Surface Chemistry of Saltpeter Crystals under Atmospheric Exposure
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Crystal Chemistry Background and Objectives
Saltpeter, scientifically known as potassium nitrate (KNO₃), has been a compound of significant historical and industrial importance for centuries. The surface chemistry of saltpeter crystals under atmospheric exposure represents a complex interplay of physical and chemical processes that have profound implications across multiple sectors including agriculture, explosives manufacturing, food preservation, and pharmaceutical applications. The evolution of our understanding of saltpeter's surface properties has progressed from rudimentary empirical observations in ancient civilizations to sophisticated molecular-level analyses in modern materials science.
The historical trajectory of saltpeter chemistry research began with its extraction from natural deposits and animal waste in the 13th century, primarily for gunpowder production. By the 18th century, systematic studies of its crystallization behavior emerged, though without molecular-level insights. The 20th century brought instrumental analysis techniques that revolutionized our understanding of saltpeter's surface properties, particularly its hygroscopic nature and reactivity with atmospheric components.
Current research trends focus on nanoscale surface characterization, environmental impact assessment, and the development of controlled-release formulations for agricultural applications. The increasing sophistication of analytical tools such as atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS), and environmental scanning electron microscopy (ESEM) has enabled unprecedented insights into the dynamic behavior of saltpeter crystal surfaces under varying atmospheric conditions.
The primary technical objectives of this investigation include: characterizing the molecular-level interactions between atmospheric moisture and saltpeter crystal surfaces; quantifying the kinetics of surface dissolution and recrystallization processes under fluctuating humidity conditions; identifying the chemical transformations occurring at the crystal-air interface during prolonged environmental exposure; and developing predictive models for saltpeter stability under diverse atmospheric conditions.
Additionally, this research aims to elucidate the mechanisms by which atmospheric pollutants, particularly sulfur and nitrogen oxides, interact with and potentially modify saltpeter crystal surfaces. Understanding these processes has significant implications for the preservation of historical artifacts and buildings where saltpeter efflorescence contributes to material degradation.
The technological significance of this research extends beyond fundamental science to practical applications in controlled-release fertilizers, pharmaceutical excipients, and food preservation systems. By comprehensively mapping the surface chemistry dynamics of saltpeter crystals, we anticipate developing novel approaches to modulating crystal stability, solubility, and reactivity for specific industrial applications.
The historical trajectory of saltpeter chemistry research began with its extraction from natural deposits and animal waste in the 13th century, primarily for gunpowder production. By the 18th century, systematic studies of its crystallization behavior emerged, though without molecular-level insights. The 20th century brought instrumental analysis techniques that revolutionized our understanding of saltpeter's surface properties, particularly its hygroscopic nature and reactivity with atmospheric components.
Current research trends focus on nanoscale surface characterization, environmental impact assessment, and the development of controlled-release formulations for agricultural applications. The increasing sophistication of analytical tools such as atomic force microscopy (AFM), X-ray photoelectron spectroscopy (XPS), and environmental scanning electron microscopy (ESEM) has enabled unprecedented insights into the dynamic behavior of saltpeter crystal surfaces under varying atmospheric conditions.
The primary technical objectives of this investigation include: characterizing the molecular-level interactions between atmospheric moisture and saltpeter crystal surfaces; quantifying the kinetics of surface dissolution and recrystallization processes under fluctuating humidity conditions; identifying the chemical transformations occurring at the crystal-air interface during prolonged environmental exposure; and developing predictive models for saltpeter stability under diverse atmospheric conditions.
Additionally, this research aims to elucidate the mechanisms by which atmospheric pollutants, particularly sulfur and nitrogen oxides, interact with and potentially modify saltpeter crystal surfaces. Understanding these processes has significant implications for the preservation of historical artifacts and buildings where saltpeter efflorescence contributes to material degradation.
The technological significance of this research extends beyond fundamental science to practical applications in controlled-release fertilizers, pharmaceutical excipients, and food preservation systems. By comprehensively mapping the surface chemistry dynamics of saltpeter crystals, we anticipate developing novel approaches to modulating crystal stability, solubility, and reactivity for specific industrial applications.
Market Applications and Demand Analysis for Saltpeter
The global market for saltpeter (potassium nitrate) continues to expand across diverse sectors, driven by its unique surface chemistry properties when exposed to atmospheric conditions. The agricultural sector represents the largest market segment, with saltpeter being extensively utilized as a nitrogen-rich fertilizer. Current market analysis indicates strong growth in regions with intensive agriculture, particularly in Asia-Pacific and South America, where precision farming techniques are gaining prominence.
The food preservation industry constitutes another significant market for saltpeter, where its surface stability under varying atmospheric conditions makes it valuable as a curing agent for meats. This application segment has shown consistent growth of late, particularly in developed economies where artisanal and specialty food products command premium prices.
Pharmaceutical applications represent a high-value market segment for saltpeter. The compound's controlled reactivity with atmospheric components makes it suitable for various medicinal formulations, including treatments for asthma and certain cardiac conditions. The pharmaceutical-grade saltpeter market demonstrates higher profit margins compared to agricultural applications, though with smaller volume requirements.
The pyrotechnics and explosives industry maintains steady demand for saltpeter, utilizing its oxidizing properties that become enhanced under specific atmospheric exposure conditions. This sector shows cyclical demand patterns influenced by construction activity and military procurement cycles across different regions.
Emerging applications in renewable energy storage systems are creating new market opportunities. Research indicates that saltpeter's surface chemistry characteristics under controlled atmospheric conditions may contribute to the development of thermal energy storage solutions, potentially opening substantial new market segments as renewable energy infrastructure expands globally.
Market challenges include price volatility due to fluctuating production costs and increasing regulatory scrutiny regarding nitrate compounds in certain regions. Environmental concerns about nitrate leaching have prompted the development of controlled-release formulations with modified surface properties that minimize environmental impact while maintaining effectiveness.
Consumer trends toward organic and natural products have created niche markets for naturally sourced saltpeter with specific surface characteristics. This premium segment, though smaller in volume, commands significantly higher pricing and shows robust growth potential in eco-conscious consumer markets.
Regional analysis reveals that developing economies are experiencing faster growth rates in saltpeter consumption, particularly in agricultural applications, while developed economies show stronger growth in specialty applications requiring precise control of surface chemistry properties under varying atmospheric conditions.
The food preservation industry constitutes another significant market for saltpeter, where its surface stability under varying atmospheric conditions makes it valuable as a curing agent for meats. This application segment has shown consistent growth of late, particularly in developed economies where artisanal and specialty food products command premium prices.
Pharmaceutical applications represent a high-value market segment for saltpeter. The compound's controlled reactivity with atmospheric components makes it suitable for various medicinal formulations, including treatments for asthma and certain cardiac conditions. The pharmaceutical-grade saltpeter market demonstrates higher profit margins compared to agricultural applications, though with smaller volume requirements.
The pyrotechnics and explosives industry maintains steady demand for saltpeter, utilizing its oxidizing properties that become enhanced under specific atmospheric exposure conditions. This sector shows cyclical demand patterns influenced by construction activity and military procurement cycles across different regions.
Emerging applications in renewable energy storage systems are creating new market opportunities. Research indicates that saltpeter's surface chemistry characteristics under controlled atmospheric conditions may contribute to the development of thermal energy storage solutions, potentially opening substantial new market segments as renewable energy infrastructure expands globally.
Market challenges include price volatility due to fluctuating production costs and increasing regulatory scrutiny regarding nitrate compounds in certain regions. Environmental concerns about nitrate leaching have prompted the development of controlled-release formulations with modified surface properties that minimize environmental impact while maintaining effectiveness.
Consumer trends toward organic and natural products have created niche markets for naturally sourced saltpeter with specific surface characteristics. This premium segment, though smaller in volume, commands significantly higher pricing and shows robust growth potential in eco-conscious consumer markets.
Regional analysis reveals that developing economies are experiencing faster growth rates in saltpeter consumption, particularly in agricultural applications, while developed economies show stronger growth in specialty applications requiring precise control of surface chemistry properties under varying atmospheric conditions.
Current Challenges in Atmospheric Exposure Research
Despite significant advancements in understanding the surface chemistry of saltpeter crystals, researchers face numerous challenges when investigating these phenomena under atmospheric exposure conditions. One primary obstacle is the dynamic nature of atmospheric conditions, which introduces multiple variables simultaneously affecting crystal surfaces. Temperature fluctuations, humidity variations, and atmospheric pollutants create a complex matrix of factors that are difficult to isolate and control in experimental settings.
The hygroscopic nature of saltpeter crystals presents another significant challenge, as these crystals readily absorb moisture from the atmosphere, leading to surface dissolution and recrystallization processes that occur continuously under ambient conditions. This property makes it exceptionally difficult to maintain stable experimental conditions and obtain reproducible results across different research facilities.
Analytical limitations constitute a major technical barrier in this field. Current surface characterization techniques often require high vacuum or controlled environments that fundamentally alter the very atmospheric interactions researchers aim to study. In-situ characterization methods capable of monitoring surface changes in real-time under atmospheric conditions remain underdeveloped, creating a significant gap between laboratory findings and real-world behavior of saltpeter crystals.
The multi-scale nature of surface interactions further complicates research efforts. Molecular-level interactions at crystal surfaces must be understood alongside macroscopic phenomena such as crystal growth and dissolution. Bridging these different scales of observation requires sophisticated modeling approaches and experimental designs that few research groups have successfully implemented.
Standardization issues plague the field, with varying experimental protocols and reporting methods making cross-study comparisons challenging. The lack of standardized reference materials and exposure conditions has led to contradictory findings in the literature, hindering systematic progress in understanding atmospheric effects on saltpeter crystal surfaces.
Interdisciplinary knowledge gaps represent another significant challenge. The comprehensive study of saltpeter crystal surface chemistry under atmospheric exposure requires expertise spanning physical chemistry, materials science, atmospheric science, and advanced analytical techniques. Few research teams possess this breadth of knowledge, limiting holistic approaches to the problem.
Long-term stability studies present particular difficulties, as the slow kinetics of some surface transformation processes require extended observation periods that exceed typical research funding cycles. This temporal challenge has resulted in a predominance of short-term studies that may miss critical long-term phenomena relevant to real-world applications of saltpeter crystals.
The hygroscopic nature of saltpeter crystals presents another significant challenge, as these crystals readily absorb moisture from the atmosphere, leading to surface dissolution and recrystallization processes that occur continuously under ambient conditions. This property makes it exceptionally difficult to maintain stable experimental conditions and obtain reproducible results across different research facilities.
Analytical limitations constitute a major technical barrier in this field. Current surface characterization techniques often require high vacuum or controlled environments that fundamentally alter the very atmospheric interactions researchers aim to study. In-situ characterization methods capable of monitoring surface changes in real-time under atmospheric conditions remain underdeveloped, creating a significant gap between laboratory findings and real-world behavior of saltpeter crystals.
The multi-scale nature of surface interactions further complicates research efforts. Molecular-level interactions at crystal surfaces must be understood alongside macroscopic phenomena such as crystal growth and dissolution. Bridging these different scales of observation requires sophisticated modeling approaches and experimental designs that few research groups have successfully implemented.
Standardization issues plague the field, with varying experimental protocols and reporting methods making cross-study comparisons challenging. The lack of standardized reference materials and exposure conditions has led to contradictory findings in the literature, hindering systematic progress in understanding atmospheric effects on saltpeter crystal surfaces.
Interdisciplinary knowledge gaps represent another significant challenge. The comprehensive study of saltpeter crystal surface chemistry under atmospheric exposure requires expertise spanning physical chemistry, materials science, atmospheric science, and advanced analytical techniques. Few research teams possess this breadth of knowledge, limiting holistic approaches to the problem.
Long-term stability studies present particular difficulties, as the slow kinetics of some surface transformation processes require extended observation periods that exceed typical research funding cycles. This temporal challenge has resulted in a predominance of short-term studies that may miss critical long-term phenomena relevant to real-world applications of saltpeter crystals.
Current Methodologies for Analyzing Crystal-Atmosphere Interactions
01 Surface modification of saltpeter crystals
Various techniques can be employed to modify the surface of saltpeter crystals to enhance their properties. These modifications can include coating with polymers, applying chemical treatments, or introducing functional groups to the crystal surface. Such modifications can improve stability, reduce hygroscopicity, and enhance the performance characteristics of the saltpeter crystals for various applications.- Surface modification of saltpeter crystals: Various techniques can be employed to modify the surface of saltpeter crystals to enhance their properties. These modifications can include coating with polymers, applying chemical treatments, or introducing functional groups to the crystal surface. Such modifications can improve stability, reduce hygroscopicity, and enhance the performance characteristics of the saltpeter crystals for specific applications.
- Crystal growth control and morphology: Methods for controlling the growth and morphology of saltpeter crystals involve manipulating crystallization conditions such as temperature, pressure, and solution composition. These techniques allow for the production of crystals with specific shapes, sizes, and surface characteristics. Controlled crystal growth is essential for applications requiring uniform crystal properties and predictable surface chemistry behavior.
- Surface analysis and characterization techniques: Various analytical methods are used to characterize the surface chemistry of saltpeter crystals, including spectroscopy, microscopy, and diffraction techniques. These methods provide insights into surface composition, structure, and reactivity. Understanding the surface properties is crucial for optimizing crystal performance in different applications and environments.
- Stabilization of saltpeter crystal surfaces: Techniques for stabilizing saltpeter crystal surfaces focus on preventing degradation, agglomeration, and unwanted reactions. These may include the application of protective coatings, incorporation of stabilizing additives, or environmental control measures. Stabilization is particularly important for maintaining crystal integrity during storage, transportation, and application in various industrial processes.
- Surface functionalization for specific applications: Saltpeter crystal surfaces can be functionalized to enhance their performance in specific applications such as catalysis, sensing, or controlled release systems. Functionalization may involve attaching reactive groups, incorporating metal ions, or creating composite structures. These modifications tailor the surface chemistry to achieve desired interactions with other materials or substances in the target application environment.
02 Crystal growth control and morphology
Methods for controlling the growth and morphology of saltpeter crystals involve manipulating crystallization conditions such as temperature, pressure, and solution composition. These techniques allow for the production of crystals with specific shapes, sizes, and surface characteristics. Controlled crystal growth is essential for applications requiring uniform crystal properties and predictable surface chemistry behavior.Expand Specific Solutions03 Surface analysis and characterization techniques
Various analytical methods are used to characterize the surface chemistry of saltpeter crystals, including spectroscopy, microscopy, and diffraction techniques. These methods provide insights into surface composition, structure, and reactivity. Understanding the surface properties is crucial for optimizing crystal performance in different applications and environments.Expand Specific Solutions04 Stabilization of saltpeter crystal surfaces
Techniques for stabilizing saltpeter crystal surfaces focus on preventing degradation, agglomeration, and moisture absorption. These methods often involve the application of protective coatings, encapsulation, or chemical treatments that modify surface energy. Stabilized crystals exhibit improved shelf life, handling properties, and performance in various environmental conditions.Expand Specific Solutions05 Surface functionalization for specific applications
Saltpeter crystal surfaces can be functionalized with specific chemical groups or compounds to enhance their performance in targeted applications. This functionalization can improve properties such as adhesion, reactivity, or compatibility with other materials. Applications include explosives, fertilizers, catalysts, and various industrial processes where controlled surface chemistry is essential for optimal performance.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The surface chemistry of saltpeter crystals under atmospheric exposure represents an emerging research field currently in its early development stage. The market is relatively small but growing, with increasing applications in environmental monitoring, cultural heritage preservation, and materials science. From a technical maturity perspective, academic institutions like Beijing University of Chemical Technology, Shandong Normal University, and Swiss Federal Institute of Technology are leading fundamental research, while companies are developing specialized applications. Nihon Parkerizing and BASF SE are advancing surface treatment technologies, Beneq Group is applying atomic layer deposition expertise, and JSR Corp is developing specialized coating solutions. Northern Technologies International and UBE Corp are exploring corrosion mitigation applications, indicating the field is transitioning from basic research to commercial applications with significant growth potential.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has pioneered innovative approaches to understanding and controlling saltpeter crystal surface chemistry under atmospheric exposure. Their research utilizes advanced surface characterization techniques including time-of-flight secondary ion mass spectrometry (TOF-SIMS) and in-situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) to monitor real-time surface changes. The university has developed proprietary humidity-controlled crystallization methods that produce saltpeter crystals with engineered surface properties resistant to atmospheric degradation. Their research has identified specific crystal facets that show enhanced stability under varying atmospheric conditions, allowing for selective crystal growth optimization. Additionally, they've created novel surface modification techniques using environmentally friendly polymeric coatings that form hydrogen bonds with surface nitrate groups, effectively sealing the crystal surface against moisture penetration while maintaining desired chemical properties. Recent publications demonstrate their success in reducing hygroscopicity by up to 75% while preserving the chemical reactivity needed for catalytic applications.
Strengths: Exceptional capabilities in surface engineering at the molecular level; strong integration of computational modeling with experimental verification. Weaknesses: Some coating technologies require specialized application equipment; long-term stability testing under extreme conditions is still ongoing.
Croda International Plc
Technical Solution: Croda International has developed sophisticated surface chemistry solutions for saltpeter crystals exposed to atmospheric conditions, focusing on sustainable and bio-based approaches. Their "EcoStable" technology platform utilizes plant-derived fatty acid derivatives that form ordered monolayers on saltpeter crystal surfaces, creating effective moisture barriers while maintaining crystal functionality. Croda's research has revealed that specific triglyceride-derived compounds can intercalate with surface nitrate groups, significantly reducing hygroscopicity without altering core crystal properties. Their approach combines surface modification with controlled crystallization techniques to engineer crystals with inherently more stable surface structures. Advanced analytical methods including dynamic vapor sorption (DVS) and surface plasmon resonance (SPR) allow precise characterization of moisture interactions at treated surfaces. Croda has also pioneered green processing methods that eliminate the need for volatile organic solvents in coating applications, reducing environmental impact while improving worker safety. Their latest innovation involves responsive polymer systems that adapt to changing atmospheric conditions, providing enhanced protection during high humidity events while maintaining normal functionality under standard conditions.
Strengths: Industry-leading expertise in sustainable surface chemistry; excellent compatibility with existing manufacturing processes; strong performance in variable humidity environments. Weaknesses: Higher initial implementation costs compared to conventional treatments; requires specific application expertise; some formulations have temperature limitations.
Key Scientific Breakthroughs in Saltpeter Surface Characterization
Salt crystals
PatentWO2013192556A2
Innovation
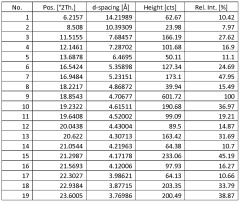
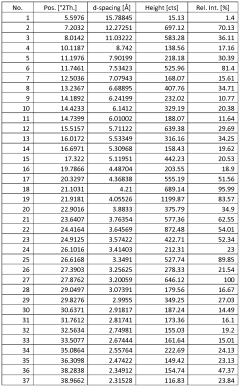
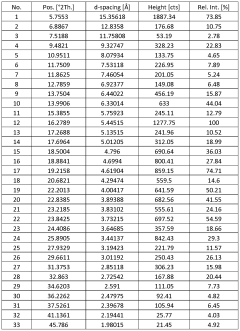
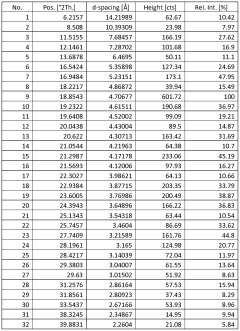
- Novel salt crystal characterization using X-ray powder diffraction patterns with specific d-spacing values and peaks, providing unique identification methods for saltpeter crystals.
- Distinctive thermal properties of salt crystals identified through Differential Scanning Calorimetry (DSC) with characteristic endotherm at about 176°C, enabling thermal fingerprinting of specific crystal forms.
- Specific preparation method involving reaction of complex organic compounds with fumaric acid in methanol, potentially creating salt crystals with unique surface properties.
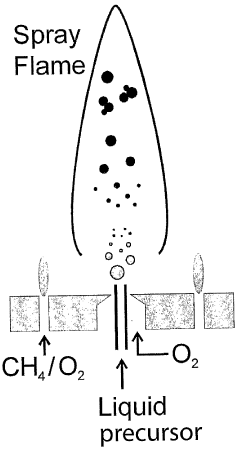
Flame synthesis of metal salt nanoparticles, in particular calcium and phosphate comprising nanoparticles
PatentWO2005087660A1
Innovation
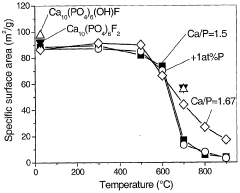
- A method involving the formation of droplets from a mixture of metal carboxylates and anionic sources, followed by high-temperature oxidation in a flame spray pyrolysis process, to produce nanoparticulate metal salts with high calcium content and controlled anionic composition, achieving high purity and specific surface area.
Environmental Impact and Sustainability Considerations
The environmental impact of saltpeter (potassium nitrate) crystals under atmospheric exposure represents a significant concern for both natural ecosystems and human health. When exposed to atmospheric conditions, saltpeter crystals undergo various chemical transformations that can release nitrogen compounds into the environment. These compounds contribute to soil acidification, water eutrophication, and air pollution through the formation of nitrogen oxides. The leaching of saltpeter from exposed surfaces during rainfall events can lead to increased nitrate concentrations in groundwater, potentially exceeding safe drinking water standards in affected areas.
Climate change factors further complicate the environmental implications of saltpeter crystal exposure. Rising temperatures accelerate the dissolution and chemical reaction rates of these crystals, potentially increasing their environmental mobility. Additionally, changing precipitation patterns may alter the distribution and concentration of saltpeter-derived compounds in various environmental compartments, creating new challenges for ecosystem management and conservation efforts.
From a sustainability perspective, the management of saltpeter-containing materials requires careful consideration of their entire lifecycle. Traditional extraction and processing methods often involve significant energy consumption and chemical inputs, resulting in substantial carbon footprints. The development of green chemistry approaches for saltpeter production and handling represents a promising avenue for reducing these environmental impacts. These approaches include the implementation of closed-loop systems that capture and reuse nitrogen compounds, minimizing waste and environmental discharge.
Remediation strategies for environments affected by saltpeter contamination must balance effectiveness with ecological sensitivity. Bioremediation techniques utilizing nitrogen-fixing bacteria have shown promise in converting excess nitrates into less harmful compounds. Phytoremediation approaches employing specific plant species capable of accumulating nitrogen compounds offer another sustainable alternative for managing affected sites.
The regulatory landscape governing saltpeter management continues to evolve, with increasing emphasis on sustainable practices. International standards for handling, storing, and disposing of nitrate-containing materials have become more stringent, reflecting growing awareness of their environmental implications. Companies working with these materials are increasingly adopting voluntary sustainability initiatives that go beyond compliance requirements, implementing comprehensive environmental management systems that address the specific challenges posed by saltpeter exposure.
Climate change factors further complicate the environmental implications of saltpeter crystal exposure. Rising temperatures accelerate the dissolution and chemical reaction rates of these crystals, potentially increasing their environmental mobility. Additionally, changing precipitation patterns may alter the distribution and concentration of saltpeter-derived compounds in various environmental compartments, creating new challenges for ecosystem management and conservation efforts.
From a sustainability perspective, the management of saltpeter-containing materials requires careful consideration of their entire lifecycle. Traditional extraction and processing methods often involve significant energy consumption and chemical inputs, resulting in substantial carbon footprints. The development of green chemistry approaches for saltpeter production and handling represents a promising avenue for reducing these environmental impacts. These approaches include the implementation of closed-loop systems that capture and reuse nitrogen compounds, minimizing waste and environmental discharge.
Remediation strategies for environments affected by saltpeter contamination must balance effectiveness with ecological sensitivity. Bioremediation techniques utilizing nitrogen-fixing bacteria have shown promise in converting excess nitrates into less harmful compounds. Phytoremediation approaches employing specific plant species capable of accumulating nitrogen compounds offer another sustainable alternative for managing affected sites.
The regulatory landscape governing saltpeter management continues to evolve, with increasing emphasis on sustainable practices. International standards for handling, storing, and disposing of nitrate-containing materials have become more stringent, reflecting growing awareness of their environmental implications. Companies working with these materials are increasingly adopting voluntary sustainability initiatives that go beyond compliance requirements, implementing comprehensive environmental management systems that address the specific challenges posed by saltpeter exposure.
Analytical Instrumentation Advancements for Surface Chemistry
Recent advancements in analytical instrumentation have revolutionized our understanding of surface chemistry phenomena, particularly in the study of saltpeter crystals under atmospheric exposure. The evolution of surface-sensitive techniques has enabled researchers to probe molecular interactions at unprecedented spatial and temporal resolutions, offering critical insights into crystallization dynamics and environmental degradation processes.
X-ray Photoelectron Spectroscopy (XPS) has undergone significant refinements, with modern instruments achieving sub-micron spatial resolution and enhanced detection limits below 0.1 atomic percent. These improvements allow for detailed chemical state analysis of saltpeter surfaces, revealing subtle oxidation state changes during atmospheric exposure that were previously undetectable.
Atomic Force Microscopy (AFM) technologies have evolved to incorporate environmental chambers that maintain precise humidity and temperature conditions while simultaneously mapping surface topography. The latest AFM systems can achieve vertical resolution below 0.1 nm while operating in controlled atmospheric conditions, enabling real-time observation of saltpeter crystal growth and dissolution processes at the molecular level.
Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) represents another breakthrough, offering chemical mapping capabilities with mass resolution exceeding 10,000 m/Δm and spatial resolution below 100 nm. This technique has proven invaluable for tracking trace contaminants on saltpeter surfaces and their influence on hygroscopic behavior under varying atmospheric conditions.
Synchrotron-based techniques have emerged as powerful tools for non-destructive analysis of saltpeter crystal surfaces. Grazing-incidence X-ray diffraction (GIXRD) and X-ray absorption spectroscopy (XAS) provide structural and electronic information about surface species with exceptional sensitivity, allowing researchers to distinguish between bulk and surface-specific phenomena during atmospheric interactions.
Ambient pressure techniques represent the newest frontier in surface analysis instrumentation. Near-ambient pressure XPS (NAP-XPS) and environmental scanning electron microscopy (ESEM) enable direct observation of saltpeter surfaces under realistic atmospheric conditions, bridging the notorious "pressure gap" that has historically limited the applicability of surface science to real-world environmental scenarios.
Machine learning algorithms have been increasingly integrated with these analytical platforms, enabling automated feature recognition and multivariate analysis of complex spectroscopic datasets. These computational approaches have dramatically improved the efficiency of data interpretation and revealed subtle patterns in surface chemistry that might otherwise remain hidden in the complexity of raw experimental data.
X-ray Photoelectron Spectroscopy (XPS) has undergone significant refinements, with modern instruments achieving sub-micron spatial resolution and enhanced detection limits below 0.1 atomic percent. These improvements allow for detailed chemical state analysis of saltpeter surfaces, revealing subtle oxidation state changes during atmospheric exposure that were previously undetectable.
Atomic Force Microscopy (AFM) technologies have evolved to incorporate environmental chambers that maintain precise humidity and temperature conditions while simultaneously mapping surface topography. The latest AFM systems can achieve vertical resolution below 0.1 nm while operating in controlled atmospheric conditions, enabling real-time observation of saltpeter crystal growth and dissolution processes at the molecular level.
Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) represents another breakthrough, offering chemical mapping capabilities with mass resolution exceeding 10,000 m/Δm and spatial resolution below 100 nm. This technique has proven invaluable for tracking trace contaminants on saltpeter surfaces and their influence on hygroscopic behavior under varying atmospheric conditions.
Synchrotron-based techniques have emerged as powerful tools for non-destructive analysis of saltpeter crystal surfaces. Grazing-incidence X-ray diffraction (GIXRD) and X-ray absorption spectroscopy (XAS) provide structural and electronic information about surface species with exceptional sensitivity, allowing researchers to distinguish between bulk and surface-specific phenomena during atmospheric interactions.
Ambient pressure techniques represent the newest frontier in surface analysis instrumentation. Near-ambient pressure XPS (NAP-XPS) and environmental scanning electron microscopy (ESEM) enable direct observation of saltpeter surfaces under realistic atmospheric conditions, bridging the notorious "pressure gap" that has historically limited the applicability of surface science to real-world environmental scenarios.
Machine learning algorithms have been increasingly integrated with these analytical platforms, enabling automated feature recognition and multivariate analysis of complex spectroscopic datasets. These computational approaches have dramatically improved the efficiency of data interpretation and revealed subtle patterns in surface chemistry that might otherwise remain hidden in the complexity of raw experimental data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!