Comparative Thermal Analysis of Nitrate-Based Oxidizers
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitrate Oxidizers Background and Research Objectives
Nitrate-based oxidizers have been fundamental components in propellant and explosive formulations since the early development of energetic materials. Dating back to the 9th century with the discovery of black powder in China, potassium nitrate (KNO3) served as one of the earliest documented oxidizers. The evolution of nitrate-based oxidizers has progressed significantly through the centuries, with ammonium nitrate (NH4NO3) emerging as a pivotal industrial oxidizer in the early 20th century, followed by the development of more specialized compounds like lithium nitrate (LiNO3) and sodium nitrate (NaNO3) for specific applications.
The technological trajectory of nitrate oxidizers has been characterized by continuous refinement aimed at enhancing stability, energy output, and safety profiles. Recent advancements have focused on nano-engineering these materials to optimize their performance characteristics, particularly thermal decomposition behaviors which directly impact their application efficacy and safety parameters.
Current research trends indicate a growing interest in comparative thermal analysis methodologies to precisely characterize the decomposition kinetics and thermodynamic properties of various nitrate-based oxidizers. These analyses are crucial for understanding how different nitrate compounds behave under various thermal conditions, which directly influences their performance in propellant formulations, pyrotechnics, and industrial applications.
The primary objective of this research is to conduct a comprehensive comparative thermal analysis of prominent nitrate-based oxidizers including ammonium nitrate, potassium nitrate, sodium nitrate, and lithium nitrate. By employing advanced thermal analysis techniques such as Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA), and Differential Thermal Analysis (DTA), we aim to establish precise decomposition profiles for each compound.
Secondary objectives include identifying the influence of particle size, crystalline structure, and additives on the thermal behavior of these oxidizers. This knowledge is essential for developing more stable and efficient formulations for various applications ranging from agricultural fertilizers to aerospace propellants.
The research also seeks to address existing knowledge gaps regarding the complex decomposition mechanisms of nitrate oxidizers under different atmospheric conditions and heating rates. Understanding these mechanisms is vital for predicting performance characteristics and mitigating potential hazards associated with their use.
By establishing standardized comparative thermal analysis protocols for nitrate-based oxidizers, this research aims to contribute to the development of safer handling procedures, more efficient manufacturing processes, and innovative applications across multiple industries where these materials play a critical role.
The technological trajectory of nitrate oxidizers has been characterized by continuous refinement aimed at enhancing stability, energy output, and safety profiles. Recent advancements have focused on nano-engineering these materials to optimize their performance characteristics, particularly thermal decomposition behaviors which directly impact their application efficacy and safety parameters.
Current research trends indicate a growing interest in comparative thermal analysis methodologies to precisely characterize the decomposition kinetics and thermodynamic properties of various nitrate-based oxidizers. These analyses are crucial for understanding how different nitrate compounds behave under various thermal conditions, which directly influences their performance in propellant formulations, pyrotechnics, and industrial applications.
The primary objective of this research is to conduct a comprehensive comparative thermal analysis of prominent nitrate-based oxidizers including ammonium nitrate, potassium nitrate, sodium nitrate, and lithium nitrate. By employing advanced thermal analysis techniques such as Differential Scanning Calorimetry (DSC), Thermogravimetric Analysis (TGA), and Differential Thermal Analysis (DTA), we aim to establish precise decomposition profiles for each compound.
Secondary objectives include identifying the influence of particle size, crystalline structure, and additives on the thermal behavior of these oxidizers. This knowledge is essential for developing more stable and efficient formulations for various applications ranging from agricultural fertilizers to aerospace propellants.
The research also seeks to address existing knowledge gaps regarding the complex decomposition mechanisms of nitrate oxidizers under different atmospheric conditions and heating rates. Understanding these mechanisms is vital for predicting performance characteristics and mitigating potential hazards associated with their use.
By establishing standardized comparative thermal analysis protocols for nitrate-based oxidizers, this research aims to contribute to the development of safer handling procedures, more efficient manufacturing processes, and innovative applications across multiple industries where these materials play a critical role.
Market Applications and Demand Analysis
The global market for nitrate-based oxidizers has experienced significant growth in recent years, driven primarily by expanding applications in pyrotechnics, mining operations, and agricultural sectors. The comparative thermal analysis of these compounds has become increasingly important as industries seek more efficient, stable, and environmentally sustainable oxidizing agents for various applications.
In the explosives and propellants industry, which currently represents approximately 40% of the total market demand, manufacturers are increasingly focused on nitrate-based oxidizers with optimized thermal decomposition profiles. This shift is largely motivated by stringent safety regulations and the need for more controlled energy release in commercial blasting operations. Mining companies specifically seek oxidizers with predictable thermal behavior under varying environmental conditions to enhance operational safety and efficiency.
The agricultural sector presents another substantial market segment, where ammonium nitrate remains the dominant nitrate-based oxidizer used in fertilizer formulations. Market research indicates growing demand for enhanced fertilizer products with controlled release properties, which directly relates to the thermal stability characteristics of the nitrate compounds used. Farmers and agricultural suppliers are increasingly willing to pay premium prices for fertilizers with verified thermal stability profiles that ensure consistent nutrient release and reduced environmental impact.
Defense applications constitute a specialized but high-value market segment for nitrate-based oxidizers. Military and defense contractors require extensive thermal analysis data to ensure propellant and explosive formulations meet strict performance and safety standards across extreme temperature ranges. This sector drives significant research investment in advanced thermal characterization techniques for nitrate compounds.
Emerging applications in renewable energy storage systems have created a new market niche for certain nitrate-based compounds, particularly those with favorable thermal energy storage properties. Molten salt energy storage systems utilizing nitrate mixtures are gaining traction in concentrated solar power installations, with market projections suggesting annual growth rates exceeding 15% over the next decade.
Regional market analysis reveals that North America and Europe currently lead in demand for thermally characterized nitrate oxidizers, primarily due to stringent regulatory frameworks and advanced industrial applications. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding manufacturing sectors and increasing adoption of precision agriculture techniques requiring advanced fertilizer formulations.
Consumer and industrial safety concerns continue to shape market demands, with customers increasingly requiring comprehensive thermal stability data before adopting new nitrate-based products. This trend has elevated the importance of comparative thermal analysis as both a research tool and a marketing differentiator for suppliers in this competitive landscape.
In the explosives and propellants industry, which currently represents approximately 40% of the total market demand, manufacturers are increasingly focused on nitrate-based oxidizers with optimized thermal decomposition profiles. This shift is largely motivated by stringent safety regulations and the need for more controlled energy release in commercial blasting operations. Mining companies specifically seek oxidizers with predictable thermal behavior under varying environmental conditions to enhance operational safety and efficiency.
The agricultural sector presents another substantial market segment, where ammonium nitrate remains the dominant nitrate-based oxidizer used in fertilizer formulations. Market research indicates growing demand for enhanced fertilizer products with controlled release properties, which directly relates to the thermal stability characteristics of the nitrate compounds used. Farmers and agricultural suppliers are increasingly willing to pay premium prices for fertilizers with verified thermal stability profiles that ensure consistent nutrient release and reduced environmental impact.
Defense applications constitute a specialized but high-value market segment for nitrate-based oxidizers. Military and defense contractors require extensive thermal analysis data to ensure propellant and explosive formulations meet strict performance and safety standards across extreme temperature ranges. This sector drives significant research investment in advanced thermal characterization techniques for nitrate compounds.
Emerging applications in renewable energy storage systems have created a new market niche for certain nitrate-based compounds, particularly those with favorable thermal energy storage properties. Molten salt energy storage systems utilizing nitrate mixtures are gaining traction in concentrated solar power installations, with market projections suggesting annual growth rates exceeding 15% over the next decade.
Regional market analysis reveals that North America and Europe currently lead in demand for thermally characterized nitrate oxidizers, primarily due to stringent regulatory frameworks and advanced industrial applications. However, the Asia-Pacific region is experiencing the fastest growth rate, driven by expanding manufacturing sectors and increasing adoption of precision agriculture techniques requiring advanced fertilizer formulations.
Consumer and industrial safety concerns continue to shape market demands, with customers increasingly requiring comprehensive thermal stability data before adopting new nitrate-based products. This trend has elevated the importance of comparative thermal analysis as both a research tool and a marketing differentiator for suppliers in this competitive landscape.
Current Thermal Analysis Techniques and Limitations
Thermal analysis techniques for nitrate-based oxidizers have evolved significantly over the past decades, with several methodologies now established as industry standards. Differential Scanning Calorimetry (DSC) remains the most widely utilized technique, offering precise measurements of heat flow during phase transitions and decomposition processes. This method provides valuable data on thermal stability, reaction kinetics, and compatibility characteristics of various nitrate compounds used in propellants and pyrotechnics.
Thermogravimetric Analysis (TGA) complements DSC by monitoring mass changes during thermal events, enabling researchers to quantify decomposition rates and identify multi-stage reaction mechanisms common in complex nitrate-based formulations. When coupled with mass spectrometry (TGA-MS), this technique further allows for real-time identification of evolved gases, providing crucial insights into decomposition pathways.
Despite these advancements, current thermal analysis methods face significant limitations when applied to nitrate-based oxidizers. Sample size restrictions represent a major challenge, as conventional instruments typically accommodate only milligram quantities, raising questions about the scalability and representativeness of results for industrial applications. This limitation becomes particularly problematic when analyzing heterogeneous mixtures where composition may vary throughout the bulk material.
Temperature control and measurement accuracy present another critical limitation. Many nitrate decomposition reactions are highly exothermic and can proceed rapidly once initiated, often exceeding the heating rate control capabilities of standard equipment. This creates difficulties in accurately capturing fast kinetic events and can lead to misinterpretation of thermal behavior under realistic application conditions.
Environmental factors also pose challenges to current analytical methods. Most thermal analysis instruments operate under controlled atmospheric conditions that may not reflect the actual deployment environment of nitrate-based materials. The influence of humidity, pressure variations, and atmospheric composition on decomposition behavior remains inadequately addressed by standard techniques.
Interpretation challenges further complicate thermal analysis of nitrate oxidizers. Complex, overlapping thermal events often characterize these materials, making deconvolution of individual processes difficult. Additionally, the influence of particle size, morphology, and crystal structure on thermal behavior is frequently overlooked in conventional analysis protocols, despite their significant impact on reactivity and stability.
Advanced techniques such as High-Pressure DSC and Fast-Scanning Calorimetry offer promising solutions to some limitations but remain specialized and not widely accessible. The development of standardized testing protocols specifically designed for nitrate-based oxidizers represents an urgent need in the field, particularly for applications requiring precise safety assessments and performance predictions.
Thermogravimetric Analysis (TGA) complements DSC by monitoring mass changes during thermal events, enabling researchers to quantify decomposition rates and identify multi-stage reaction mechanisms common in complex nitrate-based formulations. When coupled with mass spectrometry (TGA-MS), this technique further allows for real-time identification of evolved gases, providing crucial insights into decomposition pathways.
Despite these advancements, current thermal analysis methods face significant limitations when applied to nitrate-based oxidizers. Sample size restrictions represent a major challenge, as conventional instruments typically accommodate only milligram quantities, raising questions about the scalability and representativeness of results for industrial applications. This limitation becomes particularly problematic when analyzing heterogeneous mixtures where composition may vary throughout the bulk material.
Temperature control and measurement accuracy present another critical limitation. Many nitrate decomposition reactions are highly exothermic and can proceed rapidly once initiated, often exceeding the heating rate control capabilities of standard equipment. This creates difficulties in accurately capturing fast kinetic events and can lead to misinterpretation of thermal behavior under realistic application conditions.
Environmental factors also pose challenges to current analytical methods. Most thermal analysis instruments operate under controlled atmospheric conditions that may not reflect the actual deployment environment of nitrate-based materials. The influence of humidity, pressure variations, and atmospheric composition on decomposition behavior remains inadequately addressed by standard techniques.
Interpretation challenges further complicate thermal analysis of nitrate oxidizers. Complex, overlapping thermal events often characterize these materials, making deconvolution of individual processes difficult. Additionally, the influence of particle size, morphology, and crystal structure on thermal behavior is frequently overlooked in conventional analysis protocols, despite their significant impact on reactivity and stability.
Advanced techniques such as High-Pressure DSC and Fast-Scanning Calorimetry offer promising solutions to some limitations but remain specialized and not widely accessible. The development of standardized testing protocols specifically designed for nitrate-based oxidizers represents an urgent need in the field, particularly for applications requiring precise safety assessments and performance predictions.
Established Thermal Characterization Methodologies
01 Thermal stability and decomposition characteristics of nitrate oxidizers
Nitrate-based oxidizers exhibit specific thermal properties that affect their stability and decomposition behavior. These materials undergo thermal decomposition at certain temperature ranges, releasing oxygen and other gases. Understanding these thermal characteristics is crucial for safety and performance in applications such as propellants and explosives. The decomposition process can be controlled through various additives and processing methods to achieve desired reaction rates and energy release profiles.- Thermal stability and decomposition characteristics of nitrate-based oxidizers: Nitrate-based oxidizers exhibit specific thermal decomposition patterns that are crucial for their application in various systems. These materials undergo phase transitions and decomposition at characteristic temperatures, releasing oxygen and other gases. Understanding these thermal properties is essential for predicting their behavior under different temperature conditions and ensuring safe handling. Thermal analysis techniques such as differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are commonly used to study these properties.
- Nitrate-based oxidizers in energetic compositions and propellants: Nitrate-based oxidizers are widely used in energetic compositions such as propellants, explosives, and pyrotechnics due to their oxygen-rich nature. Their thermal properties significantly influence the combustion characteristics, energy output, and stability of these compositions. By carefully selecting and formulating nitrate oxidizers with appropriate binders and additives, the burning rate, ignition temperature, and overall performance of the energetic system can be optimized for specific applications.
- Modification and stabilization of nitrate-based oxidizers: Various methods are employed to modify and stabilize nitrate-based oxidizers to enhance their thermal properties. These include coating the particles, adding stabilizing agents, controlling particle size and morphology, and creating composite structures. Such modifications can improve thermal stability, reduce hygroscopicity, decrease sensitivity to thermal shock, and enhance compatibility with other components in formulations. These approaches are critical for extending shelf life and improving safety characteristics of nitrate-containing compositions.
- Thermal testing and characterization methods for nitrate oxidizers: Specialized testing methods are used to characterize the thermal properties of nitrate-based oxidizers. These include accelerated aging tests, compatibility studies, thermal cycling, and advanced analytical techniques. Such characterization is essential for understanding how these materials behave under various thermal conditions, predicting their long-term stability, and establishing safe handling protocols. The data obtained from these tests is often used to develop mathematical models that predict thermal behavior in different environments and applications.
- Environmental and safety considerations of nitrate oxidizers' thermal properties: The thermal properties of nitrate-based oxidizers have significant implications for environmental impact and safety. These materials can release nitrogen oxides and other potentially harmful gases when heated or decomposed. Understanding their thermal behavior is crucial for developing proper storage conditions, transportation regulations, and disposal methods. Additionally, knowledge of thermal stability thresholds helps prevent accidental initiation during manufacturing processes and end-use applications, reducing the risk of fires, explosions, or toxic releases.
02 Heat transfer and thermal conductivity properties
The thermal conductivity and heat transfer characteristics of nitrate-based oxidizers significantly impact their performance in various applications. These properties determine how heat is distributed throughout the material during storage and use. Enhanced thermal conductivity can improve safety by preventing localized hotspots, while controlled heat transfer rates are essential for maintaining consistent reaction rates in energetic applications. Various formulation techniques can be employed to modify these thermal properties for specific use cases.Expand Specific Solutions03 Phase transitions and crystal structure effects on thermal behavior
Nitrate-based oxidizers undergo phase transitions at specific temperatures that affect their overall thermal behavior. The crystal structure of these materials can change with temperature variations, influencing their stability, reactivity, and energy content. These phase changes may involve transitions between solid forms or melting processes that are critical to understand for proper handling and application. The thermal expansion coefficients and structural integrity during heating are important parameters that determine the performance characteristics of these oxidizers.Expand Specific Solutions04 Thermal analysis techniques for nitrate oxidizers
Various thermal analysis techniques are employed to characterize the thermal properties of nitrate-based oxidizers. These include differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and differential thermal analysis (DTA). These methods help determine critical parameters such as decomposition temperatures, heat capacity, and reaction enthalpies. The data obtained from these analyses are essential for predicting behavior under different temperature conditions and for quality control in manufacturing processes. Advanced computational models can also be used to simulate thermal behavior under various conditions.Expand Specific Solutions05 Additives and formulations to modify thermal properties
Various additives and formulation strategies can be employed to modify the thermal properties of nitrate-based oxidizers. These include stabilizers that enhance thermal stability, catalysts that affect decomposition rates, and binders that influence heat transfer characteristics. By carefully selecting additives and adjusting formulations, it is possible to tailor the thermal behavior of nitrate oxidizers for specific applications. This approach allows for optimization of performance parameters such as burn rate, ignition temperature, and energy output while maintaining safety and reliability.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The thermal analysis of nitrate-based oxidizers market is currently in a mature growth phase with an estimated global market size of $3-4 billion, driven by applications in propellants, explosives, and specialty chemicals. The competitive landscape features established players like Nippon Kayaku and China Petroleum & Chemical Corp (Sinopec) dominating commercial production, while research institutions such as Zhejiang University, Nanjing University of Science & Technology, and Toyota Central R&D Labs lead technological innovation. The technology demonstrates high maturity in traditional applications, though emerging players like Lilac Solutions and Northrop Grumman are developing advanced formulations with enhanced thermal stability and performance characteristics. Academic-industry collaborations between companies like Daicel Corp and research institutions are accelerating innovations in environmentally friendly nitrate-based oxidizer technologies.
Nippon Kayaku Co., Ltd.
Technical Solution: Nippon Kayaku has developed advanced thermal analysis methodologies specifically for nitrate-based oxidizers used in energetic materials. Their approach combines Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) to comprehensively evaluate the thermal decomposition behavior of various nitrate compounds. Their research has demonstrated that ammonium nitrate exhibits a complex multi-stage decomposition process with phase transitions occurring at approximately 32°C, 84°C, and 125°C before the main exothermic decomposition at around 230-260°C. The company has pioneered stabilization techniques using phase stabilizers that can significantly reduce the thermal sensitivity of nitrate oxidizers while maintaining their energetic performance. Their comparative studies between potassium nitrate, sodium nitrate, and ammonium nitrate have established critical safety parameters including onset temperatures, heat release rates, and activation energies that are essential for safe handling and storage.
Strengths: Extensive experience with industrial-scale applications of nitrate oxidizers; proprietary stabilization technologies that enhance thermal stability; comprehensive safety protocols based on empirical testing. Weaknesses: Their analysis methods require specialized equipment and expertise; some stabilization additives may reduce the overall energy output of the formulations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a systematic thermal analysis framework for nitrate-based oxidizers used in petroleum extraction and refining operations. Their approach integrates Simultaneous Thermal Analysis (STA) with Mass Spectrometry (MS) to identify decomposition products and reaction pathways of various nitrate compounds under different temperature regimes. Sinopec's research has revealed that metal nitrates (particularly those of transition metals) exhibit significantly different thermal stability profiles compared to ammonium nitrate, with decomposition temperatures ranging from 150°C to over 400°C depending on the cation. Their comparative studies have established that the addition of specific metal oxides can catalyze the decomposition of nitrate oxidizers, lowering activation energies by up to 30% in some formulations. This has practical applications in controlled energy release systems used in oil well stimulation. Sinopec has also pioneered methods to mitigate the hygroscopic nature of nitrate compounds through surface modification techniques, significantly improving their shelf life and handling characteristics.
Strengths: Comprehensive understanding of nitrate behavior under extreme pressure and temperature conditions relevant to petroleum applications; innovative catalytic systems that can precisely control decomposition rates; extensive field testing data. Weaknesses: Some of their formulations are specifically optimized for oil field applications and may not be directly transferable to other industries; environmental concerns regarding certain metal additives used in their systems.
Critical Patents and Scientific Literature Review
Nitrogen implementation to minimize device variation
PatentInactiveUS20040113182A1
Innovation
- Introducing a negative nitrogen concentration gradient into the oxynitride layer during the rapid thermal nitridation process by reducing the nitrous oxide flow rate, allowing excess atomic oxygen to accumulate and react with the oxynitride layer, thereby compensating for the threshold voltage variations caused by the thickness gradient.
Method of forming an oxinitride layer
PatentInactiveUS20080090424A1
Innovation
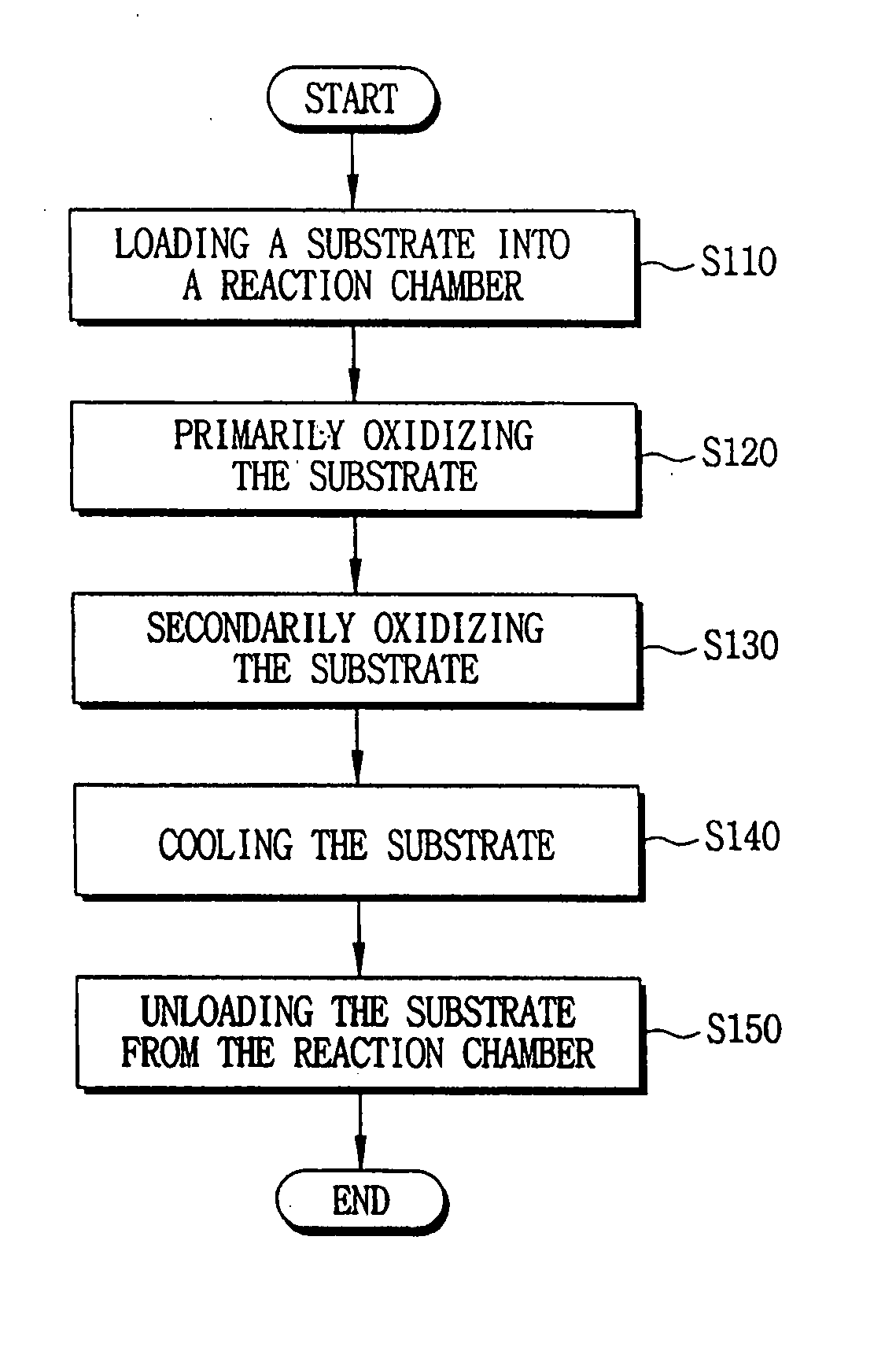
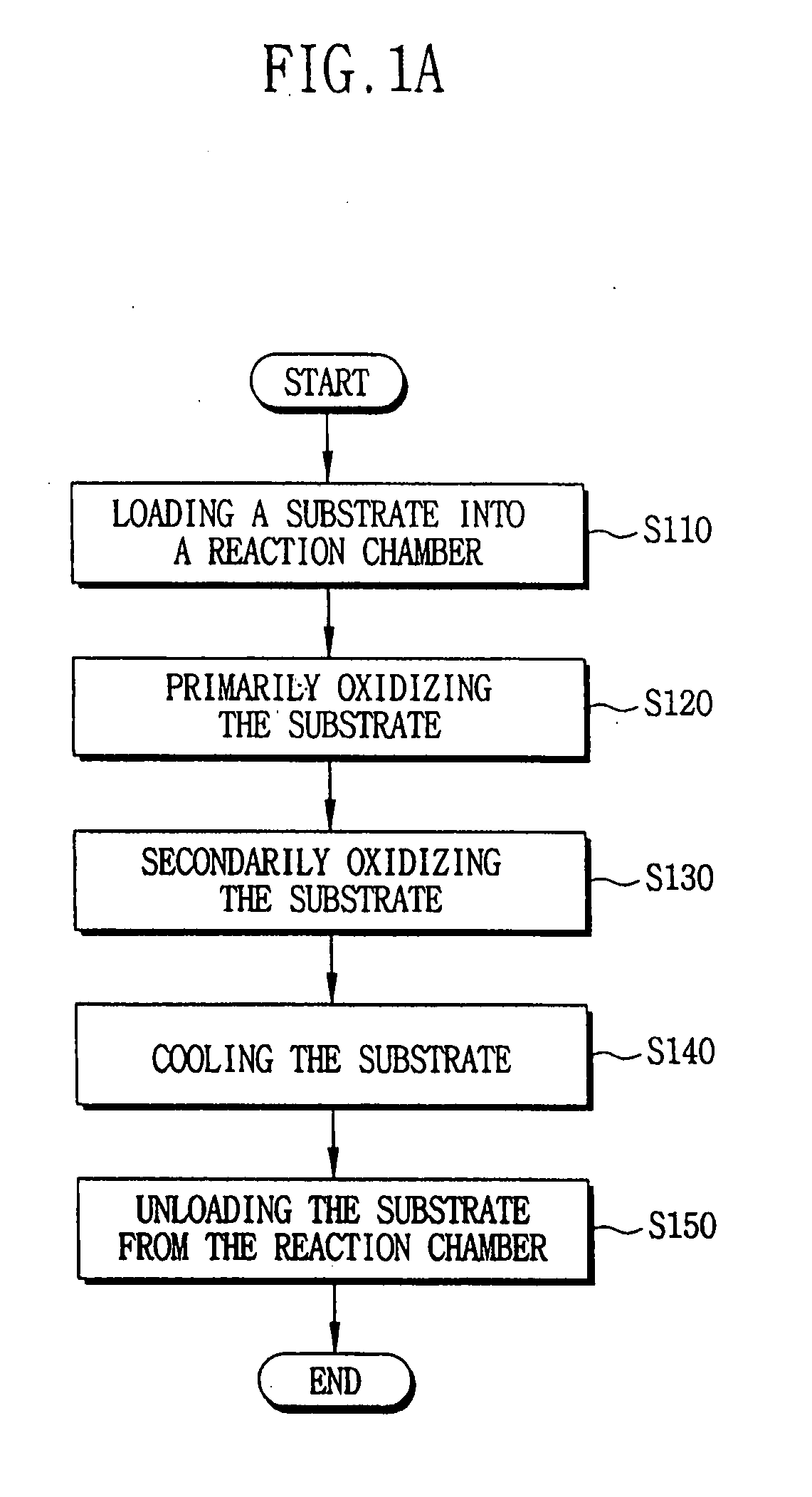
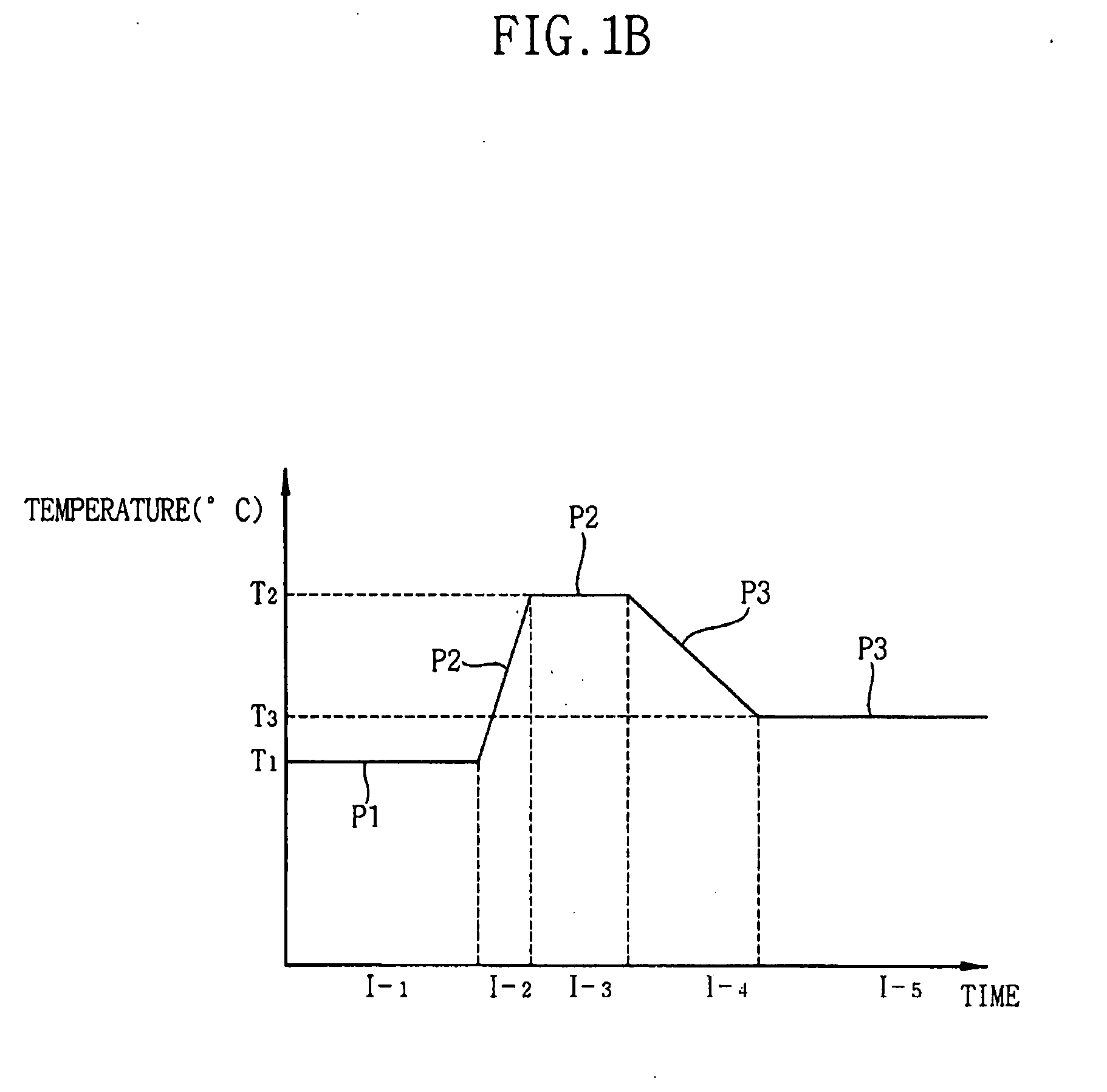
- A method involving a batch type apparatus where substrates are oxidized and nitrified in a reaction chamber at controlled pressures and temperatures, with multiple oxidation stages and the use of RTN apparatus for nitration, to form uniform and precise oxide and oxynitride layers, preventing impurity diffusion.
Safety and Regulatory Framework
The regulatory landscape governing nitrate-based oxidizers has evolved significantly in response to historical incidents and growing safety concerns. International frameworks such as the United Nations Recommendations on the Transport of Dangerous Goods classify these compounds based on their sensitivity to thermal stimuli and potential hazard profiles. In the United States, the Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) and the Department of Transportation (DOT) maintain strict oversight on the storage, handling, and transportation of nitrate-based oxidizers, particularly ammonium nitrate which has been implicated in several catastrophic industrial accidents.
European regulations, notably the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the Seveso III Directive, impose additional requirements for risk assessment and management of facilities handling substantial quantities of these materials. These frameworks mandate regular thermal stability testing using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to establish safety parameters and storage guidelines.
Safety protocols for laboratory-scale thermal analysis of nitrate oxidizers require specialized equipment including explosion-proof chambers, remote monitoring systems, and precise temperature control mechanisms. Standard operating procedures typically limit sample sizes to milligram quantities when conducting comparative thermal analyses to minimize potential hazards while maintaining scientific validity.
The implementation of hierarchical hazard controls represents best practice in this field, with engineering controls such as thermal barriers and pressure relief systems taking precedence over administrative measures. Personal protective equipment requirements typically include flame-resistant clothing, face shields, and specialized gloves when handling these thermally sensitive compounds.
Recent regulatory trends indicate a move toward more stringent decomposition temperature monitoring and accelerated aging tests to better predict long-term stability under various environmental conditions. The International Society for Thermal Analysis and Calorimetry (ISTAC) has published guidelines specifically addressing the unique challenges of nitrate oxidizer analysis, emphasizing the importance of standardized testing protocols to ensure cross-laboratory comparability of thermal data.
Emergency response planning constitutes a critical component of the safety framework, with facilities required to develop specific protocols for thermal runaway scenarios. These plans must address rapid cooling interventions, containment strategies for decomposition products, and evacuation procedures calibrated to the specific thermal properties of the nitrate compounds being handled or stored.
European regulations, notably the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the Seveso III Directive, impose additional requirements for risk assessment and management of facilities handling substantial quantities of these materials. These frameworks mandate regular thermal stability testing using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to establish safety parameters and storage guidelines.
Safety protocols for laboratory-scale thermal analysis of nitrate oxidizers require specialized equipment including explosion-proof chambers, remote monitoring systems, and precise temperature control mechanisms. Standard operating procedures typically limit sample sizes to milligram quantities when conducting comparative thermal analyses to minimize potential hazards while maintaining scientific validity.
The implementation of hierarchical hazard controls represents best practice in this field, with engineering controls such as thermal barriers and pressure relief systems taking precedence over administrative measures. Personal protective equipment requirements typically include flame-resistant clothing, face shields, and specialized gloves when handling these thermally sensitive compounds.
Recent regulatory trends indicate a move toward more stringent decomposition temperature monitoring and accelerated aging tests to better predict long-term stability under various environmental conditions. The International Society for Thermal Analysis and Calorimetry (ISTAC) has published guidelines specifically addressing the unique challenges of nitrate oxidizer analysis, emphasizing the importance of standardized testing protocols to ensure cross-laboratory comparability of thermal data.
Emergency response planning constitutes a critical component of the safety framework, with facilities required to develop specific protocols for thermal runaway scenarios. These plans must address rapid cooling interventions, containment strategies for decomposition products, and evacuation procedures calibrated to the specific thermal properties of the nitrate compounds being handled or stored.
Environmental Impact Assessment
The environmental implications of nitrate-based oxidizers extend far beyond their immediate applications in propellants and pyrotechnics. These compounds, primarily ammonium nitrate, potassium nitrate, and sodium nitrate, introduce significant environmental concerns throughout their lifecycle—from production to disposal.
Manufacturing processes for nitrate-based oxidizers typically require substantial energy inputs and generate considerable greenhouse gas emissions. The production of ammonium nitrate, for instance, involves energy-intensive processes that contribute to carbon footprints across industrial sectors. Additionally, mining operations for raw materials like potassium and sodium salts can lead to habitat disruption and soil degradation in extraction regions.
Water contamination represents one of the most pressing environmental challenges associated with these compounds. Nitrates are highly water-soluble and can readily leach into groundwater and surface water systems when improperly handled or stored. Elevated nitrate levels in drinking water sources have been linked to methemoglobinemia (blue baby syndrome) and potential carcinogenic effects through the formation of N-nitroso compounds.
Atmospheric pollution constitutes another significant concern. When nitrate-based oxidizers undergo thermal decomposition during intended use or accidental fires, they release nitrogen oxides (NOx) and other potentially harmful gases. These emissions contribute to photochemical smog formation, acid rain, and stratospheric ozone depletion, exacerbating air quality issues in affected regions.
Ecological impacts manifest through disruption of natural nitrogen cycles. Excess nitrates entering ecosystems can trigger eutrophication in aquatic environments, leading to harmful algal blooms, oxygen depletion, and subsequent damage to aquatic biodiversity. Terrestrial ecosystems may experience shifts in plant community composition, favoring nitrophilic species and potentially reducing overall biodiversity.
Regulatory frameworks worldwide have increasingly recognized these environmental hazards. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in other jurisdictions impose strict controls on nitrate compounds. These regulations mandate proper handling, storage, and disposal protocols to minimize environmental contamination.
Mitigation strategies focus on developing more environmentally benign alternatives, implementing closed-loop manufacturing systems, and improving end-of-life management. Recent advances in green chemistry have yielded promising alternatives with reduced environmental footprints, though many still face challenges in matching the performance characteristics of traditional nitrate-based oxidizers.
Manufacturing processes for nitrate-based oxidizers typically require substantial energy inputs and generate considerable greenhouse gas emissions. The production of ammonium nitrate, for instance, involves energy-intensive processes that contribute to carbon footprints across industrial sectors. Additionally, mining operations for raw materials like potassium and sodium salts can lead to habitat disruption and soil degradation in extraction regions.
Water contamination represents one of the most pressing environmental challenges associated with these compounds. Nitrates are highly water-soluble and can readily leach into groundwater and surface water systems when improperly handled or stored. Elevated nitrate levels in drinking water sources have been linked to methemoglobinemia (blue baby syndrome) and potential carcinogenic effects through the formation of N-nitroso compounds.
Atmospheric pollution constitutes another significant concern. When nitrate-based oxidizers undergo thermal decomposition during intended use or accidental fires, they release nitrogen oxides (NOx) and other potentially harmful gases. These emissions contribute to photochemical smog formation, acid rain, and stratospheric ozone depletion, exacerbating air quality issues in affected regions.
Ecological impacts manifest through disruption of natural nitrogen cycles. Excess nitrates entering ecosystems can trigger eutrophication in aquatic environments, leading to harmful algal blooms, oxygen depletion, and subsequent damage to aquatic biodiversity. Terrestrial ecosystems may experience shifts in plant community composition, favoring nitrophilic species and potentially reducing overall biodiversity.
Regulatory frameworks worldwide have increasingly recognized these environmental hazards. The European Union's REACH regulations, the United States EPA guidelines, and similar frameworks in other jurisdictions impose strict controls on nitrate compounds. These regulations mandate proper handling, storage, and disposal protocols to minimize environmental contamination.
Mitigation strategies focus on developing more environmentally benign alternatives, implementing closed-loop manufacturing systems, and improving end-of-life management. Recent advances in green chemistry have yielded promising alternatives with reduced environmental footprints, though many still face challenges in matching the performance characteristics of traditional nitrate-based oxidizers.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!