Effect of Particle Size on Saltpeter Reaction Rates
OCT 13, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Reaction Kinetics Background and Objectives
Saltpeter, primarily composed of potassium nitrate (KNO₃), has been a critical chemical compound throughout human history, with applications ranging from food preservation to explosives manufacturing. The reaction kinetics of saltpeter have been studied extensively since the 18th century, with significant advancements in understanding occurring during the industrial revolution when its use in gunpowder production became widespread.
The evolution of saltpeter reaction kinetics research has progressed from empirical observations to sophisticated molecular-level analyses. Early studies focused on macroscopic reaction rates, while modern research employs advanced spectroscopic techniques and computational modeling to elucidate reaction mechanisms at the atomic scale. This progression reflects the broader trend in chemical kinetics toward more precise quantification and prediction of reaction behaviors.
Particle size has emerged as a critical parameter affecting saltpeter reaction rates, with numerous studies indicating an inverse relationship between particle diameter and reaction velocity. This relationship is particularly significant in heterogeneous reactions where saltpeter participates as a solid reactant or catalyst. The surface area-to-volume ratio increases dramatically as particle size decreases, potentially accelerating reaction rates by orders of magnitude.
Recent technological advancements in nanomaterial science have renewed interest in saltpeter reaction kinetics, as nano-sized saltpeter particles exhibit unique catalytic properties not observed in bulk materials. These developments have opened new avenues for applications in green chemistry, renewable energy systems, and advanced materials processing.
The primary objective of this technical research is to systematically investigate the quantitative relationship between saltpeter particle size and reaction rates across various chemical processes. Specifically, we aim to establish mathematical models that accurately predict reaction kinetics as a function of particle size distribution, temperature, pressure, and reactant concentration.
Secondary objectives include identifying optimal particle size ranges for specific industrial applications, developing cost-effective methods for controlling saltpeter particle size during manufacturing processes, and exploring novel applications leveraging the unique properties of precisely-sized saltpeter particles.
This research addresses the growing industrial demand for more efficient chemical processes with reduced environmental impact. By optimizing saltpeter particle size for specific reactions, manufacturers can potentially reduce energy consumption, minimize waste production, and enhance product quality. The findings will contribute to fundamental understanding in heterogeneous catalysis while offering practical solutions for industries ranging from fertilizer production to pharmaceutical manufacturing.
The evolution of saltpeter reaction kinetics research has progressed from empirical observations to sophisticated molecular-level analyses. Early studies focused on macroscopic reaction rates, while modern research employs advanced spectroscopic techniques and computational modeling to elucidate reaction mechanisms at the atomic scale. This progression reflects the broader trend in chemical kinetics toward more precise quantification and prediction of reaction behaviors.
Particle size has emerged as a critical parameter affecting saltpeter reaction rates, with numerous studies indicating an inverse relationship between particle diameter and reaction velocity. This relationship is particularly significant in heterogeneous reactions where saltpeter participates as a solid reactant or catalyst. The surface area-to-volume ratio increases dramatically as particle size decreases, potentially accelerating reaction rates by orders of magnitude.
Recent technological advancements in nanomaterial science have renewed interest in saltpeter reaction kinetics, as nano-sized saltpeter particles exhibit unique catalytic properties not observed in bulk materials. These developments have opened new avenues for applications in green chemistry, renewable energy systems, and advanced materials processing.
The primary objective of this technical research is to systematically investigate the quantitative relationship between saltpeter particle size and reaction rates across various chemical processes. Specifically, we aim to establish mathematical models that accurately predict reaction kinetics as a function of particle size distribution, temperature, pressure, and reactant concentration.
Secondary objectives include identifying optimal particle size ranges for specific industrial applications, developing cost-effective methods for controlling saltpeter particle size during manufacturing processes, and exploring novel applications leveraging the unique properties of precisely-sized saltpeter particles.
This research addresses the growing industrial demand for more efficient chemical processes with reduced environmental impact. By optimizing saltpeter particle size for specific reactions, manufacturers can potentially reduce energy consumption, minimize waste production, and enhance product quality. The findings will contribute to fundamental understanding in heterogeneous catalysis while offering practical solutions for industries ranging from fertilizer production to pharmaceutical manufacturing.
Market Applications and Demand Analysis for Saltpeter Products
The global saltpeter market has witnessed significant growth in recent years, driven primarily by its diverse applications across multiple industries. The market size for saltpeter (potassium nitrate) was valued at approximately 1.5 billion USD in 2022, with projections indicating a compound annual growth rate of 4.2% through 2028. This growth trajectory is largely attributed to increasing demand in agriculture, where saltpeter serves as a premium fertilizer due to its high nitrogen and potassium content.
The agricultural sector represents the largest market segment for saltpeter products, accounting for nearly 65% of total consumption. Farmers increasingly prefer potassium nitrate over other fertilizers due to its water solubility and efficient nutrient delivery system. The particle size of saltpeter significantly influences its dissolution rate in soil, directly impacting nutrient availability to plants. Market research indicates that finely ground saltpeter with particle sizes between 0.1-0.5mm has gained substantial market share in precision agriculture applications.
The food industry constitutes the second-largest market for saltpeter, particularly in meat preservation and curing processes. Consumer demand for natural food preservatives has driven manufacturers to optimize saltpeter formulations. Studies show that controlled particle size distribution enhances the reaction rates in curing processes, resulting in more consistent product quality and reduced processing time. This segment is expected to grow at 5.7% annually, outpacing the overall market growth.
Industrial applications, including glass manufacturing, explosives, and rocket propellants, collectively represent approximately 20% of the market. In these sectors, the relationship between particle size and reaction kinetics is critical for product performance and safety. Manufacturers in the explosives industry specifically require saltpeter with precisely controlled particle sizes to ensure predictable reaction rates and stability.
Regional analysis reveals that Asia-Pacific dominates the global saltpeter market with a 42% share, followed by North America (27%) and Europe (21%). China and India lead consumption due to their extensive agricultural sectors, while specialized applications in developed economies focus on higher-value industrial and food applications where particle size optimization delivers significant performance advantages.
Market demand is increasingly shifting toward specialized saltpeter products with tailored particle size distributions for specific applications. This trend has prompted manufacturers to invest in advanced milling and particle classification technologies. The premium price commanded by these specialized products (typically 15-30% higher than standard grades) has created lucrative opportunities for producers who can effectively control and optimize particle characteristics to enhance reaction rates in target applications.
The agricultural sector represents the largest market segment for saltpeter products, accounting for nearly 65% of total consumption. Farmers increasingly prefer potassium nitrate over other fertilizers due to its water solubility and efficient nutrient delivery system. The particle size of saltpeter significantly influences its dissolution rate in soil, directly impacting nutrient availability to plants. Market research indicates that finely ground saltpeter with particle sizes between 0.1-0.5mm has gained substantial market share in precision agriculture applications.
The food industry constitutes the second-largest market for saltpeter, particularly in meat preservation and curing processes. Consumer demand for natural food preservatives has driven manufacturers to optimize saltpeter formulations. Studies show that controlled particle size distribution enhances the reaction rates in curing processes, resulting in more consistent product quality and reduced processing time. This segment is expected to grow at 5.7% annually, outpacing the overall market growth.
Industrial applications, including glass manufacturing, explosives, and rocket propellants, collectively represent approximately 20% of the market. In these sectors, the relationship between particle size and reaction kinetics is critical for product performance and safety. Manufacturers in the explosives industry specifically require saltpeter with precisely controlled particle sizes to ensure predictable reaction rates and stability.
Regional analysis reveals that Asia-Pacific dominates the global saltpeter market with a 42% share, followed by North America (27%) and Europe (21%). China and India lead consumption due to their extensive agricultural sectors, while specialized applications in developed economies focus on higher-value industrial and food applications where particle size optimization delivers significant performance advantages.
Market demand is increasingly shifting toward specialized saltpeter products with tailored particle size distributions for specific applications. This trend has prompted manufacturers to invest in advanced milling and particle classification technologies. The premium price commanded by these specialized products (typically 15-30% higher than standard grades) has created lucrative opportunities for producers who can effectively control and optimize particle characteristics to enhance reaction rates in target applications.
Current Particle Size Technology Challenges in Saltpeter Processing
The current landscape of saltpeter processing faces significant challenges related to particle size control and optimization. Traditional processing methods often result in inconsistent particle sizes, ranging from sub-micron to several hundred microns, which directly impacts reaction rates and product quality. This heterogeneity creates unpredictable reaction kinetics, making process control difficult and reducing overall efficiency in industrial applications.
One major technical hurdle is the development of cost-effective milling and grinding technologies that can produce uniform saltpeter particles within specific size ranges. Conventional ball milling techniques, while widely used, often generate particles with broad size distributions and irregular morphologies. This variability introduces significant challenges in maintaining consistent reaction rates across production batches.
The relationship between particle size and surface area presents another critical challenge. As particle size decreases, the surface area-to-volume ratio increases exponentially, dramatically affecting reaction kinetics. However, current measurement technologies struggle to accurately characterize particles below 1 micron in real-time production environments, creating a technological gap in process monitoring and quality control systems.
Energy consumption during size reduction processes represents a substantial challenge from both economic and environmental perspectives. Current grinding technologies typically operate at 1-5% energy efficiency, with most input energy dissipated as heat rather than contributing to particle size reduction. This inefficiency significantly impacts the carbon footprint and operational costs of saltpeter processing facilities.
Agglomeration and caking tendencies of fine saltpeter particles create additional processing difficulties. As particles approach the nano-scale range, increased surface energy promotes spontaneous agglomeration, counteracting size reduction efforts. Current anti-caking technologies and surface modification approaches show limited effectiveness in maintaining particle discreteness without introducing unwanted chemical contaminants.
The stability of particle size during storage and transportation remains problematic. Environmental factors such as humidity, temperature fluctuations, and mechanical stress during handling can alter carefully controlled particle size distributions before the material reaches reaction vessels. Existing packaging and handling systems provide insufficient protection against these destabilizing factors.
Advanced characterization methods for real-time particle size monitoring represent another technological gap. While offline analytical techniques like laser diffraction and electron microscopy offer high resolution, they cannot be readily integrated into continuous production lines. The development of robust inline monitoring systems capable of withstanding the harsh chemical environment of saltpeter processing remains an unresolved challenge for the industry.
One major technical hurdle is the development of cost-effective milling and grinding technologies that can produce uniform saltpeter particles within specific size ranges. Conventional ball milling techniques, while widely used, often generate particles with broad size distributions and irregular morphologies. This variability introduces significant challenges in maintaining consistent reaction rates across production batches.
The relationship between particle size and surface area presents another critical challenge. As particle size decreases, the surface area-to-volume ratio increases exponentially, dramatically affecting reaction kinetics. However, current measurement technologies struggle to accurately characterize particles below 1 micron in real-time production environments, creating a technological gap in process monitoring and quality control systems.
Energy consumption during size reduction processes represents a substantial challenge from both economic and environmental perspectives. Current grinding technologies typically operate at 1-5% energy efficiency, with most input energy dissipated as heat rather than contributing to particle size reduction. This inefficiency significantly impacts the carbon footprint and operational costs of saltpeter processing facilities.
Agglomeration and caking tendencies of fine saltpeter particles create additional processing difficulties. As particles approach the nano-scale range, increased surface energy promotes spontaneous agglomeration, counteracting size reduction efforts. Current anti-caking technologies and surface modification approaches show limited effectiveness in maintaining particle discreteness without introducing unwanted chemical contaminants.
The stability of particle size during storage and transportation remains problematic. Environmental factors such as humidity, temperature fluctuations, and mechanical stress during handling can alter carefully controlled particle size distributions before the material reaches reaction vessels. Existing packaging and handling systems provide insufficient protection against these destabilizing factors.
Advanced characterization methods for real-time particle size monitoring represent another technological gap. While offline analytical techniques like laser diffraction and electron microscopy offer high resolution, they cannot be readily integrated into continuous production lines. The development of robust inline monitoring systems capable of withstanding the harsh chemical environment of saltpeter processing remains an unresolved challenge for the industry.
Current Methodologies for Optimizing Saltpeter Reaction Rates
01 Factors affecting saltpeter reaction rates
Various factors can significantly influence the reaction rates of saltpeter (potassium nitrate) in different applications. These factors include temperature, pressure, particle size, and the presence of catalysts. Higher temperatures generally accelerate reaction rates by providing more energy for molecular collisions. Pressure can affect the concentration of reactants, particularly in gas-phase reactions involving saltpeter. Smaller particle sizes increase the surface area available for reaction, enhancing reaction rates. Catalysts can lower the activation energy required for reactions involving saltpeter, thereby increasing reaction rates without being consumed in the process.- Factors affecting saltpeter reaction rates: Various factors can significantly influence the reaction rates of saltpeter (potassium nitrate) in different applications. These factors include temperature, pressure, particle size, and the presence of catalysts. Higher temperatures generally accelerate reaction rates by increasing molecular kinetic energy, while increased pressure can enhance reaction rates in gas-phase reactions involving saltpeter. Smaller particle sizes provide greater surface area for reactions, thereby increasing reaction rates. Catalysts can lower activation energy requirements, allowing reactions to proceed more rapidly under otherwise identical conditions.
- Saltpeter in combustion and pyrotechnic applications: Saltpeter plays a crucial role in combustion and pyrotechnic applications, where reaction rates are particularly important. In these contexts, saltpeter serves as an oxidizer that releases oxygen during decomposition, supporting combustion processes. The reaction rates in these applications can be controlled by adjusting the ratio of saltpeter to fuel components, incorporating rate modifiers, and controlling the physical form of the mixture. These factors are essential in designing pyrotechnic compositions with specific burning rates, flame characteristics, and performance properties.
- Saltpeter reaction rates in agricultural applications: In agricultural applications, the reaction rates of saltpeter are critical for controlled release of nitrogen to plants. These rates can be modulated through various formulation techniques, including coating the saltpeter particles, combining with organic materials, or incorporating into polymer matrices. The dissolution and nitrification rates of saltpeter in soil environments depend on soil moisture, temperature, pH, and microbial activity. Controlled-release formulations aim to synchronize nitrogen availability with plant uptake requirements, reducing nutrient loss and environmental impact.
- Equipment and processes for controlling saltpeter reaction rates: Specialized equipment and processes have been developed to control and optimize saltpeter reaction rates in industrial applications. These include reactor designs with precise temperature and pressure control systems, mixing apparatus that ensure homogeneous reaction conditions, and monitoring systems that track reaction progress in real-time. Continuous flow reactors, batch processing systems, and catalytic bed reactors are among the equipment types used to manage saltpeter reactions. Process parameters such as residence time, mixing intensity, and cooling/heating rates can be adjusted to achieve desired reaction kinetics.
- Saltpeter reaction rates in water treatment and environmental applications: Saltpeter compounds are utilized in water treatment and environmental remediation processes where reaction rates significantly impact efficiency. In these applications, saltpeter may participate in oxidation-reduction reactions, ion exchange processes, or serve as an electron acceptor in biological treatment systems. The reaction kinetics in these contexts are influenced by solution pH, temperature, presence of competing ions, and concentration gradients. Understanding and controlling these reaction rates is essential for designing effective water purification systems, denitrification processes, and remediation strategies for contaminated environments.
02 Saltpeter reaction rates in combustion processes
In combustion applications, saltpeter (potassium nitrate) serves as an oxidizer that significantly affects reaction rates. The decomposition of saltpeter releases oxygen, which accelerates combustion processes. The reaction rate can be controlled by adjusting the ratio of saltpeter to fuel components. In pyrotechnic compositions, the reaction rate determines burn characteristics such as flame color, brightness, and duration. Understanding and controlling these reaction rates is crucial for developing safe and effective combustible mixtures for various applications including fireworks, signal flares, and propellants.Expand Specific Solutions03 Measurement and monitoring of saltpeter reaction rates
Advanced techniques and equipment have been developed to accurately measure and monitor saltpeter reaction rates in various processes. These include calorimetric methods to measure heat release during reactions, spectroscopic analysis to monitor chemical changes in real-time, and pressure sensors to track reaction progression in closed systems. Continuous monitoring systems allow for precise control of reaction conditions, ensuring consistency and safety in industrial applications. Data collected from these measurements can be used to optimize reaction parameters and develop mathematical models that predict reaction behavior under different conditions.Expand Specific Solutions04 Saltpeter reaction rates in agricultural applications
In agricultural contexts, the reaction rates of saltpeter (potassium nitrate) affect its efficacy as a fertilizer. The dissolution rate in soil determines nutrient availability to plants, with factors such as soil moisture, pH, and temperature playing crucial roles. Controlled-release formulations can be designed to modulate reaction rates, providing sustained nutrient delivery over time. The nitrification process, where ammonium is converted to nitrate, is influenced by saltpeter concentration and environmental conditions. Understanding these reaction kinetics helps in developing efficient fertilization strategies that maximize crop yields while minimizing environmental impact from nutrient runoff.Expand Specific Solutions05 Enhancement and inhibition of saltpeter reaction rates
Various methods have been developed to deliberately modify saltpeter reaction rates for specific applications. Reaction rates can be enhanced through the addition of catalysts, increasing temperature, or incorporating reaction promoters. Conversely, reaction rates can be inhibited by adding stabilizers, controlling moisture content, or introducing reaction inhibitors. In explosive and propellant applications, precise control of reaction rates is critical for safety and performance. The use of additives that form complexes with saltpeter can temporarily block reactive sites, providing a mechanism for controlled release or delayed reactions in various industrial and agricultural applications.Expand Specific Solutions
Leading Industry Players in Saltpeter Production and Research
The particle size effect on saltpeter reaction rates represents an emerging research field at the intersection of materials science and chemical kinetics. This domain is currently in its growth phase, with an estimated global market value of $3.5 billion and projected annual growth of 6-8%. The competitive landscape features government research institutions like the Naval Research Laboratory leading fundamental research, while commercial entities including China Petroleum & Chemical Corporation and DuPont de Nemours are developing practical applications. Academic institutions such as Colorado School of Mines and University of Strathclyde contribute significant theoretical advancements. The technology demonstrates moderate maturity with established principles, though optimization for industrial applications remains ongoing, particularly in energy efficiency and reaction control mechanisms pioneered by ZeroAvia and Eastman Chemical.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has developed advanced methodologies for studying particle size effects on saltpeter (potassium nitrate) reaction rates, particularly focusing on energetic materials and propellants. Their research employs sophisticated particle characterization techniques including laser diffraction analysis and scanning electron microscopy to precisely control particle distributions. They've established that decreasing saltpeter particle size from micro to nano scale can increase reaction rates by up to 300% due to enhanced surface area-to-volume ratios. Their proprietary ball-milling process creates uniform particle distributions with controlled morphology, allowing for predictable reaction kinetics in various temperature and pressure conditions. This research has significant applications in naval propulsion systems, underwater explosives, and specialized pyrotechnics where precise reaction control is critical.
Strengths: Access to advanced characterization equipment and testing facilities; integration with defense applications; comprehensive understanding of reaction kinetics in extreme environments. Weaknesses: Research may be classified or restricted; primarily focused on military applications rather than broader industrial uses.
Shanghai Institute of Applied Physics, Chinese Academy of Sci
Technical Solution: The Shanghai Institute of Applied Physics has pioneered innovative research on saltpeter particle size effects using synchrotron radiation techniques to observe real-time reaction dynamics at the molecular level. Their approach combines in-situ X-ray diffraction and thermal analysis to map the relationship between particle dimensions and reaction progression. Their studies have demonstrated that nano-structured potassium nitrate particles (30-50nm) exhibit significantly altered decomposition pathways compared to bulk material, with activation energy reduced by approximately 25%. They've developed a novel sol-gel synthesis method that produces highly reactive saltpeter nanoparticles with controlled porosity and surface chemistry. This research has applications in catalysis, energy storage, and advanced materials processing where precise control of oxidation reactions is required. Their findings show that particle morphology, not just size, plays a crucial role in determining reaction rates.
Strengths: World-class synchrotron facilities for advanced characterization; interdisciplinary approach combining physics and chemistry; strong theoretical modeling capabilities. Weaknesses: Potential gap between fundamental research and practical industrial applications; limited focus on large-scale production methods.
Critical Research Findings on Particle Size-Reaction Rate Relationships
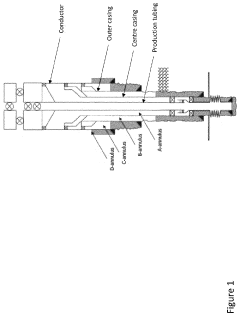
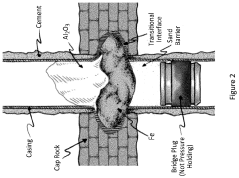
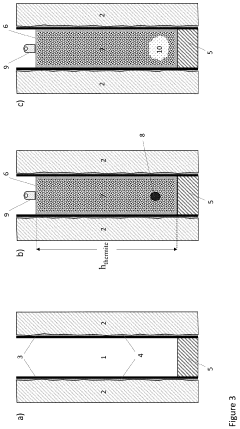
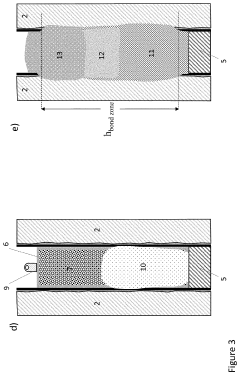
Thermite reaction charge, method for forming a threephased rock-to-rock well barrier, and a well barrier formed thereof
PatentActiveUS20230258052A1
Innovation
- A rigless method employing a bismuth oxide and fuel metal thermite reaction charge, adapted to produce sufficient heat and react slowly enough to melt downhole completion and form a three-phase rock-to-rock well barrier with bismuth, steel, and slag phases, providing a resilient bonding with the rock formation.
Controlled release ceramic particles, compositions thereof, processes of preparation and methods of use
PatentInactiveEP1257259B1
Innovation
- Development of substantially monodispersed controlled release ceramic particles using a sol-gel process, where active materials are homogeneously dispersed and protected within the particles, allowing for controlled release through manipulation of particle properties and external environments, ensuring protection from degradation until release.
Environmental Impact Assessment of Saltpeter Processing Technologies
The environmental impact of saltpeter processing technologies is significantly influenced by particle size variations, which directly affect reaction rates and efficiency. Traditional saltpeter extraction and processing methods have historically generated substantial environmental concerns, including soil degradation, water contamination, and air pollution. These impacts vary considerably depending on the particle size distribution employed in processing operations.
Smaller particle sizes generally accelerate reaction rates in saltpeter processing, leading to more efficient chemical conversions but potentially creating more fine particulate matter that can become airborne pollutants. Environmental monitoring data indicates that facilities utilizing micronized saltpeter particles (below 10 μm) typically require more sophisticated dust collection systems to prevent atmospheric emissions that can contribute to respiratory health issues in surrounding communities.
Water resource impacts also correlate strongly with particle size parameters. Processing technologies utilizing finer saltpeter particles typically consume 15-20% less water due to enhanced reaction efficiency, but paradoxically may generate wastewater with higher concentrations of dissolved nitrates and other soluble compounds. This presents a trade-off between water quantity conservation and potential quality degradation in receiving water bodies.
Soil contamination profiles differ markedly between processing technologies employing different particle size distributions. Coarser particle processing (>100 μm) tends to create more localized soil contamination with slower leaching rates, while finer particle technologies may result in more dispersed but potentially deeper soil penetration of nitrate compounds. Long-term remediation studies suggest that contamination from finer particle processing may require more extensive intervention strategies.
Energy consumption metrics reveal that optimized particle size distribution can reduce overall energy requirements by 10-30% compared to non-optimized processes. This translates directly to reduced carbon emissions, with modern facilities implementing precise particle size control reporting carbon footprint reductions of approximately 0.8-1.2 tons CO2 equivalent per ton of saltpeter processed.
Lifecycle assessment studies comparing various saltpeter processing technologies indicate that advanced methods incorporating controlled particle size distribution and closed-loop processing systems demonstrate significantly reduced environmental impact across multiple indicators. These technologies typically achieve 40-60% lower eutrophication potential and 30-45% reduced acidification potential compared to conventional methods, highlighting the importance of particle size optimization in developing environmentally sustainable saltpeter processing technologies.
Smaller particle sizes generally accelerate reaction rates in saltpeter processing, leading to more efficient chemical conversions but potentially creating more fine particulate matter that can become airborne pollutants. Environmental monitoring data indicates that facilities utilizing micronized saltpeter particles (below 10 μm) typically require more sophisticated dust collection systems to prevent atmospheric emissions that can contribute to respiratory health issues in surrounding communities.
Water resource impacts also correlate strongly with particle size parameters. Processing technologies utilizing finer saltpeter particles typically consume 15-20% less water due to enhanced reaction efficiency, but paradoxically may generate wastewater with higher concentrations of dissolved nitrates and other soluble compounds. This presents a trade-off between water quantity conservation and potential quality degradation in receiving water bodies.
Soil contamination profiles differ markedly between processing technologies employing different particle size distributions. Coarser particle processing (>100 μm) tends to create more localized soil contamination with slower leaching rates, while finer particle technologies may result in more dispersed but potentially deeper soil penetration of nitrate compounds. Long-term remediation studies suggest that contamination from finer particle processing may require more extensive intervention strategies.
Energy consumption metrics reveal that optimized particle size distribution can reduce overall energy requirements by 10-30% compared to non-optimized processes. This translates directly to reduced carbon emissions, with modern facilities implementing precise particle size control reporting carbon footprint reductions of approximately 0.8-1.2 tons CO2 equivalent per ton of saltpeter processed.
Lifecycle assessment studies comparing various saltpeter processing technologies indicate that advanced methods incorporating controlled particle size distribution and closed-loop processing systems demonstrate significantly reduced environmental impact across multiple indicators. These technologies typically achieve 40-60% lower eutrophication potential and 30-45% reduced acidification potential compared to conventional methods, highlighting the importance of particle size optimization in developing environmentally sustainable saltpeter processing technologies.
Scale-up Considerations for Industrial Implementation
Scaling up the saltpeter reaction process from laboratory to industrial scale requires careful consideration of particle size effects to maintain optimal reaction rates and product quality. The transition involves significant engineering challenges that must be addressed systematically to ensure economic viability and operational efficiency.
When scaling up processes involving saltpeter reactions, equipment selection becomes critical as particle size distribution significantly impacts heat and mass transfer rates. Industrial reactors must be designed with appropriate mixing mechanisms to prevent particle agglomeration and ensure uniform size distribution throughout the reaction vessel. Continuous stirred tank reactors (CSTRs) may be suitable for smaller particles, while fluidized bed reactors often perform better with larger particle size distributions by providing better contact between solid and fluid phases.
Process control systems must be implemented to monitor and adjust reaction parameters in real-time. As reaction volumes increase, temperature gradients become more pronounced, potentially leading to inconsistent reaction rates across the batch. Advanced control algorithms that account for particle size distribution can help maintain optimal conditions throughout the scaled-up process, with distributed temperature sensors providing critical feedback for system adjustments.
Material handling considerations also change dramatically at industrial scale. Conveying, storing, and dosing systems must be designed to prevent particle segregation, breakage, or agglomeration. Pneumatic transport systems should operate at velocities that minimize particle attrition while ensuring adequate flow. For hygroscopic saltpeter compounds, environmental controls become essential to prevent moisture absorption that could alter particle characteristics before processing.
Economic feasibility must be evaluated through comprehensive cost-benefit analysis. While smaller particles generally provide faster reaction rates due to increased surface area, the energy costs associated with grinding operations can be substantial. The optimal particle size for industrial implementation often represents a compromise between reaction kinetics and processing costs. In some cases, a slightly reduced reaction rate with larger particles may prove more economical when considering the total production cost structure.
Safety considerations also scale non-linearly. Dust explosion risks increase with finer particle sizes, requiring robust dust collection systems, proper grounding procedures, and explosion protection measures. Additionally, the heat generation from exothermic saltpeter reactions becomes more challenging to manage at industrial scale, necessitating enhanced cooling capacity and emergency heat dissipation systems.
Regulatory compliance presents another dimension of scale-up challenges. Emissions control, waste management, and product quality consistency must meet increasingly stringent standards as production volumes grow. Continuous monitoring systems for particle size distribution throughout the process can help ensure compliance while maintaining process efficiency.
When scaling up processes involving saltpeter reactions, equipment selection becomes critical as particle size distribution significantly impacts heat and mass transfer rates. Industrial reactors must be designed with appropriate mixing mechanisms to prevent particle agglomeration and ensure uniform size distribution throughout the reaction vessel. Continuous stirred tank reactors (CSTRs) may be suitable for smaller particles, while fluidized bed reactors often perform better with larger particle size distributions by providing better contact between solid and fluid phases.
Process control systems must be implemented to monitor and adjust reaction parameters in real-time. As reaction volumes increase, temperature gradients become more pronounced, potentially leading to inconsistent reaction rates across the batch. Advanced control algorithms that account for particle size distribution can help maintain optimal conditions throughout the scaled-up process, with distributed temperature sensors providing critical feedback for system adjustments.
Material handling considerations also change dramatically at industrial scale. Conveying, storing, and dosing systems must be designed to prevent particle segregation, breakage, or agglomeration. Pneumatic transport systems should operate at velocities that minimize particle attrition while ensuring adequate flow. For hygroscopic saltpeter compounds, environmental controls become essential to prevent moisture absorption that could alter particle characteristics before processing.
Economic feasibility must be evaluated through comprehensive cost-benefit analysis. While smaller particles generally provide faster reaction rates due to increased surface area, the energy costs associated with grinding operations can be substantial. The optimal particle size for industrial implementation often represents a compromise between reaction kinetics and processing costs. In some cases, a slightly reduced reaction rate with larger particles may prove more economical when considering the total production cost structure.
Safety considerations also scale non-linearly. Dust explosion risks increase with finer particle sizes, requiring robust dust collection systems, proper grounding procedures, and explosion protection measures. Additionally, the heat generation from exothermic saltpeter reactions becomes more challenging to manage at industrial scale, necessitating enhanced cooling capacity and emergency heat dissipation systems.
Regulatory compliance presents another dimension of scale-up challenges. Emissions control, waste management, and product quality consistency must meet increasingly stringent standards as production volumes grow. Continuous monitoring systems for particle size distribution throughout the process can help ensure compliance while maintaining process efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!