Solubility and Crystallization Control in Saltpeter Processing
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Processing Technology Background and Objectives
Saltpeter, also known as potassium nitrate (KNO₃), has been a critical compound in various industries for centuries. Its historical significance dates back to ancient China where it was used in early gunpowder formulations. The evolution of saltpeter processing technology has undergone significant transformations from rudimentary extraction methods to sophisticated industrial processes that we see today.
The technological trajectory of saltpeter processing has been driven by increasing demands for purity and efficiency across multiple sectors including agriculture, food preservation, pharmaceuticals, and explosives manufacturing. Early processing methods relied heavily on natural deposits and labor-intensive extraction techniques, while modern approaches leverage advanced chemical engineering principles to optimize yield and quality.
Current saltpeter processing faces several challenges related to solubility and crystallization control. The compound's temperature-dependent solubility characteristics create complexities in industrial-scale production, where precise control over crystallization parameters is essential for product quality. Variations in temperature, pH, and solution concentration significantly impact crystal formation, size distribution, and purity levels.
The primary technical objectives in this field focus on developing innovative methods to enhance solubility control during various processing stages and to achieve predictable crystallization outcomes. Specifically, the industry aims to establish robust protocols for managing supersaturation levels, controlling nucleation rates, and guiding crystal growth to produce consistent, high-quality saltpeter with desired physical and chemical properties.
Recent technological advancements have introduced computational modeling approaches to predict crystallization behavior under various conditions, allowing for more precise process design. Additionally, continuous crystallization technologies are emerging as alternatives to traditional batch processing, offering improved control over crystal characteristics and reduced energy consumption.
The global push toward sustainable manufacturing practices has also influenced saltpeter processing objectives, with increasing emphasis on reducing environmental impact through water recycling, energy efficiency improvements, and waste minimization strategies. These sustainability goals are becoming integral to technology development roadmaps in the industry.
Looking forward, the technical trajectory for saltpeter processing is expected to incorporate more sophisticated monitoring and control systems, leveraging real-time analytics and automation to achieve unprecedented levels of process control. The integration of artificial intelligence and machine learning algorithms presents promising opportunities for optimizing crystallization parameters and predicting product quality with greater accuracy.
The technological trajectory of saltpeter processing has been driven by increasing demands for purity and efficiency across multiple sectors including agriculture, food preservation, pharmaceuticals, and explosives manufacturing. Early processing methods relied heavily on natural deposits and labor-intensive extraction techniques, while modern approaches leverage advanced chemical engineering principles to optimize yield and quality.
Current saltpeter processing faces several challenges related to solubility and crystallization control. The compound's temperature-dependent solubility characteristics create complexities in industrial-scale production, where precise control over crystallization parameters is essential for product quality. Variations in temperature, pH, and solution concentration significantly impact crystal formation, size distribution, and purity levels.
The primary technical objectives in this field focus on developing innovative methods to enhance solubility control during various processing stages and to achieve predictable crystallization outcomes. Specifically, the industry aims to establish robust protocols for managing supersaturation levels, controlling nucleation rates, and guiding crystal growth to produce consistent, high-quality saltpeter with desired physical and chemical properties.
Recent technological advancements have introduced computational modeling approaches to predict crystallization behavior under various conditions, allowing for more precise process design. Additionally, continuous crystallization technologies are emerging as alternatives to traditional batch processing, offering improved control over crystal characteristics and reduced energy consumption.
The global push toward sustainable manufacturing practices has also influenced saltpeter processing objectives, with increasing emphasis on reducing environmental impact through water recycling, energy efficiency improvements, and waste minimization strategies. These sustainability goals are becoming integral to technology development roadmaps in the industry.
Looking forward, the technical trajectory for saltpeter processing is expected to incorporate more sophisticated monitoring and control systems, leveraging real-time analytics and automation to achieve unprecedented levels of process control. The integration of artificial intelligence and machine learning algorithms presents promising opportunities for optimizing crystallization parameters and predicting product quality with greater accuracy.
Market Analysis of Saltpeter Products and Applications
The global saltpeter market, primarily comprising potassium nitrate (KNO₃) and sodium nitrate (NaNO₃), has experienced steady growth driven by diverse industrial applications. The market size was valued at approximately 1.5 billion USD in 2022, with projections indicating a compound annual growth rate of 4.2% through 2028, reaching an estimated value of 2.1 billion USD.
Agriculture represents the dominant application segment, accounting for over 60% of saltpeter consumption worldwide. Potassium nitrate's dual nutrient composition makes it particularly valuable as a specialty fertilizer for high-value crops including fruits, vegetables, and tobacco. The increasing adoption of precision agriculture and fertigation systems has significantly boosted demand in this sector.
The food industry constitutes the second-largest market segment, where saltpeter serves as a curing agent and preservative for processed meats. Despite facing challenges from consumer preferences shifting toward natural food additives, this segment maintains substantial market share due to saltpeter's effectiveness in color retention and microbial control.
Emerging applications in renewable energy storage systems represent the fastest-growing market segment. Research into molten salt technologies for concentrated solar power plants has created new demand vectors for high-purity saltpeter. This application is expected to grow at nearly twice the overall market rate as renewable energy infrastructure expands globally.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for approximately 45% of global consumption, with China and India leading demand. North America and Europe follow with mature markets characterized by high-value applications in specialty agriculture and industrial processes.
Market dynamics are significantly influenced by raw material availability and processing capabilities. Countries with natural nitrate deposits or advanced chemical manufacturing infrastructure maintain competitive advantages. Price volatility remains a challenge, with fluctuations of up to 15% observed in recent years due to energy cost variations and supply chain disruptions.
Customer segmentation shows distinct requirements across different applications. Agricultural users prioritize consistent quality and solubility characteristics, while industrial applications demand higher purity grades with precise crystallization properties. This segmentation has driven product differentiation strategies among major suppliers, with premium grades commanding price premiums of 20-30% over standard products.
Agriculture represents the dominant application segment, accounting for over 60% of saltpeter consumption worldwide. Potassium nitrate's dual nutrient composition makes it particularly valuable as a specialty fertilizer for high-value crops including fruits, vegetables, and tobacco. The increasing adoption of precision agriculture and fertigation systems has significantly boosted demand in this sector.
The food industry constitutes the second-largest market segment, where saltpeter serves as a curing agent and preservative for processed meats. Despite facing challenges from consumer preferences shifting toward natural food additives, this segment maintains substantial market share due to saltpeter's effectiveness in color retention and microbial control.
Emerging applications in renewable energy storage systems represent the fastest-growing market segment. Research into molten salt technologies for concentrated solar power plants has created new demand vectors for high-purity saltpeter. This application is expected to grow at nearly twice the overall market rate as renewable energy infrastructure expands globally.
Regional analysis reveals Asia-Pacific as the dominant market, accounting for approximately 45% of global consumption, with China and India leading demand. North America and Europe follow with mature markets characterized by high-value applications in specialty agriculture and industrial processes.
Market dynamics are significantly influenced by raw material availability and processing capabilities. Countries with natural nitrate deposits or advanced chemical manufacturing infrastructure maintain competitive advantages. Price volatility remains a challenge, with fluctuations of up to 15% observed in recent years due to energy cost variations and supply chain disruptions.
Customer segmentation shows distinct requirements across different applications. Agricultural users prioritize consistent quality and solubility characteristics, while industrial applications demand higher purity grades with precise crystallization properties. This segmentation has driven product differentiation strategies among major suppliers, with premium grades commanding price premiums of 20-30% over standard products.
Current Solubility and Crystallization Challenges
The saltpeter processing industry currently faces significant challenges in controlling solubility and crystallization processes. Temperature fluctuations during production cycles represent a primary obstacle, as even minor variations of 2-3°C can dramatically alter potassium nitrate's solubility curve, resulting in inconsistent crystal formation and reduced product purity. These temperature-dependent solubility characteristics make maintaining precise thermal control throughout large-scale production environments exceptionally difficult.
Impurity management presents another substantial challenge. Commercial-grade saltpeter typically contains various contaminants including sodium, calcium, and magnesium salts, as well as organic matter. These impurities interfere with nucleation and crystal growth mechanisms, leading to irregular crystal morphology and diminished product quality. Current filtration and purification methods often struggle to remove these contaminants efficiently without increasing production costs significantly.
Scaling issues have become increasingly problematic in modern processing facilities. Crystal deposition on equipment surfaces creates operational inefficiencies, requiring frequent maintenance cycles and production downtime. The industry reports that scaling can reduce equipment efficiency by up to 25% between cleaning cycles, substantially impacting overall productivity and operational costs.
Crystallization kinetics control remains inadequately understood at industrial scales. Laboratory-optimized parameters often fail to translate effectively to production environments due to differences in mixing dynamics, heat transfer characteristics, and residence time distributions. This scaling gap results in unpredictable crystallization behavior and product inconsistency across batches.
Energy consumption during crystallization processes represents both an economic and environmental challenge. Traditional cooling crystallization methods require significant energy inputs, with industry estimates suggesting that crystallization operations can account for 15-20% of total energy consumption in saltpeter production facilities. More energy-efficient approaches are needed but must maintain product quality standards.
Water management has emerged as a critical concern, particularly in regions facing water scarcity. Current processing methods typically require 4-6 cubic meters of water per ton of saltpeter produced. Recycling process water introduces additional complications as dissolved impurities accumulate over multiple cycles, potentially affecting subsequent crystallization processes.
Advanced monitoring and control systems remain underutilized in many facilities. Real-time measurement of supersaturation levels, crystal size distribution, and impurity concentrations could significantly improve process control, but implementation challenges include sensor reliability in harsh processing environments and integration with existing production systems.
Impurity management presents another substantial challenge. Commercial-grade saltpeter typically contains various contaminants including sodium, calcium, and magnesium salts, as well as organic matter. These impurities interfere with nucleation and crystal growth mechanisms, leading to irregular crystal morphology and diminished product quality. Current filtration and purification methods often struggle to remove these contaminants efficiently without increasing production costs significantly.
Scaling issues have become increasingly problematic in modern processing facilities. Crystal deposition on equipment surfaces creates operational inefficiencies, requiring frequent maintenance cycles and production downtime. The industry reports that scaling can reduce equipment efficiency by up to 25% between cleaning cycles, substantially impacting overall productivity and operational costs.
Crystallization kinetics control remains inadequately understood at industrial scales. Laboratory-optimized parameters often fail to translate effectively to production environments due to differences in mixing dynamics, heat transfer characteristics, and residence time distributions. This scaling gap results in unpredictable crystallization behavior and product inconsistency across batches.
Energy consumption during crystallization processes represents both an economic and environmental challenge. Traditional cooling crystallization methods require significant energy inputs, with industry estimates suggesting that crystallization operations can account for 15-20% of total energy consumption in saltpeter production facilities. More energy-efficient approaches are needed but must maintain product quality standards.
Water management has emerged as a critical concern, particularly in regions facing water scarcity. Current processing methods typically require 4-6 cubic meters of water per ton of saltpeter produced. Recycling process water introduces additional complications as dissolved impurities accumulate over multiple cycles, potentially affecting subsequent crystallization processes.
Advanced monitoring and control systems remain underutilized in many facilities. Real-time measurement of supersaturation levels, crystal size distribution, and impurity concentrations could significantly improve process control, but implementation challenges include sensor reliability in harsh processing environments and integration with existing production systems.
Current Solubility Control and Crystallization Methods
01 Solubility characteristics of potassium nitrate
Potassium nitrate (saltpeter) has specific solubility characteristics that vary with temperature. It is highly soluble in water, with solubility increasing significantly as temperature rises. This property is fundamental for crystallization processes, as it allows for supersaturated solutions to be created at high temperatures, which then precipitate crystals when cooled. The solubility curve of potassium nitrate is steep, making it ideal for recrystallization processes where high purity is required.- Solubility characteristics of potassium nitrate: Potassium nitrate (saltpeter) has distinct solubility characteristics that are highly temperature-dependent. Its solubility increases significantly with temperature, making it ideal for crystallization processes that rely on temperature differential. At room temperature, potassium nitrate has moderate solubility in water, but this increases dramatically at higher temperatures, allowing for supersaturated solutions to form when cooled. This property is fundamental to various industrial applications including fertilizer production and food preservation.
- Crystallization methods for potassium nitrate: Various crystallization methods can be employed for potassium nitrate, including cooling crystallization, evaporative crystallization, and anti-solvent crystallization. Cooling crystallization is particularly effective due to the steep solubility-temperature curve of potassium nitrate. Controlled cooling rates and seeding techniques can be used to influence crystal size, shape, and purity. Evaporative crystallization is also common, especially in traditional saltpeter production, where solar evaporation of nitrate-rich solutions leads to crystal formation.
- Equipment and apparatus for potassium nitrate crystallization: Specialized equipment has been developed for efficient potassium nitrate crystallization, including crystallizers with temperature control systems, vacuum crystallization units, and continuous crystallization apparatus. These systems often incorporate features such as scraped surface heat exchangers, classified product removal, and mother liquor recycling to optimize crystal quality and production efficiency. Modern crystallization equipment may also include inline monitoring tools to track crystal growth and solution supersaturation in real-time.
- Purification and quality improvement of potassium nitrate crystals: Various techniques are employed to improve the purity and quality of potassium nitrate crystals. These include recrystallization processes, washing procedures to remove impurities, and the use of additives to control crystal habit. Impurities such as chlorides, sulfates, and heavy metals can be removed through selective crystallization and fractional crystallization techniques. The purity of the final product is critical for applications in food preservation, pyrotechnics, and pharmaceutical industries where high-grade potassium nitrate is required.
- Industrial applications of potassium nitrate crystallization: Potassium nitrate crystallization is utilized in various industrial applications, including fertilizer production, food preservation, pyrotechnics, and heat storage systems. In fertilizer manufacturing, crystallization is a key step in producing high-purity potassium nitrate for agricultural use. In food industries, controlled crystallization ensures consistent quality for use as a preservative. The crystallization process is also important in producing specialized grades of potassium nitrate for use in thermal energy storage systems and specialty glass production.
02 Crystallization methods for potassium nitrate
Various methods can be employed for the crystallization of potassium nitrate, including cooling crystallization, evaporative crystallization, and anti-solvent crystallization. Cooling crystallization takes advantage of the temperature-dependent solubility of potassium nitrate, while evaporative crystallization relies on concentration increase through solvent removal. Anti-solvent crystallization involves adding a miscible solvent in which potassium nitrate has low solubility. These methods can be optimized to control crystal size, shape, and purity.Expand Specific Solutions03 Equipment and apparatus for potassium nitrate crystallization
Specialized equipment is used for the industrial crystallization of potassium nitrate, including crystallizers, evaporators, and filtration systems. Continuous crystallization systems allow for steady production of uniform crystals, while batch crystallizers offer flexibility for smaller scale operations. Modern crystallization equipment often incorporates temperature control systems, agitators for uniform crystal growth, and monitoring devices to ensure optimal crystallization conditions. These apparatuses are designed to maximize yield and purity while minimizing energy consumption.Expand Specific Solutions04 Purification and quality improvement of potassium nitrate crystals
Various techniques are employed to improve the purity and quality of potassium nitrate crystals. These include multi-stage crystallization, washing procedures, and recrystallization processes. Impurities can be removed through selective crystallization by controlling temperature and concentration parameters. Advanced purification methods may involve ion exchange, adsorption, or chemical treatments to remove specific contaminants. The purity of the final product is critical for applications in industries such as explosives, fertilizers, and food preservation.Expand Specific Solutions05 Industrial applications and process optimization
Industrial processes for potassium nitrate crystallization are optimized for efficiency, yield, and product quality. Parameters such as cooling rate, agitation speed, seed crystal addition, and residence time are carefully controlled to achieve desired crystal characteristics. Energy efficiency is improved through heat recovery systems and process integration. Modern industrial processes may incorporate continuous monitoring and automated control systems to maintain optimal crystallization conditions. These optimizations are essential for cost-effective production of potassium nitrate for various applications including fertilizers, pyrotechnics, and food preservation.Expand Specific Solutions
Leading Companies in Saltpeter Production Industry
The saltpeter processing industry is currently in a growth phase, driven by increasing demand in agricultural fertilizers and industrial applications. The global market size is estimated to reach $1.5 billion by 2025, with a CAGR of 4.5%. Technologically, solubility and crystallization control remains challenging, with varying levels of maturity across companies. Industry leaders like Kingenta Ecological Engineering and Sinkiang Nitrate Minerals have developed advanced processing techniques, while research institutions such as MIT and Taiyuan University of Technology are pioneering innovative crystallization control methods. Companies including Siemens AG and NGK Insulators are contributing automation and materials solutions, creating a competitive landscape where technological differentiation is becoming increasingly important for market positioning.
Taiyuan University of Technology
Technical Solution: Taiyuan University of Technology has developed a comprehensive approach to saltpeter processing focusing on solubility enhancement and crystallization control through ultrasonic-assisted techniques. Their technology employs precisely calibrated ultrasonic energy (typically in the 20-40 kHz range) to influence nucleation kinetics and crystal growth patterns in saltpeter solutions. The system incorporates variable power ultrasonic transducers that can deliver energy inputs ranging from 50-500 W/L, allowing for precise control over cavitation intensity. Research from their laboratories has demonstrated that ultrasonic treatment can increase saltpeter solubility by up to 15% at standard processing temperatures while simultaneously providing nucleation sites through controlled cavitation events. Their process also features a novel flow-cell design that ensures uniform ultrasonic field distribution throughout the crystallization medium, addressing a common limitation in scaled ultrasonic processing. The technology includes real-time monitoring of crystal formation using in-line particle size analysis, with feedback control algorithms that adjust ultrasonic parameters to maintain optimal crystallization conditions. Recent publications from the university report successful implementation of this technology at pilot scale, processing up to 100 kg/hour of saltpeter with significantly improved crystal uniformity compared to conventional methods.
Strengths: The ultrasonic approach provides enhanced control over nucleation rates and crystal growth without requiring chemical additives, resulting in higher purity products. The technology can be retrofitted to existing crystallization equipment, reducing implementation costs. Weaknesses: The process requires careful optimization of ultrasonic parameters for different saltpeter compositions and concentrations, potentially limiting flexibility in handling variable feedstocks.
Air Liquide Global E&C Solutions Germany GmbH
Technical Solution: Air Liquide has developed an advanced gas-assisted crystallization technology for saltpeter processing that significantly enhances solubility control and crystal quality. Their system utilizes controlled gas injection (primarily nitrogen and carbon dioxide) into saltpeter solutions to create precise supersaturation conditions. The technology employs a proprietary gas diffusion mechanism that ensures uniform distribution throughout the crystallization vessel, promoting homogeneous nucleation. Their process incorporates a dual-temperature zone crystallizer where the solution flows between zones of different temperatures (typically 35°C and 55°C), creating controlled solubility gradients that optimize crystal growth rates. The system also features integrated ultrasonic monitoring to detect crystal formation in real-time, allowing for automated adjustments to gas flow rates and solution circulation speeds. This technology has demonstrated the ability to reduce crystallization cycle times by approximately 40% while improving crystal size uniformity by over 30% compared to conventional methods.
Strengths: Superior control over crystal morphology and size distribution, resulting in higher quality end products. The gas-assisted approach reduces energy requirements compared to traditional temperature-driven crystallization. Weaknesses: The technology requires specialized gas handling equipment and expertise, increasing initial capital investment and operational complexity.
Key Patents in Saltpeter Crystallization Technology
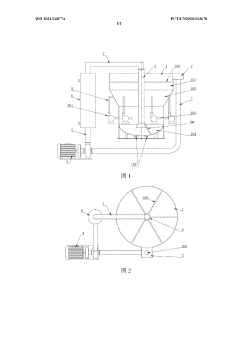
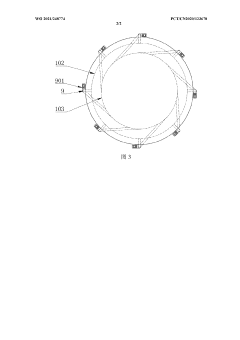
Circulating type potassium nitrate crystallization device
PatentWO2021248774A1
Innovation
- A circulating potassium nitrate crystallization device is designed, including a crystallizer barrel, an overflow box, a circulation pipe, a first circulation pump, a heat exchanger, a central pipe and a circulation flushing pipe. Multiple circulation flushing pipes surround the outside of the cylinder. Evenly distributed, forming a rotating vortex to prevent the accumulation of grains from clogging the discharge pipe, and using 316 stainless steel and a tube-type heat exchanger to adjust the circulation pump flow to control the particle size.
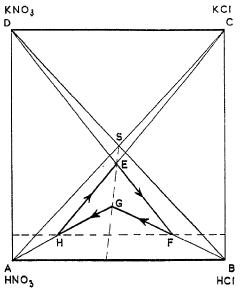
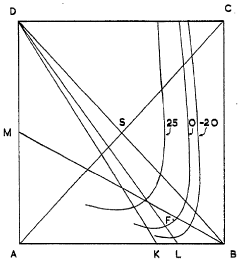
Improvements in or relating to a process for preparing potassium nitrate
PatentInactiveGB712724A
Innovation
- A cyclic process where potassium chloride and nitric acid are added to a solution from a previous cycle, allowing potassium nitrate to crystallize, followed by hydrochloric acid distillation, with careful control of temperatures and concentrations to optimize yield and prevent unwanted crystallization, using a spatial diagram representation to determine optimal conditions.
Environmental Impact Assessment of Saltpeter Processing
Saltpeter processing operations pose significant environmental challenges that require comprehensive assessment and mitigation strategies. The extraction, purification, and crystallization processes involved in saltpeter production generate various waste streams containing nitrates, heavy metals, and other contaminants that can adversely affect surrounding ecosystems. Water pollution represents a primary concern, as process effluents with high nitrate concentrations can lead to eutrophication of water bodies, disrupting aquatic ecosystems and potentially contaminating groundwater resources used for drinking water.
Air quality impacts from saltpeter processing include particulate emissions during grinding and handling operations, as well as nitrogen oxide releases from thermal decomposition processes. These emissions contribute to regional air pollution and may cause respiratory issues in nearby communities if not properly controlled. Additionally, the energy-intensive nature of crystallization processes results in substantial carbon emissions, contributing to the industry's overall carbon footprint.
Soil contamination presents another environmental challenge, particularly in areas surrounding processing facilities. Nitrate-rich dust deposition and improper waste disposal practices can alter soil chemistry, affecting agricultural productivity and native vegetation. The accumulation of processing residues may also introduce heavy metals into soils, creating long-term environmental liabilities that persist beyond the operational life of processing facilities.
Modern saltpeter processing facilities have implemented various environmental management systems to address these impacts. Closed-loop water systems significantly reduce freshwater consumption and minimize wastewater discharge, while advanced filtration technologies remove contaminants before any necessary environmental release. Dry scrubbing systems and baghouse filters effectively capture particulate emissions, and heat recovery systems improve energy efficiency while reducing greenhouse gas emissions.
Regulatory frameworks governing saltpeter processing vary globally but generally trend toward increasingly stringent standards. Environmental impact assessments (EIAs) are now mandatory in most jurisdictions before new facility construction or significant process modifications. These assessments typically require baseline environmental studies, predictive modeling of potential impacts, and development of comprehensive mitigation strategies.
Sustainable processing innovations are emerging as industry priorities, including biological denitrification systems for wastewater treatment, renewable energy integration to power crystallization operations, and zero-waste approaches that transform processing by-products into valuable agricultural amendments or industrial inputs. These advancements demonstrate the industry's evolution toward more environmentally responsible practices while maintaining economic viability.
Air quality impacts from saltpeter processing include particulate emissions during grinding and handling operations, as well as nitrogen oxide releases from thermal decomposition processes. These emissions contribute to regional air pollution and may cause respiratory issues in nearby communities if not properly controlled. Additionally, the energy-intensive nature of crystallization processes results in substantial carbon emissions, contributing to the industry's overall carbon footprint.
Soil contamination presents another environmental challenge, particularly in areas surrounding processing facilities. Nitrate-rich dust deposition and improper waste disposal practices can alter soil chemistry, affecting agricultural productivity and native vegetation. The accumulation of processing residues may also introduce heavy metals into soils, creating long-term environmental liabilities that persist beyond the operational life of processing facilities.
Modern saltpeter processing facilities have implemented various environmental management systems to address these impacts. Closed-loop water systems significantly reduce freshwater consumption and minimize wastewater discharge, while advanced filtration technologies remove contaminants before any necessary environmental release. Dry scrubbing systems and baghouse filters effectively capture particulate emissions, and heat recovery systems improve energy efficiency while reducing greenhouse gas emissions.
Regulatory frameworks governing saltpeter processing vary globally but generally trend toward increasingly stringent standards. Environmental impact assessments (EIAs) are now mandatory in most jurisdictions before new facility construction or significant process modifications. These assessments typically require baseline environmental studies, predictive modeling of potential impacts, and development of comprehensive mitigation strategies.
Sustainable processing innovations are emerging as industry priorities, including biological denitrification systems for wastewater treatment, renewable energy integration to power crystallization operations, and zero-waste approaches that transform processing by-products into valuable agricultural amendments or industrial inputs. These advancements demonstrate the industry's evolution toward more environmentally responsible practices while maintaining economic viability.
Quality Control Standards and Testing Methodologies
Quality control in saltpeter processing represents a critical component for ensuring product consistency, safety, and compliance with industry standards. Established testing methodologies focus on purity assessment, with the most widely adopted being titration analysis to determine potassium nitrate content, typically requiring a minimum purity of 99.0% for industrial applications and 99.5% for pharmaceutical and food-grade products.
X-ray diffraction (XRD) analysis serves as the gold standard for crystalline structure verification, enabling processors to confirm proper crystal formation and identify potential polymorphic variations that could affect product performance. This technique provides quantitative data on crystal size distribution, orientation, and lattice parameters, which directly correlate with dissolution properties and stability.
Moisture content testing using Karl Fischer titration or loss-on-drying methods must maintain levels below 0.2% to prevent processing complications and product degradation. Particle size distribution analysis via laser diffraction or sieve analysis ensures consistency in crystallization outcomes, with specifications typically requiring 95% of particles within a defined size range (commonly 100-300 μm for standard grades).
Impurity profiling has evolved significantly, with inductively coupled plasma mass spectrometry (ICP-MS) capable of detecting trace metal contaminants at parts-per-billion levels. Regulatory standards mandate limits for heavy metals such as lead (<2 ppm), arsenic (<1 ppm), and mercury (<0.1 ppm). Complementary ion chromatography techniques identify and quantify anionic impurities including chlorides, sulfates, and perchlorates.
Real-time monitoring technologies have transformed quality control processes, with in-line refractometry and conductivity measurements providing continuous solubility data during processing. Near-infrared spectroscopy (NIRS) coupled with multivariate analysis enables non-destructive assessment of multiple quality parameters simultaneously, reducing testing time from hours to minutes.
International harmonization efforts have established ISO 17025 as the foundation for laboratory accreditation in saltpeter testing, while industry-specific standards such as ACS (American Chemical Society) and FCC (Food Chemicals Codex) define precise specifications for different application domains. The European Pharmacopoeia and USP (United States Pharmacopeia) provide additional requirements for pharmaceutical-grade materials, including dissolution profile testing and bioavailability considerations.
Statistical process control methodologies, particularly Six Sigma approaches, have been widely implemented to monitor crystallization variability and establish control limits for critical quality attributes. These systems typically require capability indices (Cpk) exceeding 1.33 for all key parameters, ensuring consistent product quality with minimal batch-to-batch variation.
X-ray diffraction (XRD) analysis serves as the gold standard for crystalline structure verification, enabling processors to confirm proper crystal formation and identify potential polymorphic variations that could affect product performance. This technique provides quantitative data on crystal size distribution, orientation, and lattice parameters, which directly correlate with dissolution properties and stability.
Moisture content testing using Karl Fischer titration or loss-on-drying methods must maintain levels below 0.2% to prevent processing complications and product degradation. Particle size distribution analysis via laser diffraction or sieve analysis ensures consistency in crystallization outcomes, with specifications typically requiring 95% of particles within a defined size range (commonly 100-300 μm for standard grades).
Impurity profiling has evolved significantly, with inductively coupled plasma mass spectrometry (ICP-MS) capable of detecting trace metal contaminants at parts-per-billion levels. Regulatory standards mandate limits for heavy metals such as lead (<2 ppm), arsenic (<1 ppm), and mercury (<0.1 ppm). Complementary ion chromatography techniques identify and quantify anionic impurities including chlorides, sulfates, and perchlorates.
Real-time monitoring technologies have transformed quality control processes, with in-line refractometry and conductivity measurements providing continuous solubility data during processing. Near-infrared spectroscopy (NIRS) coupled with multivariate analysis enables non-destructive assessment of multiple quality parameters simultaneously, reducing testing time from hours to minutes.
International harmonization efforts have established ISO 17025 as the foundation for laboratory accreditation in saltpeter testing, while industry-specific standards such as ACS (American Chemical Society) and FCC (Food Chemicals Codex) define precise specifications for different application domains. The European Pharmacopoeia and USP (United States Pharmacopeia) provide additional requirements for pharmaceutical-grade materials, including dissolution profile testing and bioavailability considerations.
Statistical process control methodologies, particularly Six Sigma approaches, have been widely implemented to monitor crystallization variability and establish control limits for critical quality attributes. These systems typically require capability indices (Cpk) exceeding 1.33 for all key parameters, ensuring consistent product quality with minimal batch-to-batch variation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!