Optimization of Saltpeter Processing for High-Purity Outputs
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Saltpeter Purification Technology Background and Objectives
Saltpeter, also known as potassium nitrate (KNO3), has been a critical compound in various industries for centuries. Its historical significance dates back to ancient China where it was used in early gunpowder formulations. The evolution of saltpeter processing technology has undergone significant transformations from rudimentary extraction methods to sophisticated purification processes that we see today.
The technological trajectory of saltpeter purification has been driven by increasing demands for higher purity levels across multiple industries including agriculture, food preservation, pharmaceuticals, and explosives manufacturing. Traditional methods involving natural extraction from cave deposits or animal waste have gradually given way to chemical synthesis and advanced refining techniques.
Current saltpeter purification technologies face several limitations, particularly in achieving consistent high-purity outputs while maintaining cost-effectiveness and environmental sustainability. The presence of contaminants such as chlorides, sulfates, and heavy metals significantly impacts the quality and applicability of the final product, necessitating more efficient purification methodologies.
The primary objective of optimizing saltpeter processing is to develop scalable and sustainable technologies capable of consistently producing high-purity potassium nitrate (>99.5%) while minimizing energy consumption, reducing waste generation, and lowering production costs. This optimization aims to address the growing market demand for ultra-pure saltpeter in specialized applications such as crystal oscillators, specialty glass manufacturing, and advanced ceramic production.
Recent technological advancements in membrane filtration, selective crystallization, and ion exchange processes have shown promising results in improving purity levels. However, these technologies require further refinement to overcome challenges related to membrane fouling, energy intensity, and process scalability.
The global push toward greener chemical processes has also influenced saltpeter purification technology development, with increasing focus on closed-loop systems, solvent recovery, and waste valorization. Emerging approaches incorporating biotechnological solutions and electrochemical methods represent potential paradigm shifts in traditional saltpeter processing.
As we look toward future developments, the integration of digital technologies such as AI-driven process optimization, real-time monitoring systems, and automated quality control mechanisms presents opportunities for significant improvements in both efficiency and product quality. The convergence of these technological trends will likely shape the next generation of saltpeter purification methodologies.
The technological trajectory of saltpeter purification has been driven by increasing demands for higher purity levels across multiple industries including agriculture, food preservation, pharmaceuticals, and explosives manufacturing. Traditional methods involving natural extraction from cave deposits or animal waste have gradually given way to chemical synthesis and advanced refining techniques.
Current saltpeter purification technologies face several limitations, particularly in achieving consistent high-purity outputs while maintaining cost-effectiveness and environmental sustainability. The presence of contaminants such as chlorides, sulfates, and heavy metals significantly impacts the quality and applicability of the final product, necessitating more efficient purification methodologies.
The primary objective of optimizing saltpeter processing is to develop scalable and sustainable technologies capable of consistently producing high-purity potassium nitrate (>99.5%) while minimizing energy consumption, reducing waste generation, and lowering production costs. This optimization aims to address the growing market demand for ultra-pure saltpeter in specialized applications such as crystal oscillators, specialty glass manufacturing, and advanced ceramic production.
Recent technological advancements in membrane filtration, selective crystallization, and ion exchange processes have shown promising results in improving purity levels. However, these technologies require further refinement to overcome challenges related to membrane fouling, energy intensity, and process scalability.
The global push toward greener chemical processes has also influenced saltpeter purification technology development, with increasing focus on closed-loop systems, solvent recovery, and waste valorization. Emerging approaches incorporating biotechnological solutions and electrochemical methods represent potential paradigm shifts in traditional saltpeter processing.
As we look toward future developments, the integration of digital technologies such as AI-driven process optimization, real-time monitoring systems, and automated quality control mechanisms presents opportunities for significant improvements in both efficiency and product quality. The convergence of these technological trends will likely shape the next generation of saltpeter purification methodologies.
Market Analysis for High-Purity Saltpeter Products
The global market for high-purity saltpeter (potassium nitrate) has experienced significant growth over the past decade, driven primarily by expanding applications in agriculture, food preservation, and specialty chemicals. Current market valuation stands at approximately 1.2 billion USD, with projections indicating a compound annual growth rate of 4.7% through 2028.
Agricultural applications represent the largest market segment, accounting for nearly 45% of total consumption. The increasing global focus on crop yield optimization and sustainable farming practices has substantially boosted demand for high-purity potassium nitrate as a premium fertilizer. Countries with intensive agriculture systems, particularly in regions like Western Europe, North America, and East Asia, demonstrate the highest per-capita consumption rates.
The food industry constitutes the second-largest market segment at 28%, where high-purity saltpeter serves as a critical preservative and color fixative in processed meat products. Regulatory changes in major markets have established stricter purity requirements, creating premium pricing opportunities for manufacturers capable of delivering consistently high-purity outputs.
Industrial applications, including glass manufacturing, explosives, and specialty chemicals, comprise approximately 22% of the market. These sectors demand exceptionally high purity levels, often exceeding 99.5%, and represent the highest profit margin segment despite lower volume consumption.
Market distribution shows regional concentration, with Asia-Pacific leading consumption at 38%, followed by Europe (27%), North America (21%), and other regions (14%). China, India, and Brazil are experiencing the fastest growth rates, driven by agricultural modernization and expanding food processing industries.
Customer segmentation reveals three distinct tiers: large-scale agricultural cooperatives and industrial manufacturers seeking bulk quantities at competitive prices; mid-sized specialty chemical companies requiring consistent high-purity products; and niche research organizations and pharmaceutical companies demanding ultra-high purity grades at premium prices.
Pricing analysis indicates significant premiums for purity levels above 99%, with each additional 0.1% purity potentially commanding 5-8% price increases in specialty applications. This creates strong economic incentives for processing optimization technologies that can reliably achieve higher purity outputs while maintaining cost efficiency.
Market forecasts suggest particularly strong growth in pharmaceutical and electronic applications, where ultra-high purity saltpeter serves as a precursor for specialized compounds. These emerging applications, though currently representing only 5% of market volume, are projected to grow at twice the rate of traditional segments, offering attractive diversification opportunities for producers investing in advanced purification technologies.
Agricultural applications represent the largest market segment, accounting for nearly 45% of total consumption. The increasing global focus on crop yield optimization and sustainable farming practices has substantially boosted demand for high-purity potassium nitrate as a premium fertilizer. Countries with intensive agriculture systems, particularly in regions like Western Europe, North America, and East Asia, demonstrate the highest per-capita consumption rates.
The food industry constitutes the second-largest market segment at 28%, where high-purity saltpeter serves as a critical preservative and color fixative in processed meat products. Regulatory changes in major markets have established stricter purity requirements, creating premium pricing opportunities for manufacturers capable of delivering consistently high-purity outputs.
Industrial applications, including glass manufacturing, explosives, and specialty chemicals, comprise approximately 22% of the market. These sectors demand exceptionally high purity levels, often exceeding 99.5%, and represent the highest profit margin segment despite lower volume consumption.
Market distribution shows regional concentration, with Asia-Pacific leading consumption at 38%, followed by Europe (27%), North America (21%), and other regions (14%). China, India, and Brazil are experiencing the fastest growth rates, driven by agricultural modernization and expanding food processing industries.
Customer segmentation reveals three distinct tiers: large-scale agricultural cooperatives and industrial manufacturers seeking bulk quantities at competitive prices; mid-sized specialty chemical companies requiring consistent high-purity products; and niche research organizations and pharmaceutical companies demanding ultra-high purity grades at premium prices.
Pricing analysis indicates significant premiums for purity levels above 99%, with each additional 0.1% purity potentially commanding 5-8% price increases in specialty applications. This creates strong economic incentives for processing optimization technologies that can reliably achieve higher purity outputs while maintaining cost efficiency.
Market forecasts suggest particularly strong growth in pharmaceutical and electronic applications, where ultra-high purity saltpeter serves as a precursor for specialized compounds. These emerging applications, though currently representing only 5% of market volume, are projected to grow at twice the rate of traditional segments, offering attractive diversification opportunities for producers investing in advanced purification technologies.
Current Challenges in Saltpeter Processing Technologies
The saltpeter processing industry currently faces several significant technical challenges that impede the production of high-purity outputs. Traditional processing methods often result in final products containing various impurities, including heavy metals, organic compounds, and other nitrate salts, which limit their applications in sensitive industries such as pharmaceuticals, electronics, and specialty chemicals.
One major challenge is the inconsistent quality of raw materials. Saltpeter deposits vary significantly in composition depending on geographical location, with natural sources containing varying levels of contaminants such as chlorides, sulfates, and calcium compounds. This variability necessitates adaptive processing techniques, which many current systems lack the flexibility to implement effectively.
Energy consumption represents another critical concern in saltpeter processing. Conventional crystallization and purification processes require substantial thermal energy, contributing to high operational costs and significant carbon footprints. The industry has been slow to adopt energy-efficient technologies due to high capital investment requirements and uncertain return on investment timelines.
Water usage efficiency presents a growing challenge, particularly as many saltpeter processing facilities operate in water-stressed regions. Current technologies typically consume 8-12 cubic meters of water per ton of processed saltpeter, creating environmental pressures and increasing production costs as water regulations become more stringent globally.
Scaling issues during processing stages constitute a persistent technical problem. Calcium and magnesium salts present in raw materials tend to form scale deposits on equipment surfaces, reducing heat transfer efficiency, increasing maintenance requirements, and shortening equipment lifespan. Existing anti-scaling technologies have shown limited effectiveness in high-concentration saltpeter solutions.
The separation of sodium and potassium nitrates remains technically challenging, particularly when high purity levels (>99.5%) are required. Current fractional crystallization methods struggle to achieve consistent separation without multiple processing cycles, reducing throughput and increasing production costs.
Automation and process control systems in many saltpeter facilities lag behind other chemical processing industries. Manual sampling and offline analysis remain common, leading to delayed process adjustments and quality inconsistencies. Real-time monitoring technologies for nitrate concentration, impurity levels, and crystallization parameters have not been widely implemented across the industry.
Waste management presents additional challenges, as processing byproducts often contain concentrated levels of contaminants that require specialized handling. Environmental regulations increasingly restrict disposal options, forcing processors to develop more sophisticated waste treatment systems or find alternative uses for byproducts.
One major challenge is the inconsistent quality of raw materials. Saltpeter deposits vary significantly in composition depending on geographical location, with natural sources containing varying levels of contaminants such as chlorides, sulfates, and calcium compounds. This variability necessitates adaptive processing techniques, which many current systems lack the flexibility to implement effectively.
Energy consumption represents another critical concern in saltpeter processing. Conventional crystallization and purification processes require substantial thermal energy, contributing to high operational costs and significant carbon footprints. The industry has been slow to adopt energy-efficient technologies due to high capital investment requirements and uncertain return on investment timelines.
Water usage efficiency presents a growing challenge, particularly as many saltpeter processing facilities operate in water-stressed regions. Current technologies typically consume 8-12 cubic meters of water per ton of processed saltpeter, creating environmental pressures and increasing production costs as water regulations become more stringent globally.
Scaling issues during processing stages constitute a persistent technical problem. Calcium and magnesium salts present in raw materials tend to form scale deposits on equipment surfaces, reducing heat transfer efficiency, increasing maintenance requirements, and shortening equipment lifespan. Existing anti-scaling technologies have shown limited effectiveness in high-concentration saltpeter solutions.
The separation of sodium and potassium nitrates remains technically challenging, particularly when high purity levels (>99.5%) are required. Current fractional crystallization methods struggle to achieve consistent separation without multiple processing cycles, reducing throughput and increasing production costs.
Automation and process control systems in many saltpeter facilities lag behind other chemical processing industries. Manual sampling and offline analysis remain common, leading to delayed process adjustments and quality inconsistencies. Real-time monitoring technologies for nitrate concentration, impurity levels, and crystallization parameters have not been widely implemented across the industry.
Waste management presents additional challenges, as processing byproducts often contain concentrated levels of contaminants that require specialized handling. Environmental regulations increasingly restrict disposal options, forcing processors to develop more sophisticated waste treatment systems or find alternative uses for byproducts.
Current High-Purity Saltpeter Production Techniques
01 Purification methods for potassium nitrate
Various methods are employed to purify potassium nitrate (saltpeter) to achieve high purity levels. These methods include crystallization, recrystallization, washing processes, and filtration techniques. The purification process often involves removing impurities such as chlorides, sulfates, and other metal ions to obtain high-grade potassium nitrate suitable for industrial and pharmaceutical applications.- Purification methods for potassium nitrate: Various methods are employed to purify potassium nitrate (saltpeter) to achieve high purity levels. These methods include crystallization, recrystallization, washing processes, and filtration techniques. The purification process typically involves dissolving raw saltpeter in water, removing impurities through filtration, and then recrystallizing the potassium nitrate under controlled conditions to obtain a high-purity product.
- Industrial production of high-purity potassium nitrate: Industrial-scale production methods for high-purity potassium nitrate involve specialized equipment and processes. These include continuous crystallization systems, industrial filtration units, and automated production lines. The industrial processes are designed to efficiently produce large quantities of potassium nitrate with consistent purity levels, often exceeding 99%. These methods may incorporate multiple purification stages to progressively remove different types of impurities.
- Analytical methods for determining potassium nitrate purity: Various analytical techniques are used to determine the purity of potassium nitrate. These include spectroscopic methods, chromatography, titration, and thermal analysis. These analytical methods can detect and quantify impurities such as chlorides, sulfates, heavy metals, and moisture content. The purity assessment is crucial for ensuring that the potassium nitrate meets the required specifications for different applications.
- Purity standards for different applications of potassium nitrate: Different applications of potassium nitrate require different purity levels. For agricultural use as fertilizer, lower purity grades (95-98%) may be acceptable. For food preservation (E252), pharmaceutical applications, and pyrotechnics, higher purity grades (>99%) are typically required. The highest purity grades (>99.9%) are used in specialized applications such as crystal growth, electronics, and certain chemical synthesis processes. Standards organizations have established specific purity requirements for each application.
- Impurity removal techniques for potassium nitrate: Specific techniques are employed to remove particular impurities from potassium nitrate. These include ion exchange methods for removing metal ions, chemical precipitation for removing sulfates and phosphates, and activated carbon treatment for removing organic impurities. Advanced techniques such as membrane filtration and zone refining may be used for ultra-high purity requirements. The selection of impurity removal techniques depends on the nature and concentration of the impurities present and the desired final purity level.
02 Analytical methods for determining potassium nitrate purity
Various analytical techniques are used to determine the purity of potassium nitrate. These include titration methods, spectroscopic analysis, chromatography, and other instrumental methods. These analytical approaches help in quantifying the potassium nitrate content and identifying impurities present in the sample, ensuring that the product meets required purity specifications for different applications.Expand Specific Solutions03 Equipment and systems for potassium nitrate purification
Specialized equipment and systems are designed for the purification of potassium nitrate to achieve high purity levels. These include crystallizers, evaporators, filtration systems, and continuous processing equipment. The design of these systems focuses on optimizing the purification process to consistently produce high-purity potassium nitrate while minimizing energy consumption and waste generation.Expand Specific Solutions04 Industrial applications requiring high-purity potassium nitrate
High-purity potassium nitrate is essential for various industrial applications including explosives, pyrotechnics, fertilizers, food preservation, and glass manufacturing. Each application requires specific purity levels, with some demanding extremely high purity (>99.9%). The purity requirements are particularly stringent for applications in electronics, pharmaceuticals, and specialty chemicals where even trace impurities can significantly affect performance.Expand Specific Solutions05 Production of high-purity potassium nitrate from natural sources
Methods for producing high-purity potassium nitrate from natural sources such as mineral deposits, plant materials, or agricultural waste are developed to provide sustainable and cost-effective production routes. These processes often involve extraction, concentration, and purification steps to convert the raw materials into high-purity potassium nitrate. The methods aim to maximize yield while ensuring that the final product meets stringent purity specifications.Expand Specific Solutions
Leading Companies in Advanced Saltpeter Processing
The saltpeter processing optimization market is currently in a growth phase, with increasing demand for high-purity outputs across pharmaceutical, agricultural, and industrial sectors. The global market size is estimated at approximately $3.5 billion, with projected annual growth of 5-7%. Leading players include established chemical manufacturers like BASF Corp. and Henkel AG, alongside specialized companies such as Sinkiang Nitrate Minerals Co. and Kingenta Ecological Engineering Group. Technical innovation is advancing rapidly, with companies like CoorsTek and Jiangsu Jiechuangxin Material developing proprietary purification processes. Regional competition is intensifying, particularly between Western corporations (Cargill, Milliken & Co.) and Asian manufacturers (Nikko Metal Manufacturing, Shanghai Huayi Holdings), with the latter gaining market share through cost advantages and technological investments in high-purity processing methods.
Cargill, Inc.
Technical Solution: Cargill has developed a bio-assisted saltpeter purification process that leverages enzymatic reactions to selectively remove organic contaminants. Their technology combines traditional chemical processing with biotechnology, using engineered enzymes that target specific impurities without affecting the saltpeter crystals. The process operates at lower temperatures (30-45°C) compared to conventional methods (60-80°C), resulting in energy savings of approximately 40%. Cargill's system incorporates a proprietary crystallization control technology that produces uniform crystal size distribution, enhancing downstream processing efficiency and product consistency. Their integrated quality control system employs spectroscopic analysis at multiple process points, allowing for real-time adjustments to maintain purity specifications. The company has also developed environmentally friendly chelating agents that sequester metal impurities, resulting in final products with metal contaminant levels below 5 ppm, exceeding most industry standards.
Strengths: Lower energy requirements through reduced processing temperatures; environmentally friendly approach with minimal chemical waste; exceptional removal of organic contaminants; consistent crystal morphology. Weaknesses: Enzymatic components have limited shelf life and require careful storage; process is more sensitive to pH and temperature fluctuations; higher operating costs for enzyme production and replacement.
Kingenta Ecological Engineering Group Co., Ltd.
Technical Solution: Kingenta has developed a comprehensive saltpeter optimization process focused on agricultural-grade purity requirements. Their technology employs a controlled-release crystallization method that produces uniform, high-purity crystals with specific dissolution profiles tailored for agricultural applications. The company's process incorporates a multi-stage washing system using counter-current flow principles, which reduces water consumption by approximately 45% while achieving impurity removal rates of over 99%. Kingenta has implemented advanced drying technology using fluidized bed systems with precise humidity and temperature controls, resulting in products with moisture content below 0.1% without thermal degradation. Their quality control system utilizes near-infrared spectroscopy for continuous monitoring, enabling real-time process adjustments to maintain consistent purity levels. Additionally, they've developed coating technologies that protect the purified saltpeter from moisture absorption during storage and transport, extending shelf life significantly.
Strengths: Highly efficient water usage through counter-current washing systems; precise control over crystal properties including size, shape and dissolution rate; excellent moisture control in final product; specialized formulations for agricultural applications. Weaknesses: Process optimized primarily for agricultural rather than pharmaceutical or electronic grades; coating technologies add additional processing steps and costs; limited flexibility for producing multiple grades simultaneously.
Key Patents and Innovations in Saltpeter Purification
Process for the preparation of potassium nitrate
PatentInactiveCA1315233C
Innovation
- A process involving the electrochemical production of potassium carbonate from potassium chloride, using a membrane cell with a perm-selective cation-exchange membrane to generate chlorine and hydrogen, followed by conversion to potassium nitrate with nitric acid, allowing for high-purity potassium nitrate production with reduced chlorine content.
Improvements in or relating to a process for preparing potassium nitrate
PatentInactiveGB712724A
Innovation
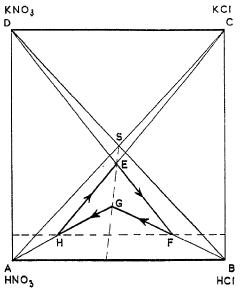
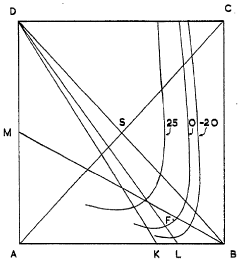
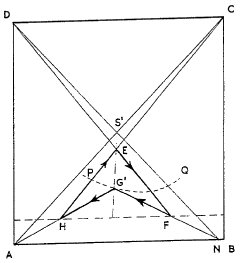
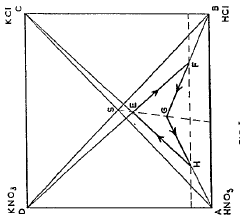
- A cyclic process where potassium chloride and nitric acid are added to a solution from a previous cycle, allowing potassium nitrate to crystallize, followed by hydrochloric acid distillation, with careful control of temperatures and concentrations to optimize yield and prevent unwanted crystallization, using a spatial diagram representation to determine optimal conditions.
Environmental Impact Assessment of Purification Processes
The environmental impact of saltpeter purification processes represents a critical consideration in the optimization of production methods for high-purity outputs. Traditional saltpeter processing techniques have historically generated significant environmental concerns, including water pollution from nitrate runoff, soil contamination, and atmospheric emissions of nitrogen oxides. These impacts necessitate comprehensive assessment to ensure sustainable production practices.
Water resource management stands as a primary environmental challenge in saltpeter processing. The purification process typically requires substantial water volumes, with conventional methods consuming 8-12 cubic meters per ton of product. Advanced filtration systems implemented in modern facilities have demonstrated potential to reduce water usage by 30-45%, while closed-loop water recycling systems can further minimize freshwater requirements and wastewater discharge.
Effluent management presents another significant environmental consideration. Untreated wastewater from saltpeter purification contains elevated levels of nitrates, heavy metals, and suspended solids that can severely impact aquatic ecosystems. Recent technological innovations, including membrane bioreactors and advanced oxidation processes, have shown promising results in reducing contaminant levels by 85-95% before discharge, substantially mitigating ecological damage.
Energy consumption patterns in purification processes directly correlate with carbon emissions and climate impact. Traditional thermal crystallization methods require 4.5-6.2 GJ of energy per ton of high-purity saltpeter produced. Implementation of energy recovery systems and transition to renewable energy sources can reduce the carbon footprint by 40-60%, as demonstrated in facilities across Northern Europe and parts of Asia.
Solid waste generation during purification represents an additional environmental burden. The processing of raw saltpeter typically generates 0.2-0.3 tons of solid waste per ton of final product, primarily consisting of insoluble minerals and organic matter. Emerging circular economy approaches have developed methods to repurpose these byproducts as soil amendments or construction materials, achieving waste reduction rates of 70-85% in optimized operations.
Regulatory frameworks governing environmental impacts vary significantly across regions, creating challenges for standardized assessment methodologies. The European Union's Industrial Emissions Directive establishes stringent requirements for Best Available Techniques (BAT) in saltpeter processing, while developing nations often operate under less restrictive environmental oversight. This regulatory disparity highlights the need for harmonized global standards to ensure consistent environmental protection across the industry.
Water resource management stands as a primary environmental challenge in saltpeter processing. The purification process typically requires substantial water volumes, with conventional methods consuming 8-12 cubic meters per ton of product. Advanced filtration systems implemented in modern facilities have demonstrated potential to reduce water usage by 30-45%, while closed-loop water recycling systems can further minimize freshwater requirements and wastewater discharge.
Effluent management presents another significant environmental consideration. Untreated wastewater from saltpeter purification contains elevated levels of nitrates, heavy metals, and suspended solids that can severely impact aquatic ecosystems. Recent technological innovations, including membrane bioreactors and advanced oxidation processes, have shown promising results in reducing contaminant levels by 85-95% before discharge, substantially mitigating ecological damage.
Energy consumption patterns in purification processes directly correlate with carbon emissions and climate impact. Traditional thermal crystallization methods require 4.5-6.2 GJ of energy per ton of high-purity saltpeter produced. Implementation of energy recovery systems and transition to renewable energy sources can reduce the carbon footprint by 40-60%, as demonstrated in facilities across Northern Europe and parts of Asia.
Solid waste generation during purification represents an additional environmental burden. The processing of raw saltpeter typically generates 0.2-0.3 tons of solid waste per ton of final product, primarily consisting of insoluble minerals and organic matter. Emerging circular economy approaches have developed methods to repurpose these byproducts as soil amendments or construction materials, achieving waste reduction rates of 70-85% in optimized operations.
Regulatory frameworks governing environmental impacts vary significantly across regions, creating challenges for standardized assessment methodologies. The European Union's Industrial Emissions Directive establishes stringent requirements for Best Available Techniques (BAT) in saltpeter processing, while developing nations often operate under less restrictive environmental oversight. This regulatory disparity highlights the need for harmonized global standards to ensure consistent environmental protection across the industry.
Quality Control Standards and Certification Requirements
Quality control in saltpeter processing represents a critical component for ensuring high-purity outputs that meet industry standards and regulatory requirements. The saltpeter industry has established comprehensive quality control frameworks that encompass various testing methodologies, certification processes, and compliance standards across different regions and applications.
International standards for saltpeter purity are primarily governed by ISO 9001:2015 for quality management systems and ISO 17025 for testing laboratory competence. These standards provide the foundation for consistent quality assessment and certification processes. Additionally, industry-specific standards such as ASTM E456 for chemical analysis of nitrate compounds and the European Standard EN 16195 for fertilizer quality have been adapted specifically for saltpeter processing.
Quality control testing protocols for high-purity saltpeter typically include quantitative analysis of nitrate content (minimum 99.5% for premium grade), moisture content (below 0.2%), and impurity profiling for heavy metals, chlorides, and sulfates. Advanced analytical techniques employed include ion chromatography, inductively coupled plasma mass spectrometry (ICP-MS), and X-ray diffraction analysis to ensure comprehensive purity verification.
Certification requirements vary by application sector. For pharmaceutical-grade saltpeter, compliance with Good Manufacturing Practice (GMP) guidelines and pharmacopeia standards (USP, EP, JP) is mandatory, requiring extensive documentation of production processes and quality testing. Food-grade applications must adhere to Food Chemical Codex (FCC) specifications and obtain certifications from relevant food safety authorities such as FDA or EFSA.
Agricultural applications of saltpeter are subject to fertilizer regulations that vary by region, with certification requirements focusing on nutrient content verification, heavy metal limits, and environmental impact assessments. The European Fertilizer Regulation (EU) 2019/1009 and the AAPFCO (Association of American Plant Food Control Officials) standards represent key regulatory frameworks in this sector.
Emerging trends in quality control standards include the implementation of real-time monitoring systems using IoT sensors for continuous quality assessment throughout the production process. This approach enables immediate detection of deviations from quality parameters and facilitates prompt corrective actions. Additionally, blockchain technology is being explored for creating immutable records of quality testing results and certification data, enhancing transparency and traceability in the supply chain.
Compliance with these quality standards and certification requirements presents significant challenges, including the high cost of sophisticated analytical equipment, the need for specialized personnel training, and the complexity of navigating different regional regulatory frameworks. However, adherence to these standards is increasingly becoming a competitive necessity rather than an optional feature in the global saltpeter market.
International standards for saltpeter purity are primarily governed by ISO 9001:2015 for quality management systems and ISO 17025 for testing laboratory competence. These standards provide the foundation for consistent quality assessment and certification processes. Additionally, industry-specific standards such as ASTM E456 for chemical analysis of nitrate compounds and the European Standard EN 16195 for fertilizer quality have been adapted specifically for saltpeter processing.
Quality control testing protocols for high-purity saltpeter typically include quantitative analysis of nitrate content (minimum 99.5% for premium grade), moisture content (below 0.2%), and impurity profiling for heavy metals, chlorides, and sulfates. Advanced analytical techniques employed include ion chromatography, inductively coupled plasma mass spectrometry (ICP-MS), and X-ray diffraction analysis to ensure comprehensive purity verification.
Certification requirements vary by application sector. For pharmaceutical-grade saltpeter, compliance with Good Manufacturing Practice (GMP) guidelines and pharmacopeia standards (USP, EP, JP) is mandatory, requiring extensive documentation of production processes and quality testing. Food-grade applications must adhere to Food Chemical Codex (FCC) specifications and obtain certifications from relevant food safety authorities such as FDA or EFSA.
Agricultural applications of saltpeter are subject to fertilizer regulations that vary by region, with certification requirements focusing on nutrient content verification, heavy metal limits, and environmental impact assessments. The European Fertilizer Regulation (EU) 2019/1009 and the AAPFCO (Association of American Plant Food Control Officials) standards represent key regulatory frameworks in this sector.
Emerging trends in quality control standards include the implementation of real-time monitoring systems using IoT sensors for continuous quality assessment throughout the production process. This approach enables immediate detection of deviations from quality parameters and facilitates prompt corrective actions. Additionally, blockchain technology is being explored for creating immutable records of quality testing results and certification data, enhancing transparency and traceability in the supply chain.
Compliance with these quality standards and certification requirements presents significant challenges, including the high cost of sophisticated analytical equipment, the need for specialized personnel training, and the complexity of navigating different regional regulatory frameworks. However, adherence to these standards is increasingly becoming a competitive necessity rather than an optional feature in the global saltpeter market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!