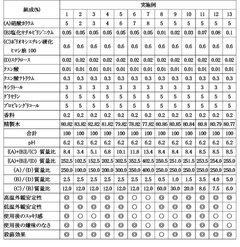
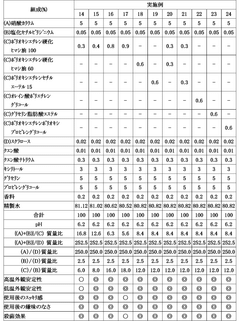
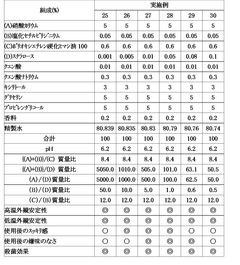
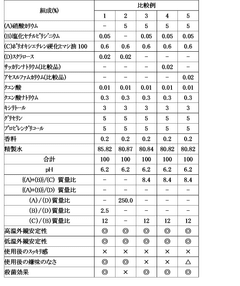
Influence of Saltpeter Purity on Propellant Stability
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Purity Background and Research Objectives
Saltpeter, chemically known as potassium nitrate (KNO₃), has been a critical component in propellant formulations since the invention of black powder in ancient China. The historical significance of saltpeter in warfare, mining, and later in industrial applications has established it as one of the most important inorganic compounds in human technological development. Throughout the centuries, the understanding of saltpeter's role in combustion processes has evolved significantly, from empirical observations to scientific comprehension of its oxidizing properties.
The purity of saltpeter has emerged as a crucial factor affecting propellant stability, performance, and safety. Early propellant manufacturers often worked with naturally occurring saltpeter, which contained various impurities including chlorides, sulfates, calcium, and magnesium compounds. These impurities were observed to cause inconsistent burning rates, reduced shelf life, and in some cases, dangerous spontaneous decomposition of propellant mixtures.
Modern propellant technology demands increasingly stringent specifications for saltpeter purity. The presence of even trace impurities can catalyze decomposition reactions, absorb moisture, or form undesirable byproducts during combustion. These effects become particularly critical in advanced propellant systems where predictable, consistent performance is essential for applications ranging from military munitions to space exploration vehicles.
The technological evolution in propellant chemistry has been marked by continuous improvements in saltpeter purification methods. From the primitive leaching techniques used historically to today's advanced crystallization and ion-exchange processes, the industry has consistently pursued higher purity standards to enhance propellant stability and performance characteristics.
This research aims to systematically investigate the correlation between saltpeter purity levels and propellant stability across various formulations and environmental conditions. Specifically, we seek to quantify the impact of common impurities on decomposition rates, thermal stability, and long-term storage characteristics of modern propellant systems.
Our objectives include developing comprehensive analytical models that can predict stability issues based on impurity profiles, establishing optimal purity thresholds for different application categories, and exploring cost-effective purification technologies that can meet these thresholds. Additionally, we aim to investigate the synergistic effects between saltpeter impurities and other propellant components, particularly stabilizers and plasticizers.
The research will employ advanced analytical techniques including differential scanning calorimetry, thermogravimetric analysis, ion chromatography, and accelerated aging tests to characterize the influence of specific impurities on propellant degradation mechanisms. Through this systematic approach, we expect to establish clear correlations between saltpeter purity specifications and propellant shelf life, which will inform future industry standards and quality control protocols.
The purity of saltpeter has emerged as a crucial factor affecting propellant stability, performance, and safety. Early propellant manufacturers often worked with naturally occurring saltpeter, which contained various impurities including chlorides, sulfates, calcium, and magnesium compounds. These impurities were observed to cause inconsistent burning rates, reduced shelf life, and in some cases, dangerous spontaneous decomposition of propellant mixtures.
Modern propellant technology demands increasingly stringent specifications for saltpeter purity. The presence of even trace impurities can catalyze decomposition reactions, absorb moisture, or form undesirable byproducts during combustion. These effects become particularly critical in advanced propellant systems where predictable, consistent performance is essential for applications ranging from military munitions to space exploration vehicles.
The technological evolution in propellant chemistry has been marked by continuous improvements in saltpeter purification methods. From the primitive leaching techniques used historically to today's advanced crystallization and ion-exchange processes, the industry has consistently pursued higher purity standards to enhance propellant stability and performance characteristics.
This research aims to systematically investigate the correlation between saltpeter purity levels and propellant stability across various formulations and environmental conditions. Specifically, we seek to quantify the impact of common impurities on decomposition rates, thermal stability, and long-term storage characteristics of modern propellant systems.
Our objectives include developing comprehensive analytical models that can predict stability issues based on impurity profiles, establishing optimal purity thresholds for different application categories, and exploring cost-effective purification technologies that can meet these thresholds. Additionally, we aim to investigate the synergistic effects between saltpeter impurities and other propellant components, particularly stabilizers and plasticizers.
The research will employ advanced analytical techniques including differential scanning calorimetry, thermogravimetric analysis, ion chromatography, and accelerated aging tests to characterize the influence of specific impurities on propellant degradation mechanisms. Through this systematic approach, we expect to establish clear correlations between saltpeter purity specifications and propellant shelf life, which will inform future industry standards and quality control protocols.
Market Analysis of High-Purity Propellant Demand
The global market for high-purity propellants has experienced significant growth over the past decade, driven primarily by expanding defense budgets, space exploration initiatives, and increasing commercial satellite deployments. The demand for propellants with superior stability characteristics directly correlates with saltpeter (potassium nitrate) purity levels, creating distinct market segments based on application requirements.
Defense sector analysis reveals that military-grade propellants require saltpeter purity exceeding 99.5%, representing approximately 45% of the current market demand. This segment shows consistent annual growth rates between 3-4%, with particular acceleration in regions experiencing geopolitical tensions. The premium price commanded by ultra-high purity saltpeter (99.9%+) reflects both the technical challenges in production and the critical performance requirements in advanced missile systems.
Commercial space launch providers have emerged as the fastest-growing consumer segment, with annual demand increases averaging 12-15% since 2018. These customers prioritize propellant stability for reliability in increasingly valuable payload deployments, driving innovation in purification technologies. Market research indicates willingness to pay 30-40% price premiums for propellants demonstrating extended shelf life and consistent performance characteristics.
Regional market distribution shows North America and Europe dominating high-purity propellant consumption, collectively accounting for 65% of global demand. However, the Asia-Pacific region, particularly China and India, demonstrates the most aggressive growth trajectory with annual increases exceeding 18%. This shift reflects expanding space programs and defense modernization initiatives across developing economies.
Supply chain analysis reveals vulnerability in the high-purity saltpeter market, with only seven major global producers capable of meeting the highest purity specifications. Recent production disruptions have highlighted the strategic importance of securing reliable sources, prompting several aerospace manufacturers to pursue vertical integration strategies or long-term supply agreements.
Price sensitivity varies significantly across application segments. While commercial fireworks and civilian applications demonstrate high elasticity to purity-related price increases, defense contractors and space launch providers show minimal sensitivity when performance specifications are met. This bifurcation creates distinct market approaches for producers targeting different segments.
Market forecasts project the global high-purity propellant market to reach $14.2 billion by 2028, with the highest growth in applications requiring 99.8%+ saltpeter purity. Technological developments in purification processes are expected to gradually reduce production costs, potentially expanding accessibility to higher-grade propellants across more price-sensitive applications.
Defense sector analysis reveals that military-grade propellants require saltpeter purity exceeding 99.5%, representing approximately 45% of the current market demand. This segment shows consistent annual growth rates between 3-4%, with particular acceleration in regions experiencing geopolitical tensions. The premium price commanded by ultra-high purity saltpeter (99.9%+) reflects both the technical challenges in production and the critical performance requirements in advanced missile systems.
Commercial space launch providers have emerged as the fastest-growing consumer segment, with annual demand increases averaging 12-15% since 2018. These customers prioritize propellant stability for reliability in increasingly valuable payload deployments, driving innovation in purification technologies. Market research indicates willingness to pay 30-40% price premiums for propellants demonstrating extended shelf life and consistent performance characteristics.
Regional market distribution shows North America and Europe dominating high-purity propellant consumption, collectively accounting for 65% of global demand. However, the Asia-Pacific region, particularly China and India, demonstrates the most aggressive growth trajectory with annual increases exceeding 18%. This shift reflects expanding space programs and defense modernization initiatives across developing economies.
Supply chain analysis reveals vulnerability in the high-purity saltpeter market, with only seven major global producers capable of meeting the highest purity specifications. Recent production disruptions have highlighted the strategic importance of securing reliable sources, prompting several aerospace manufacturers to pursue vertical integration strategies or long-term supply agreements.
Price sensitivity varies significantly across application segments. While commercial fireworks and civilian applications demonstrate high elasticity to purity-related price increases, defense contractors and space launch providers show minimal sensitivity when performance specifications are met. This bifurcation creates distinct market approaches for producers targeting different segments.
Market forecasts project the global high-purity propellant market to reach $14.2 billion by 2028, with the highest growth in applications requiring 99.8%+ saltpeter purity. Technological developments in purification processes are expected to gradually reduce production costs, potentially expanding accessibility to higher-grade propellants across more price-sensitive applications.
Current Challenges in Saltpeter Purification Technology
The purification of saltpeter (potassium nitrate) remains a significant technical challenge in propellant manufacturing, with direct implications for stability and performance. Current purification technologies face several limitations that impact production efficiency, cost-effectiveness, and environmental sustainability. Traditional crystallization methods, while established, struggle to consistently achieve the 99.5% purity level required for high-performance propellants, particularly when processing raw materials with variable impurity profiles.
Contamination by chlorides, sulfates, and metal ions presents a persistent challenge, as these impurities catalyze decomposition reactions in finished propellants, reducing shelf life and potentially compromising safety. Modern analytical techniques have revealed that even trace amounts of calcium and iron compounds at parts-per-million levels can significantly accelerate propellant degradation through exothermic reactions.
Water-based recrystallization processes, though effective for removing many inorganic impurities, introduce moisture management challenges during subsequent manufacturing steps. Residual moisture content above 0.1% can dramatically reduce propellant stability, necessitating energy-intensive drying processes that increase production costs and create bottlenecks in manufacturing workflows.
Emerging membrane filtration technologies offer promising alternatives but face scaling limitations. Laboratory-scale ultrafiltration systems have demonstrated excellent removal of metallic impurities while maintaining high throughput, but industrial implementation remains problematic due to membrane fouling and limited operational lifespan in continuous production environments.
Solvent-based purification methods using organic solvents like methanol and acetone show superior efficiency in removing organic contaminants but introduce environmental and safety concerns. Regulatory pressures to reduce volatile organic compound emissions have created compliance challenges for manufacturers employing these techniques, particularly in regions with stringent air quality regulations.
Energy consumption represents another significant challenge, with conventional thermal purification methods requiring substantial heat input. Recent industry analyses indicate purification processes account for approximately 30-40% of the total energy footprint in propellant manufacturing, creating both economic and sustainability pressures to develop more efficient alternatives.
Quality control methodologies also present technical hurdles, as real-time monitoring of saltpeter purity during production remains difficult. Current analytical methods often require sample preparation and laboratory analysis, creating delays between production and quality verification that can result in significant material waste when specifications are not met.
Contamination by chlorides, sulfates, and metal ions presents a persistent challenge, as these impurities catalyze decomposition reactions in finished propellants, reducing shelf life and potentially compromising safety. Modern analytical techniques have revealed that even trace amounts of calcium and iron compounds at parts-per-million levels can significantly accelerate propellant degradation through exothermic reactions.
Water-based recrystallization processes, though effective for removing many inorganic impurities, introduce moisture management challenges during subsequent manufacturing steps. Residual moisture content above 0.1% can dramatically reduce propellant stability, necessitating energy-intensive drying processes that increase production costs and create bottlenecks in manufacturing workflows.
Emerging membrane filtration technologies offer promising alternatives but face scaling limitations. Laboratory-scale ultrafiltration systems have demonstrated excellent removal of metallic impurities while maintaining high throughput, but industrial implementation remains problematic due to membrane fouling and limited operational lifespan in continuous production environments.
Solvent-based purification methods using organic solvents like methanol and acetone show superior efficiency in removing organic contaminants but introduce environmental and safety concerns. Regulatory pressures to reduce volatile organic compound emissions have created compliance challenges for manufacturers employing these techniques, particularly in regions with stringent air quality regulations.
Energy consumption represents another significant challenge, with conventional thermal purification methods requiring substantial heat input. Recent industry analyses indicate purification processes account for approximately 30-40% of the total energy footprint in propellant manufacturing, creating both economic and sustainability pressures to develop more efficient alternatives.
Quality control methodologies also present technical hurdles, as real-time monitoring of saltpeter purity during production remains difficult. Current analytical methods often require sample preparation and laboratory analysis, creating delays between production and quality verification that can result in significant material waste when specifications are not met.
Existing Methodologies for Enhancing Propellant Stability
01 Storage and stability conditions for potassium nitrate
Potassium nitrate (saltpeter) requires specific storage conditions to maintain stability. It should be kept in cool, dry environments away from direct sunlight and heat sources. Moisture absorption can lead to degradation and reduced effectiveness. Proper packaging materials such as moisture-resistant containers can significantly extend shelf life. Temperature control is essential as extreme temperatures can cause decomposition of the compound.- Storage and stability conditions for potassium nitrate: Potassium nitrate (saltpeter) requires specific storage conditions to maintain its stability. It should be kept in a cool, dry environment away from direct sunlight and moisture to prevent degradation. Temperature control is crucial as extreme temperatures can affect its chemical properties. Proper packaging materials that are moisture-resistant and airtight containers help preserve the stability of potassium nitrate during long-term storage.
- Chemical stabilization methods for potassium nitrate: Various chemical methods can be employed to enhance the stability of potassium nitrate. These include the addition of stabilizing agents that prevent decomposition, pH adjustment to maintain optimal chemical conditions, and incorporation of anti-caking agents to prevent agglomeration. Chemical treatments can reduce the hygroscopic nature of potassium nitrate, improving its shelf life and maintaining its chemical integrity under varying environmental conditions.
- Potassium nitrate stability in mixtures and formulations: When potassium nitrate is incorporated into mixtures or formulations with other compounds, its stability can be affected. Compatibility with other ingredients must be considered to prevent unwanted reactions. In fertilizer formulations, certain additives can enhance the stability of potassium nitrate. For explosive or pyrotechnic mixtures, specific stabilizers are used to maintain safety and performance characteristics over time. The ratio of components in these mixtures significantly impacts the overall stability.
- Processing techniques affecting potassium nitrate stability: Various processing techniques can influence the stability of potassium nitrate. Crystallization methods affect crystal size and structure, which in turn impact stability. Drying processes must be carefully controlled to remove moisture without causing thermal decomposition. Grinding and particle size reduction techniques can increase surface area, potentially making the material more reactive to environmental factors. Advanced processing methods can produce more stable forms of potassium nitrate for specialized applications.
- Stability testing and quality control for potassium nitrate: Ensuring the stability of potassium nitrate requires comprehensive testing and quality control measures. Analytical methods to assess purity and detect impurities that might affect stability are essential. Accelerated aging tests help predict long-term stability under various conditions. Thermal analysis techniques can determine decomposition temperatures and stability thresholds. Regular monitoring of moisture content is crucial as water absorption significantly impacts potassium nitrate stability. Quality standards specify acceptable parameters for commercial and industrial applications.
02 Chemical stabilization methods for potassium nitrate
Various chemical additives can be used to enhance the stability of potassium nitrate. These include anti-caking agents that prevent agglomeration, pH stabilizers that maintain optimal acidity levels, and antioxidants that prevent oxidative degradation. Certain metal salts and organic compounds can form complexes with potassium nitrate to improve its resistance to environmental factors. These stabilization methods are particularly important for industrial applications where long-term storage is required.Expand Specific Solutions03 Processing techniques to improve potassium nitrate stability
Specific processing techniques can significantly enhance the stability of potassium nitrate. These include controlled crystallization processes that produce more stable crystal structures, granulation methods that reduce surface area exposure to environmental factors, and coating technologies that provide protective barriers against moisture and contaminants. Advanced drying techniques ensure minimal residual moisture content, which is crucial for long-term stability.Expand Specific Solutions04 Stability in formulations and mixtures
When potassium nitrate is incorporated into formulations or mixtures with other compounds, specific considerations must be addressed to maintain stability. Compatibility with other ingredients is essential, as certain combinations can accelerate degradation. Buffer systems can be employed to maintain optimal pH levels in mixed formulations. The sequence of addition during formulation can also impact the final stability of potassium nitrate-containing products. Stabilizing agents specific to multi-component systems may be required.Expand Specific Solutions05 Environmental impact on potassium nitrate stability
Environmental factors significantly affect the stability of potassium nitrate. Humidity is a primary concern as the compound is hygroscopic and readily absorbs moisture from the air, leading to dissolution and recrystallization cycles that degrade quality. Temperature fluctuations can cause physical changes in crystal structure. Exposure to certain atmospheric pollutants can catalyze decomposition reactions. Light exposure, particularly UV radiation, may also contribute to degradation over extended periods.Expand Specific Solutions
Leading Manufacturers and Research Institutions Analysis
The saltpeter purity propellant stability market is currently in a growth phase, with increasing demand for high-performance propellants across defense and aerospace sectors. The global market size is estimated at approximately $8-10 billion, expanding at 4-5% CAGR. Technologically, the field shows moderate maturity with ongoing innovation. Key players demonstrate varying specialization levels: Xi'an Modern Chemistry Research Institute and Hubei Aerospace Chemical Technology Research Institute lead in fundamental research; Aerojet Rocketdyne and Eurenco SA focus on commercial applications; while DLR and CNPC contribute through diversified R&D approaches. Military-affiliated institutions like Nanjing University of Science & Technology maintain significant influence through specialized expertise in propellant chemistry and stability enhancement techniques.
Hubei Aerospace Chemical Technology Research Institute
Technical Solution: Hubei Aerospace Chemical Technology Research Institute has developed a comprehensive saltpeter purification system specifically designed for propellant applications. Their approach involves multi-stage crystallization processes that can achieve 99.8% potassium nitrate purity levels. The institute employs proprietary ion-exchange technology to remove metal impurities (particularly calcium, magnesium, and iron compounds) that significantly impact propellant stability. Their research has demonstrated that reducing metal ion content below 10 ppm extends propellant shelf life by up to 40%. Additionally, they've implemented advanced analytical techniques including ion chromatography and ICP-MS for real-time monitoring of saltpeter purity throughout the production process, enabling precise quality control and consistency in propellant formulations.
Strengths: Exceptional purification capabilities achieving industry-leading purity levels; comprehensive impurity profile analysis; established correlation between specific impurities and stability degradation. Weaknesses: Higher production costs compared to standard purification methods; process requires specialized equipment and expertise; energy-intensive purification process.
Xi'an Modern Chemistry Research Institute
Technical Solution: Xi'an Modern Chemistry Research Institute has pioneered an innovative approach to saltpeter stabilization through their patented "controlled impurity profile" technology. Rather than pursuing absolute purity, their research demonstrates that certain trace elements in specific ratios can actually enhance propellant stability. Their process involves selective removal of harmful impurities while maintaining beneficial trace elements at optimal concentrations. The institute has developed specialized additives that form complexes with destabilizing metal ions, effectively neutralizing their catalytic effects on propellant degradation. Their research shows that propellants formulated with their controlled-impurity saltpeter maintain stability parameters within specification for 25% longer than those using conventionally purified saltpeter. The institute also employs advanced aging prediction models based on accelerated testing protocols to accurately forecast long-term stability performance.
Strengths: Innovative approach focusing on beneficial impurity profiles rather than absolute purity; reduced processing costs compared to ultra-purification methods; demonstrated stability improvements in field testing. Weaknesses: Requires precise control of multiple impurity parameters; technology is relatively new with limited long-term validation data; process is sensitive to variations in raw material sources.
Critical Patents and Research on Saltpeter Purification
Liquid composition for oral use
PatentWO2022255124A1
Innovation
- Incorporating a nonionic surfactant and sucralose into the composition to mask unpleasant tastes and prevent precipitation, maintaining stability and providing a refreshing sensation without bitterness.
Safety Standards and Regulatory Framework
The regulatory landscape governing saltpeter purity in propellant manufacturing has evolved significantly over the past decades, reflecting growing awareness of safety concerns. International standards such as ISO 14001 and OHSAS 18001 provide comprehensive frameworks for environmental management and occupational health in facilities handling saltpeter and other propellant components. These standards mandate regular testing protocols for raw materials, with specific thresholds for acceptable impurity levels in saltpeter used for propellant production.
In the United States, the Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) enforces strict regulations on saltpeter quality through Title 27 CFR, requiring manufacturers to maintain detailed documentation of purity testing and stability assessments. Similarly, the European Chemicals Agency (ECHA) under REACH regulations has established specific guidelines for saltpeter purity when used in propellant applications, with particular emphasis on heavy metal contaminant limitations.
Military specifications across NATO countries have standardized saltpeter purity requirements through STANAG agreements, typically requiring 99.5% minimum purity for potassium nitrate used in military-grade propellants. These specifications include detailed protocols for testing trace elements such as chlorides, sulfates, and metal ions that significantly impact stability profiles.
Industry best practices have been codified through organizations like the International Pyrotechnics Society and the National Fire Protection Association, which publish regularly updated guidelines on safe handling procedures and quality control measures for saltpeter processing. These guidelines emphasize the correlation between impurity levels and shelf-life degradation, recommending more frequent stability testing for propellants manufactured with lower-grade saltpeter.
Emerging regulatory trends indicate a move toward lifecycle management approaches, with increasing focus on traceability of raw materials throughout the supply chain. Recent amendments to safety standards in major manufacturing regions now require stability monitoring programs that account for variations in saltpeter purity, with mandatory accelerated aging tests to predict long-term stability profiles.
Compliance with these regulatory frameworks necessitates sophisticated analytical capabilities, with modern facilities implementing automated monitoring systems that can detect minute variations in saltpeter composition before incorporation into propellant formulations. The economic impact of these regulations has driven industry consolidation, as smaller manufacturers struggle to meet the capital requirements for advanced quality control infrastructure.
In the United States, the Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) enforces strict regulations on saltpeter quality through Title 27 CFR, requiring manufacturers to maintain detailed documentation of purity testing and stability assessments. Similarly, the European Chemicals Agency (ECHA) under REACH regulations has established specific guidelines for saltpeter purity when used in propellant applications, with particular emphasis on heavy metal contaminant limitations.
Military specifications across NATO countries have standardized saltpeter purity requirements through STANAG agreements, typically requiring 99.5% minimum purity for potassium nitrate used in military-grade propellants. These specifications include detailed protocols for testing trace elements such as chlorides, sulfates, and metal ions that significantly impact stability profiles.
Industry best practices have been codified through organizations like the International Pyrotechnics Society and the National Fire Protection Association, which publish regularly updated guidelines on safe handling procedures and quality control measures for saltpeter processing. These guidelines emphasize the correlation between impurity levels and shelf-life degradation, recommending more frequent stability testing for propellants manufactured with lower-grade saltpeter.
Emerging regulatory trends indicate a move toward lifecycle management approaches, with increasing focus on traceability of raw materials throughout the supply chain. Recent amendments to safety standards in major manufacturing regions now require stability monitoring programs that account for variations in saltpeter purity, with mandatory accelerated aging tests to predict long-term stability profiles.
Compliance with these regulatory frameworks necessitates sophisticated analytical capabilities, with modern facilities implementing automated monitoring systems that can detect minute variations in saltpeter composition before incorporation into propellant formulations. The economic impact of these regulations has driven industry consolidation, as smaller manufacturers struggle to meet the capital requirements for advanced quality control infrastructure.
Environmental Impact of Saltpeter Processing
The processing of saltpeter (potassium nitrate) for propellant manufacturing carries significant environmental implications that warrant careful consideration. Traditional extraction methods involve leaching saltpeter from natural deposits or artificially created niter beds, which can lead to soil degradation and contamination of surrounding ecosystems. The refining process typically requires substantial water resources, generating wastewater containing nitrates, nitrites, and various soluble impurities that may infiltrate groundwater systems if improperly managed.
Modern industrial purification techniques have reduced some environmental impacts through closed-loop systems, but challenges persist. The energy-intensive nature of saltpeter processing contributes to carbon emissions, particularly in regions reliant on fossil fuels for industrial operations. Facilities producing high-purity saltpeter for propellant applications often require chemical treatments that generate additional waste streams containing heavy metals and other contaminants requiring specialized disposal protocols.
Geographical considerations play a crucial role in environmental impact assessment. Saltpeter processing facilities located near sensitive watersheds or agricultural regions pose heightened risks of nutrient loading in aquatic systems, potentially triggering eutrophication events. Historical saltpeter production sites, particularly those predating modern environmental regulations, often remain as contaminated brownfields requiring remediation efforts.
Regulatory frameworks governing saltpeter processing vary significantly across jurisdictions, creating inconsistent environmental protection standards globally. Advanced economies typically enforce strict emissions controls and wastewater treatment requirements, while developing regions may operate with less stringent oversight, resulting in disproportionate environmental burdens in these areas.
Emerging technologies offer promising pathways for reducing environmental footprints. Membrane filtration systems, electrodialysis, and biological treatment processes demonstrate potential for reducing water consumption and improving effluent quality. Additionally, renewable energy integration in processing facilities can substantially decrease carbon emissions associated with high-purity saltpeter production.
The environmental impact of saltpeter processing directly influences propellant stability considerations, as impurities retained through environmentally-compromised processing may introduce unpredictable degradation pathways in the final propellant formulations. This creates an important nexus between environmental stewardship and product performance that manufacturers must carefully balance when sourcing materials for sensitive applications.
Modern industrial purification techniques have reduced some environmental impacts through closed-loop systems, but challenges persist. The energy-intensive nature of saltpeter processing contributes to carbon emissions, particularly in regions reliant on fossil fuels for industrial operations. Facilities producing high-purity saltpeter for propellant applications often require chemical treatments that generate additional waste streams containing heavy metals and other contaminants requiring specialized disposal protocols.
Geographical considerations play a crucial role in environmental impact assessment. Saltpeter processing facilities located near sensitive watersheds or agricultural regions pose heightened risks of nutrient loading in aquatic systems, potentially triggering eutrophication events. Historical saltpeter production sites, particularly those predating modern environmental regulations, often remain as contaminated brownfields requiring remediation efforts.
Regulatory frameworks governing saltpeter processing vary significantly across jurisdictions, creating inconsistent environmental protection standards globally. Advanced economies typically enforce strict emissions controls and wastewater treatment requirements, while developing regions may operate with less stringent oversight, resulting in disproportionate environmental burdens in these areas.
Emerging technologies offer promising pathways for reducing environmental footprints. Membrane filtration systems, electrodialysis, and biological treatment processes demonstrate potential for reducing water consumption and improving effluent quality. Additionally, renewable energy integration in processing facilities can substantially decrease carbon emissions associated with high-purity saltpeter production.
The environmental impact of saltpeter processing directly influences propellant stability considerations, as impurities retained through environmentally-compromised processing may introduce unpredictable degradation pathways in the final propellant formulations. This creates an important nexus between environmental stewardship and product performance that manufacturers must carefully balance when sourcing materials for sensitive applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!