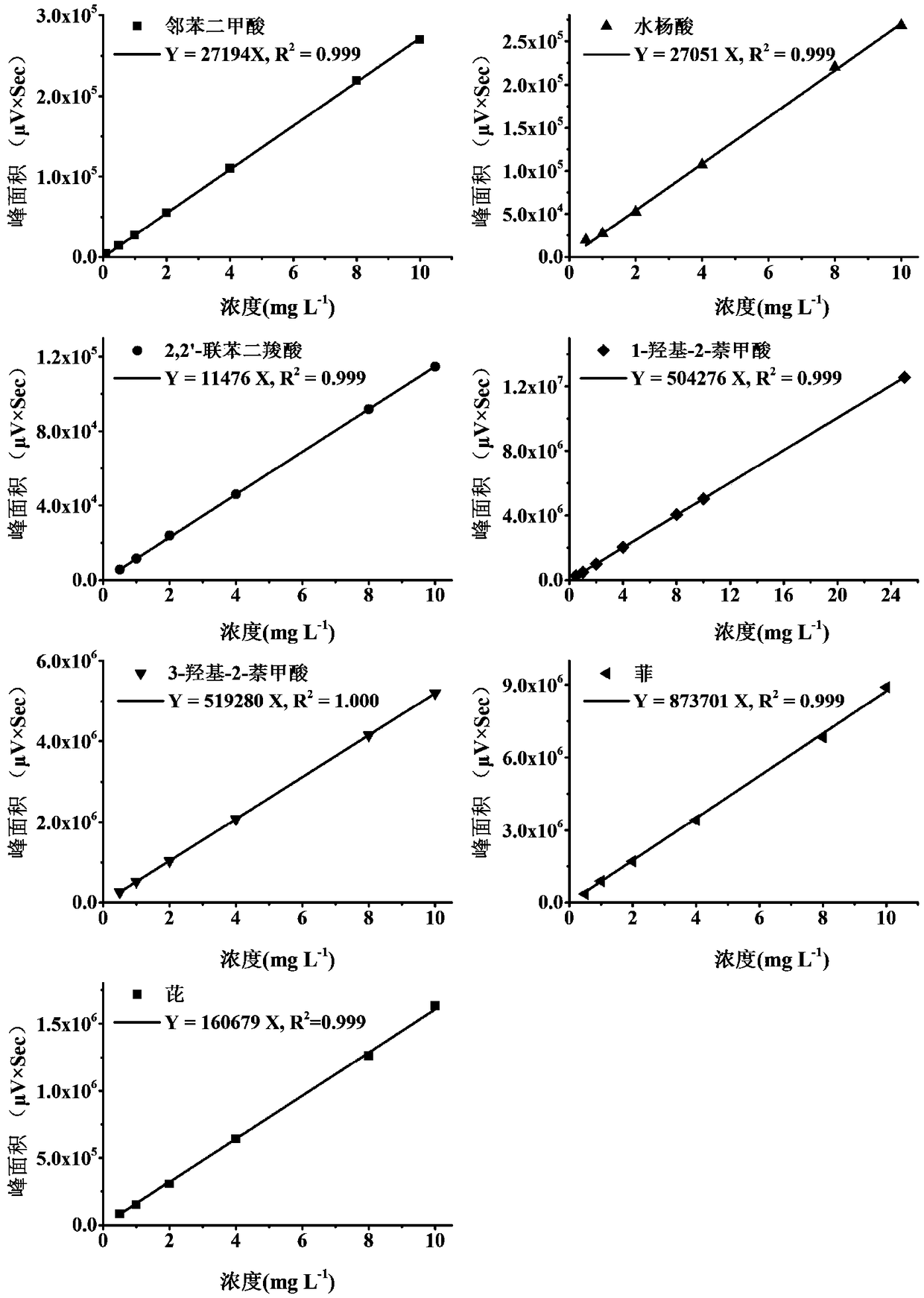
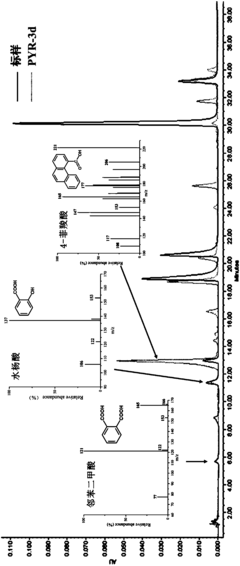
Impurities and Degradation Pathways in Stored Saltpeter Samples
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Storage Degradation Background and Objectives
Saltpeter, also known as potassium nitrate (KNO₃), has been a critical compound throughout human history, serving essential roles in agriculture, food preservation, medicine, and most notably, as a key component in gunpowder. The study of degradation pathways in stored saltpeter represents a crucial intersection of chemistry, materials science, and historical preservation technology. Over centuries, the understanding of saltpeter's properties has evolved from alchemical curiosity to precise scientific characterization.
The evolution of saltpeter technology traces back to ancient China around the 9th century, where it was first utilized in explosive compositions. By the 13th century, knowledge of saltpeter applications had spread to Europe, revolutionizing warfare and later industrial processes. Modern applications extend beyond explosives to fertilizers, food preservatives, and specialty chemicals, highlighting the compound's continued relevance despite its ancient origins.
Recent technological advances have enabled more sophisticated analysis of saltpeter degradation mechanisms. Modern analytical techniques including X-ray diffraction (XRD), ion chromatography, and mass spectrometry now allow researchers to identify trace impurities and degradation products at previously undetectable levels. This technological progression provides unprecedented opportunities to understand the complex chemical transformations occurring during long-term storage.
The primary objective of this research is to comprehensively characterize the impurity profiles and degradation pathways in saltpeter samples stored under various conditions and timeframes. Specifically, we aim to identify the chemical mechanisms responsible for quality deterioration, quantify the rate of degradation under different environmental parameters, and establish correlations between storage conditions and impurity formation.
Secondary objectives include developing predictive models for saltpeter stability, establishing improved storage protocols to minimize degradation, and creating analytical standards for quality assessment of historical and contemporary samples. These goals address both fundamental scientific questions and practical applications in fields ranging from historical conservation to modern industrial processes.
The significance of this research extends across multiple domains. For historical preservation, understanding degradation mechanisms enables better conservation of artifacts containing saltpeter. In industrial applications, improved storage protocols can enhance product stability and reduce economic losses. From a scientific perspective, elucidating these complex degradation pathways contributes to fundamental knowledge in inorganic chemistry and materials science.
Current challenges in this field include limited standardization of analytical methods, incomplete understanding of catalytic effects from trace contaminants, and difficulties in accurately dating and authenticating historical samples. Addressing these challenges requires interdisciplinary collaboration between chemists, materials scientists, and preservation specialists.
The evolution of saltpeter technology traces back to ancient China around the 9th century, where it was first utilized in explosive compositions. By the 13th century, knowledge of saltpeter applications had spread to Europe, revolutionizing warfare and later industrial processes. Modern applications extend beyond explosives to fertilizers, food preservatives, and specialty chemicals, highlighting the compound's continued relevance despite its ancient origins.
Recent technological advances have enabled more sophisticated analysis of saltpeter degradation mechanisms. Modern analytical techniques including X-ray diffraction (XRD), ion chromatography, and mass spectrometry now allow researchers to identify trace impurities and degradation products at previously undetectable levels. This technological progression provides unprecedented opportunities to understand the complex chemical transformations occurring during long-term storage.
The primary objective of this research is to comprehensively characterize the impurity profiles and degradation pathways in saltpeter samples stored under various conditions and timeframes. Specifically, we aim to identify the chemical mechanisms responsible for quality deterioration, quantify the rate of degradation under different environmental parameters, and establish correlations between storage conditions and impurity formation.
Secondary objectives include developing predictive models for saltpeter stability, establishing improved storage protocols to minimize degradation, and creating analytical standards for quality assessment of historical and contemporary samples. These goals address both fundamental scientific questions and practical applications in fields ranging from historical conservation to modern industrial processes.
The significance of this research extends across multiple domains. For historical preservation, understanding degradation mechanisms enables better conservation of artifacts containing saltpeter. In industrial applications, improved storage protocols can enhance product stability and reduce economic losses. From a scientific perspective, elucidating these complex degradation pathways contributes to fundamental knowledge in inorganic chemistry and materials science.
Current challenges in this field include limited standardization of analytical methods, incomplete understanding of catalytic effects from trace contaminants, and difficulties in accurately dating and authenticating historical samples. Addressing these challenges requires interdisciplinary collaboration between chemists, materials scientists, and preservation specialists.
Market Analysis of Stored Saltpeter Applications
The global market for saltpeter (potassium nitrate) applications has shown remarkable resilience despite technological advancements in alternative compounds. Current market valuation stands at approximately 1.2 billion USD, with a compound annual growth rate of 3.7% projected through 2028. This steady growth is primarily driven by saltpeter's continued importance in agriculture, food preservation, and specialty chemical applications.
Agricultural applications represent the largest market segment, accounting for roughly 65% of total saltpeter consumption. The compound's role as a nitrogen-rich fertilizer remains crucial for crop production in regions with specific soil conditions. Particularly notable is the increasing demand in precision agriculture, where controlled-release fertilizers containing purified saltpeter compounds are gaining traction.
The food preservation sector constitutes about 18% of the market, with particular strength in regions where traditional preservation methods remain economically viable. This segment has seen modest but consistent growth of 2.5% annually, primarily in developing economies where cold chain infrastructure is still developing. The quality and purity of stored saltpeter directly impacts its efficacy in this application.
Specialty chemical applications, including pyrotechnics, glass manufacturing, and certain pharmaceutical formulations, make up approximately 14% of the market. This segment shows the highest growth potential at 5.2% annually, driven by innovation in high-purity applications requiring minimal impurities.
Regional analysis reveals that Asia-Pacific dominates consumption at 42% of global demand, followed by North America (24%) and Europe (21%). Emerging markets in South America and Africa are showing accelerated adoption rates, particularly in agricultural applications.
Market dynamics are increasingly influenced by purity standards and degradation concerns. Premium pricing of up to 40% is observed for high-purity saltpeter with minimal degradation products, particularly for pharmaceutical and food applications. This price differential underscores the economic importance of understanding and controlling impurity profiles and degradation pathways in stored samples.
Consumer and regulatory trends indicate growing preference for natural preservatives with established safety profiles, positioning well-preserved saltpeter favorably against newer synthetic alternatives in certain applications. However, this advantage is contingent upon proper storage and handling to prevent formation of potentially harmful degradation compounds.
Market forecasts suggest that applications requiring high-purity saltpeter will see the strongest growth, highlighting the commercial relevance of research into impurity identification and degradation prevention in stored samples. Companies investing in advanced purification and stabilization technologies are positioned to capture premium market segments where product integrity is paramount.
Agricultural applications represent the largest market segment, accounting for roughly 65% of total saltpeter consumption. The compound's role as a nitrogen-rich fertilizer remains crucial for crop production in regions with specific soil conditions. Particularly notable is the increasing demand in precision agriculture, where controlled-release fertilizers containing purified saltpeter compounds are gaining traction.
The food preservation sector constitutes about 18% of the market, with particular strength in regions where traditional preservation methods remain economically viable. This segment has seen modest but consistent growth of 2.5% annually, primarily in developing economies where cold chain infrastructure is still developing. The quality and purity of stored saltpeter directly impacts its efficacy in this application.
Specialty chemical applications, including pyrotechnics, glass manufacturing, and certain pharmaceutical formulations, make up approximately 14% of the market. This segment shows the highest growth potential at 5.2% annually, driven by innovation in high-purity applications requiring minimal impurities.
Regional analysis reveals that Asia-Pacific dominates consumption at 42% of global demand, followed by North America (24%) and Europe (21%). Emerging markets in South America and Africa are showing accelerated adoption rates, particularly in agricultural applications.
Market dynamics are increasingly influenced by purity standards and degradation concerns. Premium pricing of up to 40% is observed for high-purity saltpeter with minimal degradation products, particularly for pharmaceutical and food applications. This price differential underscores the economic importance of understanding and controlling impurity profiles and degradation pathways in stored samples.
Consumer and regulatory trends indicate growing preference for natural preservatives with established safety profiles, positioning well-preserved saltpeter favorably against newer synthetic alternatives in certain applications. However, this advantage is contingent upon proper storage and handling to prevent formation of potentially harmful degradation compounds.
Market forecasts suggest that applications requiring high-purity saltpeter will see the strongest growth, highlighting the commercial relevance of research into impurity identification and degradation prevention in stored samples. Companies investing in advanced purification and stabilization technologies are positioned to capture premium market segments where product integrity is paramount.
Current Challenges in Saltpeter Preservation Technology
Saltpeter (potassium nitrate) preservation faces significant challenges due to its hygroscopic nature and susceptibility to chemical degradation. Current storage methods often fail to prevent moisture absorption, leading to physical and chemical alterations that compromise sample integrity. Traditional containers, including glass jars and plastic bags, provide insufficient protection against environmental humidity fluctuations, particularly in regions with variable climate conditions.
Temperature control represents another critical challenge, as saltpeter samples stored above 30°C show accelerated degradation rates. Research indicates that for every 10°C increase in storage temperature, degradation rates approximately double, significantly reducing shelf life and analytical reliability. Most current storage facilities lack precise temperature regulation systems capable of maintaining the optimal 15-20°C range.
Contamination from storage containers presents an ongoing issue, with studies revealing that certain plastic polymers release compounds that interact with saltpeter over time. These interactions catalyze degradation pathways and introduce impurities that confound analytical results. Metal containers similarly pose problems through oxidative reactions, particularly when samples contain trace acidic compounds.
Light exposure, especially in the UV spectrum, accelerates photodegradation processes in stored saltpeter. Current preservation protocols often overlook this factor, with many storage facilities utilizing transparent or translucent containers that permit light penetration. Research demonstrates that samples exposed to laboratory lighting conditions for six months exhibit significantly higher levels of nitrite conversion compared to those stored in darkness.
Microbial contamination represents an underappreciated challenge in saltpeter preservation. Despite its antimicrobial properties at high concentrations, diluted or impure saltpeter can support microbial growth. Current sterilization procedures before storage are inconsistent across research facilities, leading to variable contamination levels that affect degradation rates and pathways.
Analytical challenges compound these preservation issues, as current methodologies struggle to distinguish between original impurities and degradation products formed during storage. Standard chromatographic techniques lack the sensitivity to detect early-stage degradation markers, often resulting in false negatives when assessing sample integrity. This analytical limitation hampers efforts to develop improved preservation protocols.
Economic constraints further complicate advancement in preservation technology. High-quality storage systems with controlled atmospheres and temperature regulation remain prohibitively expensive for many research institutions, leading to compromises in storage conditions that accelerate sample degradation. The cost-benefit analysis of advanced preservation methods versus sample replacement continues to favor suboptimal storage practices in many settings.
Temperature control represents another critical challenge, as saltpeter samples stored above 30°C show accelerated degradation rates. Research indicates that for every 10°C increase in storage temperature, degradation rates approximately double, significantly reducing shelf life and analytical reliability. Most current storage facilities lack precise temperature regulation systems capable of maintaining the optimal 15-20°C range.
Contamination from storage containers presents an ongoing issue, with studies revealing that certain plastic polymers release compounds that interact with saltpeter over time. These interactions catalyze degradation pathways and introduce impurities that confound analytical results. Metal containers similarly pose problems through oxidative reactions, particularly when samples contain trace acidic compounds.
Light exposure, especially in the UV spectrum, accelerates photodegradation processes in stored saltpeter. Current preservation protocols often overlook this factor, with many storage facilities utilizing transparent or translucent containers that permit light penetration. Research demonstrates that samples exposed to laboratory lighting conditions for six months exhibit significantly higher levels of nitrite conversion compared to those stored in darkness.
Microbial contamination represents an underappreciated challenge in saltpeter preservation. Despite its antimicrobial properties at high concentrations, diluted or impure saltpeter can support microbial growth. Current sterilization procedures before storage are inconsistent across research facilities, leading to variable contamination levels that affect degradation rates and pathways.
Analytical challenges compound these preservation issues, as current methodologies struggle to distinguish between original impurities and degradation products formed during storage. Standard chromatographic techniques lack the sensitivity to detect early-stage degradation markers, often resulting in false negatives when assessing sample integrity. This analytical limitation hampers efforts to develop improved preservation protocols.
Economic constraints further complicate advancement in preservation technology. High-quality storage systems with controlled atmospheres and temperature regulation remain prohibitively expensive for many research institutions, leading to compromises in storage conditions that accelerate sample degradation. The cost-benefit analysis of advanced preservation methods versus sample replacement continues to favor suboptimal storage practices in many settings.
Existing Methodologies for Impurity Detection and Analysis
01 Common impurities in saltpeter samples
Saltpeter (potassium nitrate) samples often contain various impurities that can affect their quality and performance. These impurities typically include chlorides, sulfates, heavy metals, and organic matter. The presence of these contaminants can impact the stability, hygroscopicity, and reactivity of saltpeter. Detection and quantification of these impurities are crucial for quality control in industries where saltpeter is used, such as fertilizers, explosives, and food preservation.- Common impurities in saltpeter samples: Saltpeter (potassium nitrate) samples often contain various impurities that can affect their quality and performance. These impurities typically include chlorides, sulfates, heavy metals, and organic matter. Detection and quantification of these impurities are essential for quality control in industries where saltpeter is used. Advanced analytical techniques such as chromatography, spectroscopy, and elemental analysis are employed to identify and measure these contaminants in saltpeter samples.
- Degradation pathways of saltpeter under environmental conditions: Saltpeter undergoes degradation through various pathways when exposed to environmental factors. Moisture absorption is a primary concern, leading to dissolution and recrystallization that can alter the physical properties. Thermal decomposition occurs at elevated temperatures, producing nitrogen oxides and oxygen. Photochemical degradation can happen when exposed to UV light. Microbial action may also contribute to the breakdown of saltpeter, particularly in the presence of organic matter. Understanding these degradation mechanisms is crucial for proper storage and handling of saltpeter-containing materials.
- Analytical methods for impurity detection in saltpeter: Various analytical methods have been developed for detecting and quantifying impurities in saltpeter samples. These include ion chromatography for anion analysis, atomic absorption spectroscopy for metal impurities, infrared spectroscopy for organic contaminants, and X-ray diffraction for crystalline impurity identification. Modern techniques like ICP-MS (Inductively Coupled Plasma Mass Spectrometry) offer high sensitivity for trace element analysis. Sample preparation techniques such as extraction, filtration, and pre-concentration are often employed to enhance detection limits and accuracy in impurity analysis.
- Stabilization techniques to prevent saltpeter degradation: Various stabilization techniques have been developed to prevent or minimize the degradation of saltpeter. These include coating with hydrophobic materials to prevent moisture absorption, addition of antioxidants to inhibit oxidative degradation, pH adjustment to maintain chemical stability, and incorporation of UV stabilizers to prevent photodegradation. Controlled storage conditions, including temperature, humidity, and light exposure management, are also critical for maintaining the integrity of saltpeter samples. These stabilization approaches extend the shelf life and maintain the efficacy of saltpeter-containing products.
- Impact of impurities on saltpeter performance and applications: Impurities in saltpeter can significantly impact its performance across various applications. In pyrotechnics and explosives, impurities can alter burning rates, sensitivity, and stability. For agricultural applications as fertilizer, certain impurities may affect nutrient availability or introduce toxicity to plants. In food preservation, impurities can influence preservation efficacy and may introduce unwanted flavors or health concerns. Industrial applications may experience reduced efficiency or equipment corrosion due to specific contaminants. Understanding these impacts drives the development of purification processes and quality standards for different saltpeter applications.
02 Degradation pathways of saltpeter under environmental conditions
Saltpeter undergoes various degradation pathways when exposed to environmental factors. Moisture absorption leads to dissolution and recrystallization, altering the physical structure. Temperature fluctuations can accelerate decomposition, particularly at elevated temperatures where it may break down into potassium nitrite and oxygen. Light exposure, especially UV radiation, can catalyze photochemical degradation. Microbial activity in the presence of organic matter can reduce nitrates to nitrites and further to nitrogen compounds through denitrification processes.Expand Specific Solutions03 Analytical methods for impurity detection in saltpeter
Various analytical techniques are employed to detect and quantify impurities in saltpeter samples. These include chromatographic methods such as ion chromatography and HPLC for ionic impurities, spectroscopic techniques including ICP-MS and atomic absorption spectroscopy for metal contaminants, and thermal analysis methods like DSC and TGA to evaluate thermal stability and decomposition behavior. Advanced techniques such as XRD and SEM-EDX are used to characterize crystalline structure and elemental composition, while FTIR spectroscopy helps identify organic contaminants.Expand Specific Solutions04 Stabilization techniques to prevent saltpeter degradation
Various stabilization techniques have been developed to prevent or minimize saltpeter degradation. These include coating with hydrophobic materials to reduce moisture absorption, addition of anti-caking agents to prevent agglomeration, incorporation of antioxidants to inhibit oxidative degradation, pH adjustment to maintain optimal stability, and controlled storage conditions with regulated temperature and humidity. Some formulations also include metal oxide stabilizers that can trap decomposition products and prevent autocatalytic degradation reactions.Expand Specific Solutions05 Impact of impurities on saltpeter applications
Impurities in saltpeter significantly impact its applications across various industries. In agricultural applications, metal contaminants can affect soil health and plant uptake. For pyrotechnic and explosive uses, impurities can alter burning rates, sensitivity, and stability, potentially creating safety hazards. In food preservation, certain impurities may introduce toxicity concerns or affect preservation efficacy. Industrial applications such as glass manufacturing and heat treatment processes require specific purity levels to ensure product quality. Pharmaceutical applications demand high-purity saltpeter to meet regulatory standards and ensure product safety.Expand Specific Solutions
Key Industry Players in Saltpeter Production and Storage
The research on impurities and degradation pathways in stored saltpeter samples is currently in an early development stage, with the market showing promising growth potential due to increasing applications in pharmaceuticals, agriculture, and industrial chemicals. The competitive landscape features established analytical companies like Bio-Rad Laboratories and Applied Materials providing sophisticated testing equipment, while chemical specialists such as Air Liquide, Kemira, and Tokuyama contribute expertise in chemical processing and purification. The technology remains moderately mature, with companies like Baxter International and Fresenius Kabi focusing on pharmaceutical-grade applications, while research institutions like Max Planck Society and IFP Energies Nouvelles drive fundamental scientific understanding of degradation mechanisms. Samsung Electronics and SUMCO represent the semiconductor industry's interest in high-purity saltpeter applications.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed a comprehensive analytical platform specifically for characterizing organic and biological impurities in stored saltpeter samples. Their approach combines high-performance liquid chromatography (HPLC) with advanced mass spectrometry to identify trace organic contaminants that can catalyze degradation. Bio-Rad's research has revealed previously unidentified microbial degradation pathways, demonstrating that certain bacterial species can utilize nitrate as an electron acceptor under oxygen-limited storage conditions, leading to reduction of nitrate to nitrite and eventually to nitrogen gas. Their proprietary sample preparation protocols enable extraction and concentration of both water-soluble and lipophilic impurities from saltpeter matrices. Bio-Rad has also developed specialized biomarker assays that can determine the biological origin of historical saltpeter samples based on residual DNA and protein fragments, providing valuable information for archaeological and historical research.
Strengths: Unparalleled capabilities in biological impurity characterization; innovative approaches to microbial degradation pathway identification; specialized expertise in biomarker analysis for historical samples. Weaknesses: Less focused on inorganic impurities and physical degradation mechanisms compared to competitors with more traditional chemical analysis backgrounds.
Max Planck Gesellschaft zur Förderung der Wissenschaften eV
Technical Solution: The Max Planck Institute has pioneered multidisciplinary research on saltpeter degradation, combining advanced analytical chemistry with materials science and historical preservation approaches. Their research utilizes synchrotron-based X-ray absorption spectroscopy (XAS) and X-ray diffraction (XRD) to characterize crystalline structure changes during degradation at the molecular level. This has enabled identification of intermediate degradation products previously undetectable by conventional methods. The Institute has developed a comprehensive degradation pathway model that accounts for both chemical and physical transformation processes, including hygroscopic water absorption, crystal structure rearrangement, and chemical decomposition. Their work with historical saltpeter samples from European gunpowder archives has established chronological markers for dating samples based on specific impurity profiles and degradation products. Additionally, they've created environmental aging chambers that can simulate centuries of natural aging in controlled laboratory conditions, allowing for accelerated testing of preservation strategies.
Strengths: World-class analytical capabilities combining multiple advanced techniques; interdisciplinary approach integrating chemistry, materials science, and historical preservation; unique access to historical sample collections for comparative studies. Weaknesses: Research is primarily academic in nature and may lack immediate commercial applications; some of their most advanced analytical techniques require specialized equipment not widely available.
Critical Research on Saltpeter Degradation Mechanisms
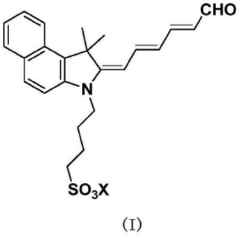
Degradation impurity of indocyanine green, preparation method therefor, and use thereof
PatentWO2024017404A9
Innovation
- An indocyanine dye degradation impurity and its related analysis method were prepared. The compound was prepared through degradation reaction under alkali, heat, and light conditions, and technical means such as silica gel thin-layer chromatography and liquid chromatography were used for separation and analysis. Purification, impurity controls are provided for quality control.
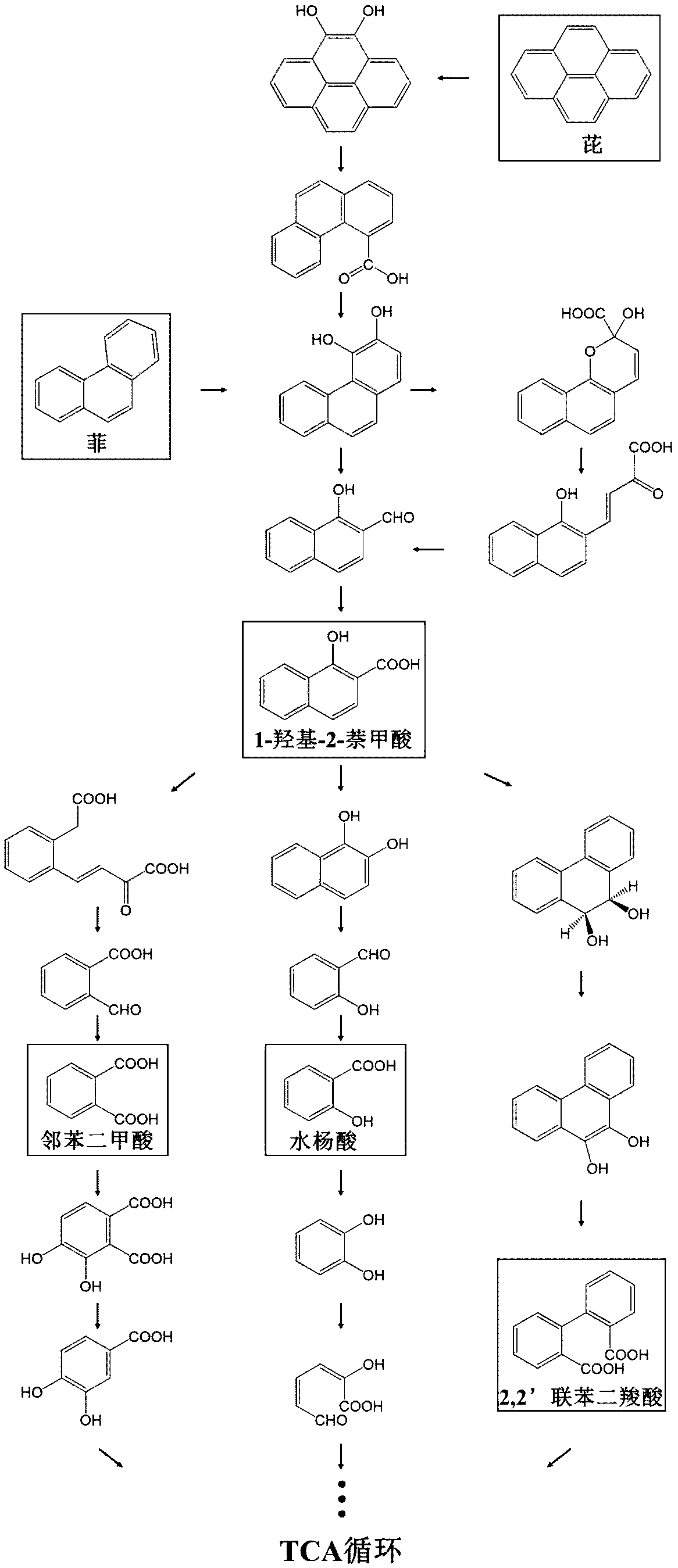
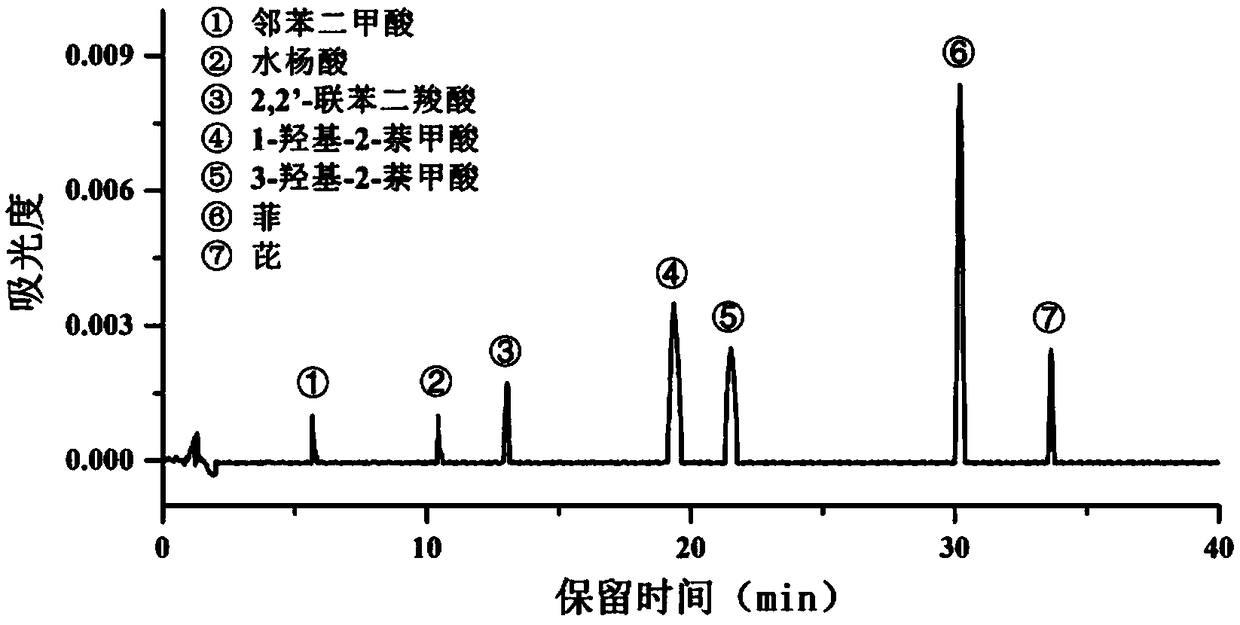
High-efficiency extraction of bacterial polycyclic aromatic hydrocarbon metabolites and identification of degradation pathways
PatentActiveCN107941938B
Innovation
- Degrading bacteria were cultured using phenanthrene/pyrene as the sole carbon source, intermediate degradation products were extracted through alkaline lysis, HPLC gradient elution conditions were optimized, and HPLC-MS technology was used for qualitative and quantitative analysis to determine the proportion of degradation pathways.
Environmental Factors Affecting Saltpeter Stability
The stability of saltpeter (potassium nitrate) in storage is significantly influenced by various environmental factors, which can accelerate degradation processes and lead to the formation of impurities. Temperature fluctuations represent one of the most critical factors affecting saltpeter stability. Research indicates that elevated temperatures accelerate chemical reactions within stored saltpeter, potentially leading to decomposition and the formation of nitrites and other nitrogen-containing compounds. Conversely, extremely low temperatures may cause physical stress through freeze-thaw cycles, potentially disrupting the crystalline structure.
Humidity levels play an equally important role in saltpeter degradation. As a hygroscopic substance, saltpeter readily absorbs moisture from the surrounding environment. This absorption can lead to partial dissolution and subsequent recrystallization, which often incorporates impurities from the storage environment. Studies have demonstrated that relative humidity exceeding 75% significantly increases the rate of moisture absorption, leading to accelerated degradation pathways.
Light exposure, particularly UV radiation, contributes to photochemical reactions in stored saltpeter. These reactions can catalyze the breakdown of nitrate compounds and facilitate the formation of nitrites and other degradation products. Research has shown that samples stored in transparent containers or exposed to direct sunlight exhibit higher levels of impurities compared to those stored in opaque containers or dark environments.
Atmospheric contaminants represent another significant environmental factor. Exposure to airborne pollutants, including sulfur dioxide, carbon dioxide, and various particulates, can lead to chemical reactions with saltpeter. These reactions often result in the formation of sulfates, carbonates, and other compounds that compromise the purity of the saltpeter. Industrial environments with high levels of atmospheric pollutants have been shown to accelerate degradation rates significantly.
Storage container materials can also influence saltpeter stability through potential chemical interactions. Metals such as iron, copper, and zinc may catalyze redox reactions with saltpeter, leading to the formation of metal nitrates and other compounds. Research indicates that inert materials such as glass or certain polymers provide superior protection against such contamination pathways.
Microbial activity, while often overlooked, can significantly impact saltpeter stability in environments with sufficient moisture. Certain bacteria can utilize nitrate as an electron acceptor in their metabolic processes, reducing it to nitrite and other nitrogen compounds. This biological pathway of degradation becomes particularly relevant in storage conditions with inadequate moisture control or in historical samples exposed to environmental microorganisms over extended periods.
Humidity levels play an equally important role in saltpeter degradation. As a hygroscopic substance, saltpeter readily absorbs moisture from the surrounding environment. This absorption can lead to partial dissolution and subsequent recrystallization, which often incorporates impurities from the storage environment. Studies have demonstrated that relative humidity exceeding 75% significantly increases the rate of moisture absorption, leading to accelerated degradation pathways.
Light exposure, particularly UV radiation, contributes to photochemical reactions in stored saltpeter. These reactions can catalyze the breakdown of nitrate compounds and facilitate the formation of nitrites and other degradation products. Research has shown that samples stored in transparent containers or exposed to direct sunlight exhibit higher levels of impurities compared to those stored in opaque containers or dark environments.
Atmospheric contaminants represent another significant environmental factor. Exposure to airborne pollutants, including sulfur dioxide, carbon dioxide, and various particulates, can lead to chemical reactions with saltpeter. These reactions often result in the formation of sulfates, carbonates, and other compounds that compromise the purity of the saltpeter. Industrial environments with high levels of atmospheric pollutants have been shown to accelerate degradation rates significantly.
Storage container materials can also influence saltpeter stability through potential chemical interactions. Metals such as iron, copper, and zinc may catalyze redox reactions with saltpeter, leading to the formation of metal nitrates and other compounds. Research indicates that inert materials such as glass or certain polymers provide superior protection against such contamination pathways.
Microbial activity, while often overlooked, can significantly impact saltpeter stability in environments with sufficient moisture. Certain bacteria can utilize nitrate as an electron acceptor in their metabolic processes, reducing it to nitrite and other nitrogen compounds. This biological pathway of degradation becomes particularly relevant in storage conditions with inadequate moisture control or in historical samples exposed to environmental microorganisms over extended periods.
Regulatory Standards for Stored Chemical Compounds
The regulatory landscape for stored chemical compounds, particularly saltpeter (potassium nitrate), is governed by multiple international and national frameworks that establish specific standards for storage, handling, and quality control. The International Organization for Standardization (ISO) has developed ISO 14001 and ISO 9001 standards that indirectly apply to chemical storage practices, emphasizing environmental management systems and quality control processes respectively.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) have established comprehensive guidelines for chemical storage. OSHA's 29 CFR 1910.1200 Hazard Communication Standard requires proper labeling and safety data sheets for stored chemicals, while EPA regulations under the Resource Conservation and Recovery Act (RCRA) govern the management of hazardous waste that may result from degraded compounds.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation provides stringent requirements for chemical safety assessment and documentation. For saltpeter specifically, which is classified as an oxidizing agent, the EU's Seveso III Directive applies when stored in quantities exceeding certain thresholds, requiring facilities to implement major accident prevention policies.
Storage standards for saltpeter are particularly stringent due to its oxidizing properties. The National Fire Protection Association (NFPA) code 400 classifies potassium nitrate as a Class 1 oxidizer, requiring specific storage conditions including segregation from combustible materials, temperature and humidity controls, and proper ventilation systems. These standards are critical as impurities and degradation can significantly alter the chemical's properties and safety profile.
Pharmacopoeias worldwide, including the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), establish purity standards for potassium nitrate used in pharmaceutical applications. These standards typically specify maximum allowable limits for impurities such as chlorides, sulfates, and heavy metals, with regular testing requirements to ensure compliance throughout the storage period.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, particularly ICH Q3A and Q3B, provide frameworks for establishing acceptable levels of impurities in chemical compounds and for validating analytical procedures used to detect degradation products. These guidelines are essential for research on impurities and degradation pathways in stored saltpeter samples.
Regulatory bodies also mandate specific documentation requirements for stored chemicals, including certificates of analysis, stability data, and degradation studies. The FDA's current Good Manufacturing Practices (cGMP) and the WHO's Good Storage and Distribution Practices provide additional guidance on documentation systems necessary for tracking the quality of stored chemical compounds throughout their lifecycle.
In the United States, the Environmental Protection Agency (EPA) and the Occupational Safety and Health Administration (OSHA) have established comprehensive guidelines for chemical storage. OSHA's 29 CFR 1910.1200 Hazard Communication Standard requires proper labeling and safety data sheets for stored chemicals, while EPA regulations under the Resource Conservation and Recovery Act (RCRA) govern the management of hazardous waste that may result from degraded compounds.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation provides stringent requirements for chemical safety assessment and documentation. For saltpeter specifically, which is classified as an oxidizing agent, the EU's Seveso III Directive applies when stored in quantities exceeding certain thresholds, requiring facilities to implement major accident prevention policies.
Storage standards for saltpeter are particularly stringent due to its oxidizing properties. The National Fire Protection Association (NFPA) code 400 classifies potassium nitrate as a Class 1 oxidizer, requiring specific storage conditions including segregation from combustible materials, temperature and humidity controls, and proper ventilation systems. These standards are critical as impurities and degradation can significantly alter the chemical's properties and safety profile.
Pharmacopoeias worldwide, including the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.), establish purity standards for potassium nitrate used in pharmaceutical applications. These standards typically specify maximum allowable limits for impurities such as chlorides, sulfates, and heavy metals, with regular testing requirements to ensure compliance throughout the storage period.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, particularly ICH Q3A and Q3B, provide frameworks for establishing acceptable levels of impurities in chemical compounds and for validating analytical procedures used to detect degradation products. These guidelines are essential for research on impurities and degradation pathways in stored saltpeter samples.
Regulatory bodies also mandate specific documentation requirements for stored chemicals, including certificates of analysis, stability data, and degradation studies. The FDA's current Good Manufacturing Practices (cGMP) and the WHO's Good Storage and Distribution Practices provide additional guidance on documentation systems necessary for tracking the quality of stored chemical compounds throughout their lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!