Surface Morphology Changes in Saltpeter during Thermal Cycling
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Thermal Cycling Background and Objectives
Saltpeter, chemically known as potassium nitrate (KNO₃), has been a compound of significant historical and industrial importance for centuries. Its applications span from traditional uses in gunpowder and food preservation to modern applications in fertilizers, pyrotechnics, and as a component in various industrial processes. The thermal behavior of saltpeter has garnered increasing attention due to its critical role in numerous thermal energy storage systems and industrial applications where materials undergo repeated heating and cooling cycles.
The evolution of saltpeter's surface morphology during thermal cycling represents a complex interplay of physical and chemical transformations that significantly impact material performance and longevity. Historical research has primarily focused on the bulk properties of saltpeter, with less emphasis on surface-level changes that occur during thermal stress. Recent technological advancements in microscopy and thermal analysis have enabled more detailed investigations into these surface phenomena.
Current technological trends indicate a growing interest in understanding how repeated thermal cycling affects the crystalline structure, porosity, and overall surface integrity of saltpeter. This interest is driven by the need to enhance the efficiency and durability of systems utilizing this compound, particularly in renewable energy storage applications and specialized industrial processes where thermal stability is paramount.
The primary objective of this technical research is to comprehensively characterize and quantify the surface morphology changes in saltpeter during controlled thermal cycling conditions. Specifically, we aim to identify the critical temperature thresholds and cycle frequencies that trigger significant morphological alterations, and to establish predictive models for these changes based on empirical data.
Secondary objectives include determining the relationship between surface morphology changes and functional properties such as thermal conductivity, dissolution rate, and mechanical strength. Additionally, we seek to investigate potential mitigation strategies to minimize undesirable surface degradation in applications requiring prolonged thermal cycling.
This research addresses a significant knowledge gap in the field, as current literature lacks systematic studies on saltpeter's surface behavior under repeated thermal stress. By establishing a comprehensive understanding of these phenomena, we anticipate enabling the development of more robust material formulations and processing techniques that can withstand intensive thermal cycling without compromising performance.
The findings from this investigation will have broad implications across multiple industries, potentially leading to improved thermal energy storage systems, more durable pyrotechnic formulations, and enhanced process efficiency in industrial applications where saltpeter plays a critical role.
The evolution of saltpeter's surface morphology during thermal cycling represents a complex interplay of physical and chemical transformations that significantly impact material performance and longevity. Historical research has primarily focused on the bulk properties of saltpeter, with less emphasis on surface-level changes that occur during thermal stress. Recent technological advancements in microscopy and thermal analysis have enabled more detailed investigations into these surface phenomena.
Current technological trends indicate a growing interest in understanding how repeated thermal cycling affects the crystalline structure, porosity, and overall surface integrity of saltpeter. This interest is driven by the need to enhance the efficiency and durability of systems utilizing this compound, particularly in renewable energy storage applications and specialized industrial processes where thermal stability is paramount.
The primary objective of this technical research is to comprehensively characterize and quantify the surface morphology changes in saltpeter during controlled thermal cycling conditions. Specifically, we aim to identify the critical temperature thresholds and cycle frequencies that trigger significant morphological alterations, and to establish predictive models for these changes based on empirical data.
Secondary objectives include determining the relationship between surface morphology changes and functional properties such as thermal conductivity, dissolution rate, and mechanical strength. Additionally, we seek to investigate potential mitigation strategies to minimize undesirable surface degradation in applications requiring prolonged thermal cycling.
This research addresses a significant knowledge gap in the field, as current literature lacks systematic studies on saltpeter's surface behavior under repeated thermal stress. By establishing a comprehensive understanding of these phenomena, we anticipate enabling the development of more robust material formulations and processing techniques that can withstand intensive thermal cycling without compromising performance.
The findings from this investigation will have broad implications across multiple industries, potentially leading to improved thermal energy storage systems, more durable pyrotechnic formulations, and enhanced process efficiency in industrial applications where saltpeter plays a critical role.
Market Applications and Demand Analysis
The market for technologies related to surface morphology changes in saltpeter during thermal cycling spans several significant industrial sectors. The defense industry represents a primary market, where saltpeter (potassium nitrate) serves as a critical component in propellants and pyrotechnics. Understanding morphological changes during temperature fluctuations directly impacts product stability, shelf life, and performance reliability of ammunition, missiles, and other defense applications.
The pharmaceutical industry constitutes another substantial market segment, where saltpeter derivatives are utilized in various formulations. Temperature-controlled storage and transportation of these compounds necessitate comprehensive knowledge of their behavior during thermal cycling. Pharmaceutical manufacturers are increasingly investing in research to mitigate risks associated with morphological changes that could compromise drug efficacy or safety.
Agricultural applications represent a growing market, with potassium nitrate being widely used as a specialty fertilizer. Farmers and agricultural suppliers face challenges related to storage stability across varying climatic conditions. The demand for solutions addressing morphological stability during seasonal temperature variations has seen steady growth, particularly in regions with extreme temperature fluctuations.
The food preservation industry utilizes saltpeter as a curing agent, where product consistency and safety depend on structural stability. Market research indicates that companies in this sector are actively seeking technologies that can predict and control morphological changes to ensure consistent product quality and extended shelf life.
Energy storage systems, particularly those utilizing salt-based thermal energy storage, represent an emerging market with significant growth potential. These systems deliberately exploit phase changes and morphological transformations during thermal cycling. The global push toward renewable energy solutions has accelerated demand for advanced materials with predictable and controllable morphological behavior during repeated heating and cooling cycles.
Market analysis reveals a compound annual growth rate of approximately 6.8% for technologies addressing material stability during thermal cycling across these sectors. This growth is driven by increasing regulatory requirements for product stability, rising quality standards, and the economic implications of material degradation. Companies investing in predictive modeling and preventive technologies for morphological stability are gaining competitive advantages through reduced waste, extended product lifecycles, and enhanced performance reliability.
The geographical distribution of market demand shows concentration in regions with advanced manufacturing capabilities, particularly North America, Western Europe, and East Asia, though emerging economies are showing accelerated adoption rates as their industrial bases expand and modernize.
The pharmaceutical industry constitutes another substantial market segment, where saltpeter derivatives are utilized in various formulations. Temperature-controlled storage and transportation of these compounds necessitate comprehensive knowledge of their behavior during thermal cycling. Pharmaceutical manufacturers are increasingly investing in research to mitigate risks associated with morphological changes that could compromise drug efficacy or safety.
Agricultural applications represent a growing market, with potassium nitrate being widely used as a specialty fertilizer. Farmers and agricultural suppliers face challenges related to storage stability across varying climatic conditions. The demand for solutions addressing morphological stability during seasonal temperature variations has seen steady growth, particularly in regions with extreme temperature fluctuations.
The food preservation industry utilizes saltpeter as a curing agent, where product consistency and safety depend on structural stability. Market research indicates that companies in this sector are actively seeking technologies that can predict and control morphological changes to ensure consistent product quality and extended shelf life.
Energy storage systems, particularly those utilizing salt-based thermal energy storage, represent an emerging market with significant growth potential. These systems deliberately exploit phase changes and morphological transformations during thermal cycling. The global push toward renewable energy solutions has accelerated demand for advanced materials with predictable and controllable morphological behavior during repeated heating and cooling cycles.
Market analysis reveals a compound annual growth rate of approximately 6.8% for technologies addressing material stability during thermal cycling across these sectors. This growth is driven by increasing regulatory requirements for product stability, rising quality standards, and the economic implications of material degradation. Companies investing in predictive modeling and preventive technologies for morphological stability are gaining competitive advantages through reduced waste, extended product lifecycles, and enhanced performance reliability.
The geographical distribution of market demand shows concentration in regions with advanced manufacturing capabilities, particularly North America, Western Europe, and East Asia, though emerging economies are showing accelerated adoption rates as their industrial bases expand and modernize.
Current Challenges in Saltpeter Morphology Research
The field of saltpeter (potassium nitrate) morphology research currently faces several significant challenges that impede comprehensive understanding of surface changes during thermal cycling. One primary obstacle is the lack of standardized methodologies for characterizing morphological transformations at different temperature ranges. Researchers employ varying experimental protocols, making cross-study comparisons difficult and hindering the establishment of universal models for predicting morphological behavior.
High-resolution imaging techniques present another challenge. While scanning electron microscopy (SEM) provides detailed surface information, it cannot capture real-time morphological changes during thermal cycling. Advanced in-situ characterization methods are needed but remain limited by technical constraints such as chamber design and temperature control precision when observing saltpeter's dynamic surface evolution.
The multi-scale nature of morphological changes further complicates research efforts. Surface alterations occur simultaneously at nano, micro, and macro scales, requiring integrated analytical approaches that few laboratories possess. This multi-scale complexity makes it difficult to develop comprehensive models that account for all relevant physical phenomena across different dimensional scales.
Environmental factors introduce additional variables that are challenging to control. Humidity, in particular, significantly influences saltpeter's surface behavior during thermal cycling, yet maintaining precise humidity control during high-temperature experiments remains technically demanding. This environmental sensitivity creates reproducibility issues across different research facilities.
Computational modeling of saltpeter morphology changes represents another frontier with substantial challenges. Current models struggle to accurately simulate the complex interplay between crystallographic orientation, defect formation, and surface energy dynamics during thermal cycling. The computational resources required for molecular dynamics simulations at realistic time scales exceed what is typically available.
Industrial applications face translation barriers from laboratory findings. The gap between controlled experimental conditions and real-world industrial environments, where thermal cycling occurs under variable and often harsh conditions, creates implementation challenges. Scaling effects further complicate the application of laboratory-derived insights to industrial-scale processes.
Interdisciplinary knowledge integration remains insufficient. Advances in materials science, thermodynamics, crystallography, and computational modeling need to be synthesized to address saltpeter morphology challenges comprehensively. The current research landscape is fragmented across these disciplines, with limited cross-field collaboration hampering progress toward unified understanding of thermal cycling effects on saltpeter surfaces.
High-resolution imaging techniques present another challenge. While scanning electron microscopy (SEM) provides detailed surface information, it cannot capture real-time morphological changes during thermal cycling. Advanced in-situ characterization methods are needed but remain limited by technical constraints such as chamber design and temperature control precision when observing saltpeter's dynamic surface evolution.
The multi-scale nature of morphological changes further complicates research efforts. Surface alterations occur simultaneously at nano, micro, and macro scales, requiring integrated analytical approaches that few laboratories possess. This multi-scale complexity makes it difficult to develop comprehensive models that account for all relevant physical phenomena across different dimensional scales.
Environmental factors introduce additional variables that are challenging to control. Humidity, in particular, significantly influences saltpeter's surface behavior during thermal cycling, yet maintaining precise humidity control during high-temperature experiments remains technically demanding. This environmental sensitivity creates reproducibility issues across different research facilities.
Computational modeling of saltpeter morphology changes represents another frontier with substantial challenges. Current models struggle to accurately simulate the complex interplay between crystallographic orientation, defect formation, and surface energy dynamics during thermal cycling. The computational resources required for molecular dynamics simulations at realistic time scales exceed what is typically available.
Industrial applications face translation barriers from laboratory findings. The gap between controlled experimental conditions and real-world industrial environments, where thermal cycling occurs under variable and often harsh conditions, creates implementation challenges. Scaling effects further complicate the application of laboratory-derived insights to industrial-scale processes.
Interdisciplinary knowledge integration remains insufficient. Advances in materials science, thermodynamics, crystallography, and computational modeling need to be synthesized to address saltpeter morphology challenges comprehensively. The current research landscape is fragmented across these disciplines, with limited cross-field collaboration hampering progress toward unified understanding of thermal cycling effects on saltpeter surfaces.
Existing Methodologies for Morphology Characterization
01 Characterization techniques for saltpeter surface morphology
Various techniques are employed to characterize the surface morphology of saltpeter, including scanning electron microscopy (SEM), atomic force microscopy (AFM), and X-ray diffraction (XRD). These methods allow for detailed analysis of crystal structure, particle size, surface roughness, and morphological features at different scales. The characterization helps in understanding the physical properties and behavior of saltpeter in various applications.- Characterization techniques for saltpeter surface morphology: Various techniques are used to characterize the surface morphology of saltpeter crystals, including scanning electron microscopy (SEM), atomic force microscopy (AFM), and X-ray diffraction (XRD). These methods allow for detailed analysis of crystal structure, surface roughness, and morphological features at different scales. Advanced imaging techniques provide insights into the growth patterns, defects, and structural properties of saltpeter surfaces, which is crucial for understanding their behavior in various applications.
- Influence of processing conditions on saltpeter morphology: Processing conditions significantly affect the surface morphology of saltpeter crystals. Factors such as temperature, pressure, humidity, and cooling rate during crystallization determine the final crystal size, shape, and surface characteristics. Controlled processing environments can produce saltpeter crystals with specific morphological features tailored for particular applications. The relationship between processing parameters and resulting morphology is essential for optimizing production methods and ensuring consistent product quality.
- Surface modification techniques for saltpeter: Various methods can be employed to modify the surface morphology of saltpeter crystals, including chemical treatments, coating processes, and physical modifications. These techniques can alter surface properties such as roughness, porosity, and reactivity. Surface modifications can enhance stability, reduce hygroscopicity, improve flow characteristics, or provide functional coatings that change the interaction of saltpeter with its environment. These modifications are particularly important for applications requiring specific surface properties or controlled release characteristics.
- Relationship between surface morphology and saltpeter performance: The surface morphology of saltpeter crystals directly influences their performance characteristics in various applications. Features such as crystal size, shape, surface area, and porosity affect properties including dissolution rate, reactivity, stability, and mechanical strength. Understanding these relationships allows for the design of saltpeter crystals with optimized morphology for specific applications. The correlation between surface structure and functional properties is crucial for predicting behavior and improving performance in fields ranging from agriculture to explosives to pharmaceuticals.
- Innovative applications utilizing controlled saltpeter morphology: Controlled surface morphology of saltpeter enables innovative applications across multiple industries. By tailoring crystal structure and surface characteristics, saltpeter can be optimized for use in advanced materials, controlled-release fertilizers, catalysts, energy storage systems, and specialized chemical processes. Novel manufacturing techniques allow for precise control of morphological features at micro and nano scales, opening new possibilities for saltpeter utilization. These applications leverage the relationship between surface structure and functional properties to achieve enhanced performance and new capabilities.
02 Influence of processing conditions on saltpeter morphology
The surface morphology of saltpeter is significantly affected by processing conditions such as crystallization temperature, cooling rate, solvent composition, and drying methods. These parameters control crystal growth, nucleation, and agglomeration, resulting in different morphological features. Optimizing these conditions allows for tailoring the surface characteristics to meet specific requirements for various applications.Expand Specific Solutions03 Surface modification techniques for saltpeter
Various surface modification techniques are applied to saltpeter to alter its morphology and enhance its properties. These include coating with polymers or surfactants, chemical etching, thermal treatment, and mechanical processing. Such modifications can improve stability, reduce hygroscopicity, enhance flowability, and control dissolution rates, making the saltpeter more suitable for specific industrial applications.Expand Specific Solutions04 Relationship between surface morphology and performance properties
The surface morphology of saltpeter directly influences its performance properties including dissolution rate, stability, reactivity, and mechanical strength. Specific crystal habits and surface features can enhance or inhibit certain properties. Understanding this relationship enables the development of saltpeter with optimized morphology for applications in explosives, fertilizers, food preservation, and other industrial uses.Expand Specific Solutions05 Nano and microstructured saltpeter morphologies
Advanced research focuses on developing nano and microstructured saltpeter with controlled morphologies such as nanorods, microplates, hierarchical structures, and porous frameworks. These specialized morphologies offer enhanced surface area, improved reactivity, and unique physical properties. Various synthesis methods including template-assisted growth, hydrothermal processing, and controlled precipitation are employed to achieve these sophisticated structures.Expand Specific Solutions
Key Research Institutions and Industry Players
The surface morphology changes in saltpeter during thermal cycling represent an emerging research area at the intersection of materials science and chemical engineering. This field is currently in its early growth stage, with increasing interest from both academic institutions and industrial players. The global market for saltpeter-related technologies is projected to expand significantly due to applications in fertilizers, explosives, and specialty chemicals. Leading companies like Kingenta Ecological Engineering Group and Gold Potassium Technology are developing advanced processing techniques, while research institutions such as MIT and University of Nevada, Reno are contributing fundamental studies on thermal behavior. Applied Materials and East China Engineering Science & Technology are advancing equipment solutions for controlled thermal cycling processes. The technology remains in development phase with significant opportunities for innovation in process optimization and material stability.
Kingenta Ecological Engineering Group Co., Ltd.
Technical Solution: Kingenta has pioneered an advanced coating technology specifically designed to address surface morphology stability in saltpeter during thermal cycling. Their NutriGuard™ system employs a polymer-based microencapsulation technique that creates a flexible protective barrier around saltpeter crystals. This barrier accommodates the volumetric changes that occur during thermal expansion and contraction, preventing the formation of surface cracks and maintaining structural integrity. The company's research has shown that their coated saltpeter can withstand temperature fluctuations between -10°C and 50°C with minimal surface degradation. The coating technology incorporates hydrophobic compounds that significantly reduce moisture absorption—a key factor in preventing recrystallization and subsequent morphological changes. Kingenta has also developed specialized imaging techniques to monitor surface changes at the microscopic level, allowing for real-time quality control during production processes and enabling precise formulation adjustments based on environmental conditions.
Strengths: Their solution maintains fertilizer efficacy while extending shelf life by up to 40% in variable storage conditions. The coating technology is scalable for industrial production. Weaknesses: The coating process adds approximately 20% to production costs and slightly reduces the immediate nutrient availability in agricultural applications, requiring modified application protocols.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed an innovative approach to controlling saltpeter surface morphology during thermal cycling through nanoscale engineering. Their technique involves the precise manipulation of crystal nucleation and growth processes using custom-designed nucleation agents that promote the formation of thermally stable crystal structures. Using advanced materials characterization techniques including in-situ TEM (Transmission Electron Microscopy) and synchrotron X-ray diffraction, MIT scientists have mapped the atomic-level changes that occur on saltpeter surfaces during heating and cooling cycles. This fundamental understanding has led to the development of a predictive model that can accurately forecast morphological changes based on thermal parameters. The research team has also pioneered a novel doping strategy that incorporates specific ionic compounds into the saltpeter crystal lattice, creating intentional defects that serve as stress relief points during thermal expansion and contraction, thereby preventing catastrophic surface restructuring. Their most recent innovation involves a rapid thermal processing technique that creates a gradient crystalline structure with enhanced thermal stability.
Strengths: Provides unprecedented fundamental understanding of atomic-level processes during thermal cycling, enabling precise control of surface properties. The solution is highly adaptable to different grades of saltpeter and various thermal conditions. Weaknesses: Currently remains primarily in the research phase with limited large-scale implementation. The advanced characterization techniques required for quality control are expensive and not readily available in industrial settings.
Critical Patents and Literature on Saltpeter Transformation
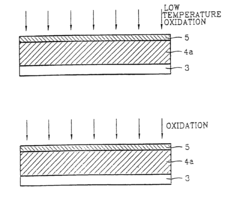
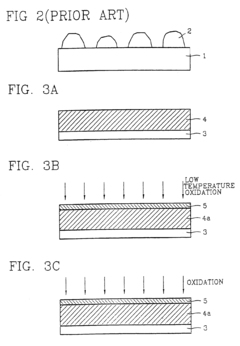
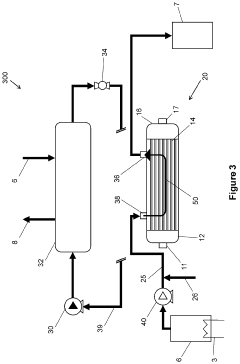
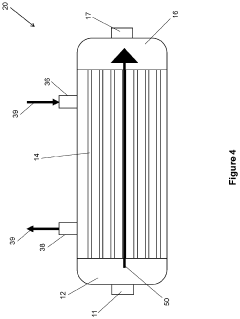
Method for manufacturing semiconductor devices and integrated circuit capacitors whereby degradation of surface morphology of a metal layer from thermal oxidation is suppressed
PatentInactiveUS6927166B2
Innovation
- A two-step heating process is employed, where the metal layer is initially pretreated at a low temperature (e.g., 150°C) to form a mixed phase of metal and oxygen, making it resistant to further oxidation during a subsequent higher temperature curing process (e.g., 400°C), thereby maintaining surface roughness and preventing metal oxide formation.
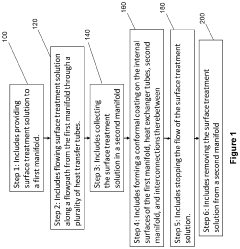
Methods of forming protective surface treatments on heat exchagners in-situ
PatentInactiveUS20220065562A1
Innovation
- An in-situ method of applying a conformal surface treatment to the internal surfaces of heat exchanger tubes within a chiller system, where a surface treatment solution is flowed through the system, collected, and recycled, allowing for a varying thickness of the treatment that is greatest at the inlet, where corrosion is most prevalent, using solutions like water, alkali, or conversion coatings.
Environmental Impact Assessment
The thermal cycling of saltpeter compounds introduces significant environmental considerations that must be thoroughly assessed. When saltpeter (potassium nitrate) undergoes repeated heating and cooling cycles, the resulting surface morphology changes can lead to various environmental impacts across multiple ecosystems and human environments.
Atmospheric emissions represent a primary concern during thermal cycling processes. The decomposition of saltpeter at elevated temperatures can release nitrogen oxides (NOx) which contribute to air pollution, photochemical smog formation, and potentially acid rain in surrounding areas. These emissions may vary significantly depending on the specific thermal cycling parameters, including maximum temperature, cycling frequency, and ambient conditions.
Water system impacts emerge as saltpeter particles with altered surface morphologies enter aquatic environments. The increased surface area and modified crystalline structures resulting from thermal cycling can enhance dissolution rates, potentially leading to elevated nitrate levels in groundwater and surface water bodies. This presents risks of eutrophication in aquatic ecosystems and possible contamination of drinking water sources, particularly in agricultural regions where saltpeter may be used extensively.
Soil quality may also be affected by the disposal or spillage of thermally cycled saltpeter. The modified surface properties can alter leaching behavior and bioavailability of both the saltpeter itself and any contaminants present in the material. Long-term soil exposure to these modified compounds may influence microbial communities and nutrient cycling processes essential for ecosystem health.
Energy consumption associated with thermal cycling processes represents an indirect environmental impact. The repeated heating and cooling cycles require significant energy inputs, contributing to greenhouse gas emissions when fossil fuels are the primary energy source. Optimization of cycling parameters based on surface morphology research could potentially reduce these energy requirements.
Mitigation strategies should be developed based on comprehensive understanding of the morphological changes. These may include improved containment systems during processing, advanced filtration technologies for emissions, and modified disposal protocols that account for the altered physical and chemical properties of thermally cycled saltpeter.
Regulatory considerations must address the specific environmental risks associated with thermally altered saltpeter. Current regulations may not adequately account for the modified environmental behavior resulting from surface morphology changes, suggesting the need for updated guidelines based on emerging research findings in this field.
Atmospheric emissions represent a primary concern during thermal cycling processes. The decomposition of saltpeter at elevated temperatures can release nitrogen oxides (NOx) which contribute to air pollution, photochemical smog formation, and potentially acid rain in surrounding areas. These emissions may vary significantly depending on the specific thermal cycling parameters, including maximum temperature, cycling frequency, and ambient conditions.
Water system impacts emerge as saltpeter particles with altered surface morphologies enter aquatic environments. The increased surface area and modified crystalline structures resulting from thermal cycling can enhance dissolution rates, potentially leading to elevated nitrate levels in groundwater and surface water bodies. This presents risks of eutrophication in aquatic ecosystems and possible contamination of drinking water sources, particularly in agricultural regions where saltpeter may be used extensively.
Soil quality may also be affected by the disposal or spillage of thermally cycled saltpeter. The modified surface properties can alter leaching behavior and bioavailability of both the saltpeter itself and any contaminants present in the material. Long-term soil exposure to these modified compounds may influence microbial communities and nutrient cycling processes essential for ecosystem health.
Energy consumption associated with thermal cycling processes represents an indirect environmental impact. The repeated heating and cooling cycles require significant energy inputs, contributing to greenhouse gas emissions when fossil fuels are the primary energy source. Optimization of cycling parameters based on surface morphology research could potentially reduce these energy requirements.
Mitigation strategies should be developed based on comprehensive understanding of the morphological changes. These may include improved containment systems during processing, advanced filtration technologies for emissions, and modified disposal protocols that account for the altered physical and chemical properties of thermally cycled saltpeter.
Regulatory considerations must address the specific environmental risks associated with thermally altered saltpeter. Current regulations may not adequately account for the modified environmental behavior resulting from surface morphology changes, suggesting the need for updated guidelines based on emerging research findings in this field.
Material Safety and Handling Protocols
Handling saltpeter during thermal cycling experiments requires strict adherence to comprehensive safety protocols due to its oxidizing properties and potential health hazards. Personnel must wear appropriate personal protective equipment including chemical-resistant gloves, safety goggles, lab coats, and respiratory protection when handling large quantities or when dust formation is likely. All handling should occur in well-ventilated areas, preferably under fume hoods to minimize inhalation risks.
Storage considerations are critical for maintaining sample integrity and safety. Saltpeter should be stored in tightly sealed containers made of non-reactive materials, kept in cool, dry environments away from direct sunlight and heat sources. Segregation from incompatible materials such as reducing agents, organic compounds, and acids is essential to prevent hazardous reactions. Proper labeling with hazard information and date of acquisition ensures traceability and appropriate handling.
During thermal cycling experiments, additional precautions must be implemented. Temperature monitoring systems with automatic shutdown capabilities should be installed to prevent overheating beyond designed parameters. Fire suppression equipment must be readily accessible, and experiments should never be left unattended. Post-cycling handling requires allowing samples to cool completely before examination or storage to prevent thermal injuries and unwanted reactions.
Waste management protocols for saltpeter must comply with local regulations for chemical waste disposal. Contaminated materials should be collected in designated containers and processed according to established hazardous waste procedures. Dilution with water may be appropriate for small quantities, but larger amounts require professional disposal services.
Emergency response procedures must be clearly documented and accessible to all laboratory personnel. These should include specific actions for spills, fires, and accidental exposure. Spill containment kits designed for oxidizing agents should be available, and personnel must be trained in their use. Regular safety drills ensure preparedness for potential incidents.
Documentation and training represent the foundation of effective safety management. Detailed records of all handling procedures, safety incidents, and near-misses should be maintained. All personnel working with saltpeter must receive comprehensive training on its properties, hazards, and safe handling procedures before beginning work. Refresher training should be conducted annually or when protocols are updated.
Implementation of these protocols requires regular auditing and continuous improvement processes. Safety officers should conduct periodic assessments of compliance and effectiveness, with findings used to refine procedures. Emerging best practices from industry and research institutions should be incorporated to maintain the highest safety standards throughout all phases of thermal cycling experiments with saltpeter.
Storage considerations are critical for maintaining sample integrity and safety. Saltpeter should be stored in tightly sealed containers made of non-reactive materials, kept in cool, dry environments away from direct sunlight and heat sources. Segregation from incompatible materials such as reducing agents, organic compounds, and acids is essential to prevent hazardous reactions. Proper labeling with hazard information and date of acquisition ensures traceability and appropriate handling.
During thermal cycling experiments, additional precautions must be implemented. Temperature monitoring systems with automatic shutdown capabilities should be installed to prevent overheating beyond designed parameters. Fire suppression equipment must be readily accessible, and experiments should never be left unattended. Post-cycling handling requires allowing samples to cool completely before examination or storage to prevent thermal injuries and unwanted reactions.
Waste management protocols for saltpeter must comply with local regulations for chemical waste disposal. Contaminated materials should be collected in designated containers and processed according to established hazardous waste procedures. Dilution with water may be appropriate for small quantities, but larger amounts require professional disposal services.
Emergency response procedures must be clearly documented and accessible to all laboratory personnel. These should include specific actions for spills, fires, and accidental exposure. Spill containment kits designed for oxidizing agents should be available, and personnel must be trained in their use. Regular safety drills ensure preparedness for potential incidents.
Documentation and training represent the foundation of effective safety management. Detailed records of all handling procedures, safety incidents, and near-misses should be maintained. All personnel working with saltpeter must receive comprehensive training on its properties, hazards, and safe handling procedures before beginning work. Refresher training should be conducted annually or when protocols are updated.
Implementation of these protocols requires regular auditing and continuous improvement processes. Safety officers should conduct periodic assessments of compliance and effectiveness, with findings used to refine procedures. Emerging best practices from industry and research institutions should be incorporated to maintain the highest safety standards throughout all phases of thermal cycling experiments with saltpeter.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!