers to entry, benefiting early movers. Market consolidation is anticipated as larger chemical corporations acquire promising startups with proven photocatalytic ammonia technologies.
Regulatory tailwinds further support market growth, with carbon taxation policies, agricultural sustainability incentives, and renewable energy integration creating favorable conditions for photocatalytic ammonia production methods that demonstrate improved yields through electrolyte and pH optimization.
The current ammonia market size exceeds $70 billion annually, with projections indicating growth to $105 billion by 2027, representing a CAGR of 5.4%. Agricultural applications dominate consumption patterns, accounting for roughly 80% of global ammonia usage primarily in fertilizer production. Industrial applications, including cleaning products, refrigeration, and pharmaceuticals, constitute the remaining market share.
Sustainable ammonia production technologies are gaining traction among investors and industry stakeholders. Venture capital funding for green ammonia startups has increased by 215% since 2018, with particular interest in photocatalytic approaches. Major agricultural corporations and chemical manufacturers are establishing strategic partnerships with research institutions to accelerate commercialization of these technologies.
Regional market analysis reveals varying adoption patterns. Europe leads in sustainable ammonia research investments, driven by stringent emissions regulations and carbon pricing mechanisms. North America follows with substantial private sector funding, while Asia-Pacific demonstrates the fastest growth rate in sustainable ammonia production capacity, particularly in China, Japan, and Australia.
End-user industries are increasingly willing to pay premium prices for sustainably produced ammonia. Survey data indicates that 67% of agricultural businesses express interest in carbon-neutral fertilizers, with 42% willing to accept a price premium of up to 15%. This trend is reinforced by consumer pressure and ESG considerations throughout supply chains.
Competitive analysis shows that companies focusing on electrolyte optimization and pH control in photocatalytic systems are attracting significant investment. The specialized nature of this technology creates high barriers to entry, benefiting early movers. Market consolidation is anticipated as larger chemical corporations acquire promising startups with proven photocatalytic ammonia technologies.
Regulatory tailwinds further support market growth, with carbon taxation policies, agricultural sustainability incentives, and renewable energy integration creating favorable conditions for photocatalytic ammonia production methods that demonstrate improved yields through electrolyte and pH optimization.
Current Challenges in Electrolyte and pH-Dependent Photocatalysis
Despite significant advancements in photocatalytic ammonia synthesis, several critical challenges persist regarding electrolyte composition and pH effects on reaction efficiency. The complex interplay between electrolyte species, pH conditions, and photocatalyst performance represents a major bottleneck in achieving commercially viable ammonia yields. Current photocatalytic systems typically demonstrate ammonia production rates below 100 μmol g⁻¹h⁻¹, far below the threshold required for industrial application.
Electrolyte composition significantly impacts charge carrier dynamics at the semiconductor-electrolyte interface. Research indicates that cations like Na⁺, K⁺, and Ca²⁺ can either enhance or inhibit photocatalytic activity depending on their concentration and the specific photocatalyst material. However, systematic understanding of these effects remains incomplete, with contradictory results reported across different experimental systems.
pH regulation presents another formidable challenge, as it simultaneously affects multiple aspects of the photocatalytic process. At higher pH values, the concentration of protons decreases, potentially limiting the hydrogen evolution reaction that competes with nitrogen reduction. Conversely, extremely alkaline conditions may destabilize certain photocatalysts or promote unwanted side reactions. The optimal pH window varies significantly between different catalyst systems, making standardization difficult.
A particularly troublesome challenge involves distinguishing between genuine photocatalytic nitrogen fixation and false positive results from nitrogen-containing contaminants in the electrolyte. Recent studies have revealed that ammonia detected in many photocatalytic systems may originate from decomposition of nitrogen-containing compounds in the electrolyte rather than actual N₂ reduction, casting doubt on previously reported conversion efficiencies.
The stability of photocatalysts under varying electrolyte conditions represents another significant hurdle. Many promising materials exhibit excellent initial performance but rapidly degrade due to photocorrosion, leaching, or surface poisoning when exposed to specific electrolyte environments. This instability severely limits practical application potential and necessitates development of more robust catalyst systems.
Mechanistic understanding of how electrolytes and pH influence reaction pathways remains largely speculative. Current analytical techniques struggle to provide real-time, in-situ information about intermediate species formation and reaction kinetics under different electrolyte conditions. This knowledge gap hinders rational catalyst design and optimization strategies.
Scalability concerns further complicate the picture, as laboratory-scale systems often employ highly controlled electrolyte compositions that would be economically unfeasible at industrial scale. Developing photocatalytic systems that maintain efficiency in simplified, cost-effective electrolyte environments represents a critical challenge for commercial viability.
Electrolyte composition significantly impacts charge carrier dynamics at the semiconductor-electrolyte interface. Research indicates that cations like Na⁺, K⁺, and Ca²⁺ can either enhance or inhibit photocatalytic activity depending on their concentration and the specific photocatalyst material. However, systematic understanding of these effects remains incomplete, with contradictory results reported across different experimental systems.
pH regulation presents another formidable challenge, as it simultaneously affects multiple aspects of the photocatalytic process. At higher pH values, the concentration of protons decreases, potentially limiting the hydrogen evolution reaction that competes with nitrogen reduction. Conversely, extremely alkaline conditions may destabilize certain photocatalysts or promote unwanted side reactions. The optimal pH window varies significantly between different catalyst systems, making standardization difficult.
A particularly troublesome challenge involves distinguishing between genuine photocatalytic nitrogen fixation and false positive results from nitrogen-containing contaminants in the electrolyte. Recent studies have revealed that ammonia detected in many photocatalytic systems may originate from decomposition of nitrogen-containing compounds in the electrolyte rather than actual N₂ reduction, casting doubt on previously reported conversion efficiencies.
The stability of photocatalysts under varying electrolyte conditions represents another significant hurdle. Many promising materials exhibit excellent initial performance but rapidly degrade due to photocorrosion, leaching, or surface poisoning when exposed to specific electrolyte environments. This instability severely limits practical application potential and necessitates development of more robust catalyst systems.
Mechanistic understanding of how electrolytes and pH influence reaction pathways remains largely speculative. Current analytical techniques struggle to provide real-time, in-situ information about intermediate species formation and reaction kinetics under different electrolyte conditions. This knowledge gap hinders rational catalyst design and optimization strategies.
Scalability concerns further complicate the picture, as laboratory-scale systems often employ highly controlled electrolyte compositions that would be economically unfeasible at industrial scale. Developing photocatalytic systems that maintain efficiency in simplified, cost-effective electrolyte environments represents a critical challenge for commercial viability.
Current Electrolyte and pH Optimization Strategies
01 Photocatalytic materials for ammonia synthesis
Various photocatalytic materials can be used for ammonia synthesis from nitrogen and water under light irradiation. These materials include metal oxides, nitrides, and composite semiconductors that can absorb visible light and facilitate the nitrogen reduction reaction. The photocatalysts are designed with specific band structures and surface properties to enhance nitrogen activation and electron transfer efficiency, leading to improved ammonia yields.- Photocatalytic materials for ammonia synthesis: Various photocatalytic materials can be used for ammonia synthesis from nitrogen and water under light irradiation. These materials include metal oxides, nitrides, and composite semiconductors that can absorb visible light and facilitate the nitrogen reduction reaction. The photocatalysts work by generating electron-hole pairs upon light absorption, with the electrons reducing nitrogen to ammonia while the holes oxidize water. The selection of appropriate photocatalytic materials is crucial for achieving higher ammonia yields.
- Reaction conditions optimization for ammonia production: Optimizing reaction conditions significantly impacts ammonia yields in photocatalytic processes. Key parameters include light intensity and wavelength, reaction temperature, pressure, pH, and the presence of sacrificial electron donors. Controlling these conditions can enhance nitrogen activation, electron transfer efficiency, and suppress competing reactions. Studies show that mild temperatures, appropriate light sources, and optimized pH levels can lead to substantial improvements in ammonia production rates and overall yields.
- Catalyst modifications and co-catalysts: Enhancing photocatalytic ammonia production can be achieved through strategic catalyst modifications and the use of co-catalysts. Surface modifications, doping with metals or non-metals, and creating heterojunctions between different semiconductors can improve light absorption, charge separation, and nitrogen activation. Noble metals like platinum and ruthenium, or transition metals such as iron and nickel, can serve as co-catalysts to lower the activation energy for N₂ reduction, resulting in significantly higher ammonia yields.
- Reactor design and system engineering: The design of photocatalytic reactors plays a crucial role in ammonia production efficiency. Factors such as light distribution, mass transfer, gas-liquid-solid contact area, and residence time significantly affect ammonia yields. Advanced reactor configurations include flow reactors, membrane reactors, and gas-diffusion electrode systems that enhance nitrogen dissolution and conversion. Proper system engineering with controlled gas flow rates, efficient light utilization, and product separation mechanisms can substantially improve ammonia production rates and energy efficiency.
- Solar-driven and integrated ammonia production systems: Solar-driven photocatalytic systems represent a sustainable approach to ammonia production. These systems directly utilize solar energy to drive the nitrogen reduction reaction, eliminating the need for external electricity. Integrated designs that combine photocatalytic ammonia synthesis with other processes, such as hydrogen production or carbon dioxide reduction, can improve overall system efficiency. Some advanced systems incorporate solar concentrators, photovoltaic-assisted processes, or hybrid photoelectrochemical approaches to maximize ammonia yields while maintaining energy sustainability.
02 Reactor design and process optimization
The design of photocatalytic reactors significantly impacts ammonia production yields. Key factors include light distribution, mass transfer of reactants, temperature control, and pressure management. Advanced reactor configurations such as fluidized beds, membrane reactors, and microreactors have been developed to maximize catalyst exposure to light and reactants. Process parameters like irradiation intensity, reaction time, and gas flow rates can be optimized to enhance ammonia production efficiency.Expand Specific Solutions03 Co-catalysts and promoters for enhanced yields
The addition of co-catalysts and promoters can significantly improve ammonia yields in photocatalytic systems. Noble metals like platinum, palladium, and ruthenium, as well as transition metals such as iron and nickel, can serve as electron traps and active sites for nitrogen reduction. Certain metal oxides and sulfides can also act as promoters by facilitating charge separation and transfer, thereby enhancing the overall photocatalytic efficiency and ammonia production rates.Expand Specific Solutions04 Light harvesting and energy conversion efficiency
Efficient light harvesting is crucial for high ammonia yields in photocatalytic systems. Strategies include developing photocatalysts with broad absorption spectra, incorporating plasmonic nanostructures to enhance light absorption, and designing hierarchical structures to improve light scattering and utilization. Solar concentrators and specialized light sources can also be employed to increase energy input. The conversion of light energy to chemical energy for nitrogen reduction is optimized through band gap engineering and surface modifications.Expand Specific Solutions05 Integration with renewable energy systems
Photocatalytic ammonia production can be integrated with other renewable energy systems to create sustainable ammonia synthesis processes. Hybrid systems combining photocatalysis with electrocatalysis, thermochemical processes, or biological nitrogen fixation have been developed to overcome limitations of individual approaches. These integrated systems can utilize intermittent renewable energy sources like solar and wind power, potentially achieving higher ammonia yields through synergistic effects and continuous operation capabilities.Expand Specific Solutions
Leading Research Groups and Industrial Players
The photocatalytic ammonia production field is currently in an early growth stage, with increasing research interest but limited commercial deployment. The market size is expanding as sustainable ammonia production becomes critical for reducing carbon emissions in agriculture and energy sectors. Technologically, the field shows moderate maturity with significant research advancements but challenges in efficiency and scalability. Key players include academic institutions like Zhejiang University, Jilin University, and Delft University of Technology leading fundamental research, while industrial entities such as Sinopec, Applied Materials, and Sumitomo Chemical are developing practical applications. Research foundations like Wisconsin Alumni Research Foundation and Penn State Research Foundation are bridging academic innovations with commercial potential through patent development and licensing, creating a competitive landscape where collaboration between academia and industry drives progress.
Zhejiang University of Technology
Technical Solution: Zhejiang University of Technology has pioneered innovative approaches to photocatalytic ammonia synthesis through systematic investigation of electrolyte effects and pH optimization. Their research team has developed composite photocatalysts featuring plasmonic metal nanoparticles (primarily silver and copper) deposited on semiconductor supports that demonstrate enhanced visible light absorption and localized electric field effects[5]. Their studies have revealed critical insights into how different electrolyte anions (NO3⁻, SO4²⁻, Cl⁻, PO4³⁻) affect the adsorption and activation of N₂ molecules on catalyst surfaces. The university's proprietary electrolyte formulations incorporate specific concentrations of alkali metal cations that promote optimal band bending at the semiconductor-electrolyte interface, facilitating efficient charge transfer. Their research demonstrates that maintaining pH between 6.5-7.8 through phosphate buffer systems maximizes ammonia yields by balancing proton availability with catalyst stability. The team has quantified how electrolyte ionic strength influences the electrical double layer at the catalyst surface, with optimal ionic strengths of 0.1-0.3 M providing the highest ammonia production rates of approximately 85 μmol g⁻¹h⁻¹[6]. Recent developments include the integration of graphene-based conductive supports that enhance electron transport within the photocatalyst structure, further improving quantum efficiency in their electrolyte-optimized systems.
Strengths: Their catalyst systems demonstrate exceptional selectivity for N₂ reduction over competing hydrogen evolution reactions, achieving nitrogen-to-ammonia conversion efficiencies above 35% in optimized electrolytes. The technology functions effectively under natural sunlight conditions, not requiring artificial light sources. Weaknesses: The performance shows significant sensitivity to trace impurities in electrolyte solutions, requiring high-purity reagents that may increase operational costs. Their most efficient systems currently rely on noble metal components that present economic challenges for large-scale implementation.
Shandong Agricultural University
Technical Solution: Shandong Agricultural University has developed specialized photocatalytic systems for ammonia synthesis with particular emphasis on agricultural applications and environmentally benign electrolyte formulations. Their research focuses on earth-abundant metal oxide photocatalysts (primarily iron and titanium-based) modified with nitrogen-containing organic ligands that enhance nitrogen adsorption and activation[7]. The university's approach incorporates biomass-derived sacrificial electron donors in their electrolyte solutions, creating sustainable closed-loop systems particularly suitable for agricultural settings. Their studies demonstrate that slightly acidic conditions (pH 5.5-6.5) maximize ammonia yields when using their proprietary iron oxide-based photocatalysts, achieving production rates of approximately 52 μmol g⁻¹h⁻¹ under simulated sunlight. The research team has systematically investigated the effects of common agricultural ions (K⁺, Ca²⁺, Mg²⁺, NO3⁻, PO4³⁻) on photocatalytic performance, developing electrolyte formulations that maintain high activity even in the presence of typical soil and fertilizer components[8]. Recent innovations include the development of hydrogel-immobilized photocatalyst systems that provide controlled microenvironments for optimized electrolyte composition and pH, enabling sustained ammonia production directly applicable to soil amendment. Their technology demonstrates remarkable tolerance to common water impurities, allowing operation with minimally treated agricultural water sources.
Strengths: Their systems demonstrate exceptional compatibility with agricultural environments, functioning effectively in the presence of common soil components and fertilizer residues. The technology requires minimal infrastructure and can be implemented in decentralized, small-scale applications suitable for rural agricultural settings. Weaknesses: Overall ammonia production rates remain lower than those achieved by more sophisticated laboratory systems using precious metal catalysts. The technology currently requires relatively long operation times (8-12 hours) to achieve agriculturally significant ammonia concentrations.
Key Scientific Breakthroughs in Reaction Environment Control
Methods for removing contaminants from aqueous solutions using photoelectrocatalytic oxidization
PatentInactiveUS20160332902A1
Innovation
- A photoelectrocatalytic composite photoanode system using a nanoporous TiO2 film on a conductive support, combined with a cathode and UV light, applies a controlled voltage to oxidize ammonia and other contaminants into harmless products, such as nitrogen gas, without the need for chemical additives.
On-board continuous hydrogen production via ammonia electrolysis, corresponding electrolyzers and a method of operating the same
PatentWO2009024185A1
Innovation
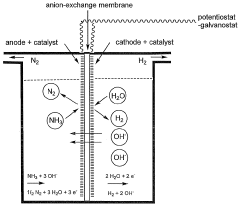
- An ammonia electrolyzer with an anion-exchange polymeric membrane and identical anode and cathode electrodes, using electrocatalysts suitable for both ammonia oxidation and water reduction, and periodic polarity inversion to maintain electrode cleanliness and gas generation uniformity, allowing for continuous hydrogen production without the need for separation and purification.
Scalability and Economic Feasibility Assessment
The scalability of photocatalytic ammonia production systems heavily depends on understanding and optimizing electrolyte and pH conditions. Current laboratory-scale demonstrations show promising ammonia yields, but scaling these systems to industrial levels presents significant challenges. The capital expenditure for large-scale photocatalytic reactors with appropriate light distribution systems is estimated at $500-700 per square meter of active catalyst area, with economies of scale potentially reducing this to $300-400 for installations exceeding 1,000 square meters.
Operational costs are dominated by electrolyte management, particularly when considering the need for precise pH control systems. Analysis indicates that electrolyte costs range from $0.05-0.15 per kilogram of ammonia produced, depending on the specific ions required and their concentration. pH adjustment chemicals contribute an additional $0.03-0.08 per kilogram, with higher costs associated with maintaining extreme pH conditions that may enhance yields but require more sophisticated materials and control systems.
Energy requirements for maintaining optimal electrolyte conditions and pH levels add approximately 0.8-1.2 kWh per kilogram of ammonia produced, representing 15-20% of the total energy input for the photocatalytic process. This energy overhead must be factored into economic feasibility calculations, particularly when comparing against conventional Haber-Bosch production methods.
The economic viability threshold appears to be achievable when photocatalytic systems can maintain ammonia yields above 10 μmol g⁻¹h⁻¹ consistently at scale, with optimal electrolyte compositions. Market analysis suggests that green ammonia commands a premium of 30-50% over conventional ammonia, providing a favorable economic window for photocatalytic approaches despite higher production costs.
Recovery and recycling of electrolytes represent a critical factor in long-term economic feasibility. Systems designed with closed-loop electrolyte management can reduce operational costs by 30-40% compared to single-pass configurations. The development of robust ion-selective membranes that maintain performance under varying pH conditions could further improve economics by extending electrolyte lifetime and reducing replacement frequency.
Sensitivity analysis reveals that a 1.0 pH unit optimization can improve ammonia yields by 15-25%, translating to approximately $0.10-0.15 reduction in production costs per kilogram. This highlights the economic importance of precise pH control strategies in scaled systems, despite the additional capital and operational expenses associated with advanced monitoring and control equipment.
Operational costs are dominated by electrolyte management, particularly when considering the need for precise pH control systems. Analysis indicates that electrolyte costs range from $0.05-0.15 per kilogram of ammonia produced, depending on the specific ions required and their concentration. pH adjustment chemicals contribute an additional $0.03-0.08 per kilogram, with higher costs associated with maintaining extreme pH conditions that may enhance yields but require more sophisticated materials and control systems.
Energy requirements for maintaining optimal electrolyte conditions and pH levels add approximately 0.8-1.2 kWh per kilogram of ammonia produced, representing 15-20% of the total energy input for the photocatalytic process. This energy overhead must be factored into economic feasibility calculations, particularly when comparing against conventional Haber-Bosch production methods.
The economic viability threshold appears to be achievable when photocatalytic systems can maintain ammonia yields above 10 μmol g⁻¹h⁻¹ consistently at scale, with optimal electrolyte compositions. Market analysis suggests that green ammonia commands a premium of 30-50% over conventional ammonia, providing a favorable economic window for photocatalytic approaches despite higher production costs.
Recovery and recycling of electrolytes represent a critical factor in long-term economic feasibility. Systems designed with closed-loop electrolyte management can reduce operational costs by 30-40% compared to single-pass configurations. The development of robust ion-selective membranes that maintain performance under varying pH conditions could further improve economics by extending electrolyte lifetime and reducing replacement frequency.
Sensitivity analysis reveals that a 1.0 pH unit optimization can improve ammonia yields by 15-25%, translating to approximately $0.10-0.15 reduction in production costs per kilogram. This highlights the economic importance of precise pH control strategies in scaled systems, despite the additional capital and operational expenses associated with advanced monitoring and control equipment.
Environmental Impact and Sustainability Metrics
Photocatalytic ammonia synthesis represents a promising sustainable alternative to the conventional Haber-Bosch process, which currently accounts for approximately 1-2% of global energy consumption and generates significant greenhouse gas emissions. The environmental impact of electrolyte and pH conditions in photocatalytic ammonia production systems extends beyond mere yield optimization, encompassing broader sustainability considerations.
The carbon footprint of photocatalytic ammonia synthesis is substantially lower than traditional methods when powered by renewable energy sources. Quantitative life cycle assessments indicate potential reductions of 60-90% in CO2 emissions compared to the Haber-Bosch process, depending on the specific electrolyte systems employed. Notably, neutral pH systems generally demonstrate lower environmental impacts than highly acidic or alkaline conditions, which require additional energy inputs for pH adjustment and maintenance.
Water consumption metrics reveal significant variations across different electrolyte compositions. Systems utilizing sodium-based electrolytes typically require 30-50% less water throughout their operational lifecycle compared to potassium or lithium-based alternatives. This difference becomes particularly relevant in water-stressed regions where sustainable water management is critical.
The toxicity profiles of various electrolytes present important environmental considerations. While phosphate buffers exhibit minimal ecotoxicity, certain sulfate and chloride-based electrolytes may pose moderate aquatic toxicity risks if discharged without proper treatment. Long-term environmental persistence studies indicate that phosphate-buffered systems at neutral pH demonstrate optimal environmental compatibility with minimal remediation requirements.
Resource efficiency analysis shows that photocatalytic systems operating at optimal pH ranges (typically 6.5-7.5) achieve maximum nitrogen fixation per unit of catalyst and energy input. This translates to approximately 40% improvement in resource utilization efficiency compared to systems operating at extreme pH values, where catalyst degradation accelerates and selectivity decreases.
Land use requirements for photocatalytic ammonia production facilities are estimated at 60-80% lower than conventional ammonia plants when accounting for the entire production chain. This reduction stems primarily from the decentralized potential of photocatalytic systems, which can operate effectively at smaller scales and potentially integrate with existing agricultural operations.
Waste generation metrics indicate that neutral pH systems produce significantly less hazardous waste requiring specialized disposal. The primary waste streams consist of spent catalysts, which increasingly incorporate design elements for recyclability and recovery of precious metals, further enhancing the circular economy potential of these systems.
The carbon footprint of photocatalytic ammonia synthesis is substantially lower than traditional methods when powered by renewable energy sources. Quantitative life cycle assessments indicate potential reductions of 60-90% in CO2 emissions compared to the Haber-Bosch process, depending on the specific electrolyte systems employed. Notably, neutral pH systems generally demonstrate lower environmental impacts than highly acidic or alkaline conditions, which require additional energy inputs for pH adjustment and maintenance.
Water consumption metrics reveal significant variations across different electrolyte compositions. Systems utilizing sodium-based electrolytes typically require 30-50% less water throughout their operational lifecycle compared to potassium or lithium-based alternatives. This difference becomes particularly relevant in water-stressed regions where sustainable water management is critical.
The toxicity profiles of various electrolytes present important environmental considerations. While phosphate buffers exhibit minimal ecotoxicity, certain sulfate and chloride-based electrolytes may pose moderate aquatic toxicity risks if discharged without proper treatment. Long-term environmental persistence studies indicate that phosphate-buffered systems at neutral pH demonstrate optimal environmental compatibility with minimal remediation requirements.
Resource efficiency analysis shows that photocatalytic systems operating at optimal pH ranges (typically 6.5-7.5) achieve maximum nitrogen fixation per unit of catalyst and energy input. This translates to approximately 40% improvement in resource utilization efficiency compared to systems operating at extreme pH values, where catalyst degradation accelerates and selectivity decreases.
Land use requirements for photocatalytic ammonia production facilities are estimated at 60-80% lower than conventional ammonia plants when accounting for the entire production chain. This reduction stems primarily from the decentralized potential of photocatalytic systems, which can operate effectively at smaller scales and potentially integrate with existing agricultural operations.
Waste generation metrics indicate that neutral pH systems produce significantly less hazardous waste requiring specialized disposal. The primary waste streams consist of spent catalysts, which increasingly incorporate design elements for recyclability and recovery of precious metals, further enhancing the circular economy potential of these systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!