Compare GC-MS vs NMR for Structure Elucidation
SEP 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Structure Elucidation Technology Background and Objectives
Structure elucidation, the process of determining the molecular structure of chemical compounds, has evolved significantly over the past century. Initially relying on chemical degradation methods and elemental analysis, the field underwent revolutionary changes with the introduction of spectroscopic techniques in the mid-20th century. Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR) spectroscopy emerged as cornerstone technologies that transformed structure elucidation from a time-consuming, material-intensive process to a more efficient and accurate analytical approach.
GC-MS technology, developed in the 1950s, combines the separation capabilities of gas chromatography with the identification power of mass spectrometry. This hybrid technique quickly gained prominence for its ability to analyze complex mixtures and provide molecular weight and fragmentation pattern information. The evolution of GC-MS has been marked by improvements in resolution, sensitivity, and data processing capabilities, making it an indispensable tool in various fields including pharmaceuticals, environmental monitoring, and forensic science.
NMR spectroscopy, first applied to structure elucidation in the 1960s, offers unique insights into molecular architecture by measuring the magnetic properties of certain atomic nuclei. The development of higher field strength magnets, pulse sequences, and multidimensional techniques has dramatically enhanced NMR's capabilities. Modern NMR can provide detailed information about molecular connectivity, spatial relationships, and dynamic behaviors that are crucial for complete structure determination.
The technological trajectory of both methods has been characterized by increasing automation, miniaturization, and integration with computational tools. Machine learning algorithms and spectral databases have significantly improved the speed and accuracy of structure elucidation, reducing the expertise barrier for utilizing these sophisticated techniques.
The primary objective in comparing GC-MS and NMR for structure elucidation is to establish a comprehensive understanding of their respective strengths, limitations, and complementary nature. This includes evaluating their analytical capabilities across different compound classes, sample requirements, data interpretation complexities, and cost-effectiveness. Additionally, this comparison aims to identify optimal application scenarios for each technique and explore how their combined use can provide more robust structural determinations.
Future technological goals include enhancing sensitivity for trace analysis, improving compatibility with diverse sample matrices, developing more intuitive data interpretation tools, and reducing instrument size and cost to expand accessibility. The ultimate aim is to establish protocols that maximize the synergistic potential of these techniques while minimizing their individual limitations in structure elucidation workflows.
GC-MS technology, developed in the 1950s, combines the separation capabilities of gas chromatography with the identification power of mass spectrometry. This hybrid technique quickly gained prominence for its ability to analyze complex mixtures and provide molecular weight and fragmentation pattern information. The evolution of GC-MS has been marked by improvements in resolution, sensitivity, and data processing capabilities, making it an indispensable tool in various fields including pharmaceuticals, environmental monitoring, and forensic science.
NMR spectroscopy, first applied to structure elucidation in the 1960s, offers unique insights into molecular architecture by measuring the magnetic properties of certain atomic nuclei. The development of higher field strength magnets, pulse sequences, and multidimensional techniques has dramatically enhanced NMR's capabilities. Modern NMR can provide detailed information about molecular connectivity, spatial relationships, and dynamic behaviors that are crucial for complete structure determination.
The technological trajectory of both methods has been characterized by increasing automation, miniaturization, and integration with computational tools. Machine learning algorithms and spectral databases have significantly improved the speed and accuracy of structure elucidation, reducing the expertise barrier for utilizing these sophisticated techniques.
The primary objective in comparing GC-MS and NMR for structure elucidation is to establish a comprehensive understanding of their respective strengths, limitations, and complementary nature. This includes evaluating their analytical capabilities across different compound classes, sample requirements, data interpretation complexities, and cost-effectiveness. Additionally, this comparison aims to identify optimal application scenarios for each technique and explore how their combined use can provide more robust structural determinations.
Future technological goals include enhancing sensitivity for trace analysis, improving compatibility with diverse sample matrices, developing more intuitive data interpretation tools, and reducing instrument size and cost to expand accessibility. The ultimate aim is to establish protocols that maximize the synergistic potential of these techniques while minimizing their individual limitations in structure elucidation workflows.
Market Applications and Demand Analysis for Analytical Techniques
The analytical instrumentation market has witnessed substantial growth in recent years, driven by increasing demand for accurate structure elucidation techniques across various industries. The global market for analytical instruments was valued at approximately $85 billion in 2022, with structure elucidation technologies comprising a significant segment of this market. Both GC-MS and NMR technologies hold substantial market shares within this domain, serving distinct yet overlapping application needs.
Pharmaceutical and biotechnology sectors represent the largest market segments for structure elucidation technologies, accounting for nearly 40% of the total market demand. These industries rely heavily on both GC-MS and NMR for drug discovery, development, and quality control processes. The increasing complexity of drug molecules and the growing emphasis on biologics have further accelerated the demand for advanced analytical techniques capable of providing comprehensive structural information.
The food and beverage industry constitutes another significant market for these analytical techniques, particularly for quality control, authenticity verification, and contaminant detection. GC-MS has traditionally dominated this sector due to its sensitivity in detecting volatile compounds and contaminants. However, NMR is gaining traction for its ability to provide comprehensive metabolomic profiles without sample destruction, especially valuable for high-value products like wine, olive oil, and honey authentication.
Environmental monitoring represents a rapidly growing application area, with increasing regulatory requirements driving demand for sensitive analytical methods. GC-MS maintains a strong position in this sector due to its excellent sensitivity for detecting trace environmental contaminants, while NMR finds specialized applications in studying environmental processes and transformations.
Academic and research institutions constitute approximately 25% of the market, utilizing both technologies for fundamental research across chemistry, biochemistry, materials science, and related disciplines. The demand in this sector is increasingly shifting toward more accessible, user-friendly systems that require less specialized expertise to operate.
Market trends indicate a growing preference for hybrid or complementary analytical approaches that combine the strengths of multiple techniques. This has led to the development of integrated analytical workflows incorporating both GC-MS and NMR data, supported by advanced chemometric software platforms. The market is also witnessing increased demand for portable and benchtop instruments, particularly in GC-MS, driven by needs for on-site analysis in environmental monitoring, food safety, and forensic applications.
Regionally, North America and Europe currently dominate the market for advanced structure elucidation technologies, accounting for approximately 60% of global demand. However, Asia-Pacific represents the fastest-growing market, with annual growth rates exceeding 8%, driven by expanding pharmaceutical manufacturing, increasing environmental regulations, and growing investment in research infrastructure across China, India, Japan, and South Korea.
Pharmaceutical and biotechnology sectors represent the largest market segments for structure elucidation technologies, accounting for nearly 40% of the total market demand. These industries rely heavily on both GC-MS and NMR for drug discovery, development, and quality control processes. The increasing complexity of drug molecules and the growing emphasis on biologics have further accelerated the demand for advanced analytical techniques capable of providing comprehensive structural information.
The food and beverage industry constitutes another significant market for these analytical techniques, particularly for quality control, authenticity verification, and contaminant detection. GC-MS has traditionally dominated this sector due to its sensitivity in detecting volatile compounds and contaminants. However, NMR is gaining traction for its ability to provide comprehensive metabolomic profiles without sample destruction, especially valuable for high-value products like wine, olive oil, and honey authentication.
Environmental monitoring represents a rapidly growing application area, with increasing regulatory requirements driving demand for sensitive analytical methods. GC-MS maintains a strong position in this sector due to its excellent sensitivity for detecting trace environmental contaminants, while NMR finds specialized applications in studying environmental processes and transformations.
Academic and research institutions constitute approximately 25% of the market, utilizing both technologies for fundamental research across chemistry, biochemistry, materials science, and related disciplines. The demand in this sector is increasingly shifting toward more accessible, user-friendly systems that require less specialized expertise to operate.
Market trends indicate a growing preference for hybrid or complementary analytical approaches that combine the strengths of multiple techniques. This has led to the development of integrated analytical workflows incorporating both GC-MS and NMR data, supported by advanced chemometric software platforms. The market is also witnessing increased demand for portable and benchtop instruments, particularly in GC-MS, driven by needs for on-site analysis in environmental monitoring, food safety, and forensic applications.
Regionally, North America and Europe currently dominate the market for advanced structure elucidation technologies, accounting for approximately 60% of global demand. However, Asia-Pacific represents the fastest-growing market, with annual growth rates exceeding 8%, driven by expanding pharmaceutical manufacturing, increasing environmental regulations, and growing investment in research infrastructure across China, India, Japan, and South Korea.
Current Status and Challenges of GC-MS and NMR Technologies
Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR) represent two cornerstone analytical techniques in modern structure elucidation. Currently, GC-MS technology has achieved remarkable sensitivity, with detection limits reaching parts per trillion levels in optimal conditions. The technique excels in analyzing volatile and semi-volatile compounds, particularly in complex mixtures, and has become increasingly accessible with benchtop instruments costing between $50,000-150,000, making it a standard tool in many analytical laboratories.
Recent advances in GC-MS include the development of comprehensive two-dimensional gas chromatography (GC×GC), which has dramatically improved separation capabilities for complex mixtures. Additionally, high-resolution time-of-flight mass spectrometers have enhanced the accuracy of molecular formula determination, addressing previous limitations in distinguishing compounds with similar mass spectra.
NMR technology has similarly progressed significantly, with superconducting magnets now routinely operating at field strengths of 800-1000 MHz for proton resonance. Modern NMR spectrometers incorporate cryoprobe technology, enhancing sensitivity by cooling the detection coils to near absolute zero temperatures, resulting in signal-to-noise improvements of 3-4 fold compared to conventional probes.
Despite these advancements, both technologies face substantial challenges. GC-MS remains limited by the requirement for compound volatility, making it unsuitable for analyzing large biomolecules, polymers, and thermally labile compounds. Sample derivatization can partially address this issue but introduces additional complexity and potential artifacts. Furthermore, structural isomers often produce similar fragmentation patterns, complicating definitive identification.
NMR confronts sensitivity limitations, typically requiring microgram to milligram quantities of sample—orders of magnitude more than GC-MS. This presents significant obstacles when analyzing trace components or working with limited sample quantities. Additionally, the high cost of advanced NMR instrumentation (ranging from $500,000 to several million dollars) restricts access for many research institutions and companies, particularly in developing regions.
The geographical distribution of these technologies reveals interesting patterns. High-end NMR facilities are predominantly concentrated in North America, Western Europe, and East Asia, while GC-MS has achieved broader global penetration due to its lower cost and versatility. This disparity creates challenges for collaborative research and technology transfer in regions with limited access to advanced NMR capabilities.
Integration challenges persist between these complementary techniques. While combining data from both methods provides comprehensive structural information, standardized workflows and data integration platforms remain underdeveloped, often requiring specialized expertise to effectively interpret multi-technique analytical results.
Recent advances in GC-MS include the development of comprehensive two-dimensional gas chromatography (GC×GC), which has dramatically improved separation capabilities for complex mixtures. Additionally, high-resolution time-of-flight mass spectrometers have enhanced the accuracy of molecular formula determination, addressing previous limitations in distinguishing compounds with similar mass spectra.
NMR technology has similarly progressed significantly, with superconducting magnets now routinely operating at field strengths of 800-1000 MHz for proton resonance. Modern NMR spectrometers incorporate cryoprobe technology, enhancing sensitivity by cooling the detection coils to near absolute zero temperatures, resulting in signal-to-noise improvements of 3-4 fold compared to conventional probes.
Despite these advancements, both technologies face substantial challenges. GC-MS remains limited by the requirement for compound volatility, making it unsuitable for analyzing large biomolecules, polymers, and thermally labile compounds. Sample derivatization can partially address this issue but introduces additional complexity and potential artifacts. Furthermore, structural isomers often produce similar fragmentation patterns, complicating definitive identification.
NMR confronts sensitivity limitations, typically requiring microgram to milligram quantities of sample—orders of magnitude more than GC-MS. This presents significant obstacles when analyzing trace components or working with limited sample quantities. Additionally, the high cost of advanced NMR instrumentation (ranging from $500,000 to several million dollars) restricts access for many research institutions and companies, particularly in developing regions.
The geographical distribution of these technologies reveals interesting patterns. High-end NMR facilities are predominantly concentrated in North America, Western Europe, and East Asia, while GC-MS has achieved broader global penetration due to its lower cost and versatility. This disparity creates challenges for collaborative research and technology transfer in regions with limited access to advanced NMR capabilities.
Integration challenges persist between these complementary techniques. While combining data from both methods provides comprehensive structural information, standardized workflows and data integration platforms remain underdeveloped, often requiring specialized expertise to effectively interpret multi-technique analytical results.
Comparative Analysis of GC-MS and NMR Methodologies
01 Combined GC-MS and NMR techniques for comprehensive structure elucidation
The integration of Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR) spectroscopy provides complementary analytical data for comprehensive structure elucidation of organic compounds. GC-MS offers information about molecular weight and fragmentation patterns, while NMR provides detailed structural information about atomic connectivity and spatial arrangement. This combined approach enhances the accuracy and reliability of structure determination, particularly for complex organic molecules and natural products.- Combined GC-MS and NMR techniques for structural analysis: The integration of Gas Chromatography-Mass Spectrometry (GC-MS) and Nuclear Magnetic Resonance (NMR) spectroscopy provides complementary data for comprehensive structure elucidation. GC-MS offers molecular weight and fragmentation pattern information, while NMR provides detailed structural connectivity and spatial arrangement data. This combined approach enhances the accuracy of compound identification and structural determination, particularly for complex organic molecules and natural products.
- Advanced data processing algorithms for spectral interpretation: Sophisticated algorithms and computational methods have been developed to automate and enhance the interpretation of GC-MS and NMR spectral data. These algorithms can identify patterns in complex spectra, compare results against databases, and suggest structural possibilities with statistical confidence levels. Machine learning approaches are increasingly being applied to improve the accuracy of structure elucidation, reducing the time and expertise required for manual interpretation.
- Sample preparation techniques for improved analysis: Specialized sample preparation methods enhance the quality of GC-MS and NMR data for structure elucidation. These include derivatization techniques for GC-MS to improve volatility and separation of compounds, and various extraction and purification protocols to minimize interference from matrix components. For NMR, deuterated solvents selection and concentration optimization are critical for obtaining high-resolution spectra necessary for accurate structure determination.
- Integrated analytical systems and workflows: Integrated systems that combine GC-MS and NMR capabilities within unified workflows improve efficiency in structure elucidation. These systems often include automated sample handling, data acquisition, and processing components that streamline the analytical process. Standardized workflows ensure consistency in results and facilitate the correlation of complementary data from multiple analytical techniques, leading to more reliable structural assignments.
- Application-specific structure elucidation methods: Specialized structure elucidation approaches have been developed for specific applications, such as metabolomics, natural product discovery, and pharmaceutical analysis. These methods often involve customized GC-MS and NMR parameters, targeted databases, and application-specific interpretation strategies. For example, in metabolomics, rapid screening methods combine with detailed structural analysis to identify and characterize metabolites in complex biological samples.
02 Advanced data processing algorithms for spectral analysis
Sophisticated algorithms and computational methods have been developed to process and interpret the complex data generated by GC-MS and NMR analyses. These algorithms facilitate automated peak identification, spectral deconvolution, and structural assignment, significantly reducing the time and expertise required for structure elucidation. Machine learning and artificial intelligence approaches are increasingly being applied to improve the accuracy of structural predictions based on spectroscopic data.Expand Specific Solutions03 Specialized instrumentation for enhanced structural analysis
Innovative instrumentation designs have been developed to enhance the capabilities of GC-MS and NMR for structure elucidation. These include hyphenated techniques such as GC-MS-MS, high-resolution mass spectrometry, and multi-dimensional NMR spectroscopy. Advanced probe designs, higher magnetic field strengths, and improved detector sensitivity contribute to better spectral resolution and more detailed structural information, enabling the elucidation of increasingly complex molecular structures.Expand Specific Solutions04 Sample preparation techniques for improved spectral quality
Effective sample preparation methods are crucial for obtaining high-quality GC-MS and NMR data for structure elucidation. These techniques include various extraction procedures, derivatization methods for GC-MS, and deuteration protocols for NMR. Proper sample preparation enhances signal-to-noise ratios, reduces spectral interference, and improves the overall quality of analytical data, leading to more accurate structure determinations.Expand Specific Solutions05 Application-specific protocols for different compound classes
Specialized protocols have been developed for the structure elucidation of specific classes of compounds using GC-MS and NMR. These protocols are tailored to address the unique challenges presented by different molecular types, such as stereoisomers, natural products, metabolites, and synthetic compounds. They incorporate optimized instrument parameters, specific pulse sequences for NMR, and targeted fragmentation techniques for MS, ensuring more accurate and efficient structure determination for particular compound classes.Expand Specific Solutions
Major Instrument Manufacturers and Research Institutions
The structure elucidation landscape using GC-MS versus NMR is currently in a mature market phase, with an estimated global analytical instrumentation market exceeding $5 billion. NMR technology offers superior structural information but requires higher investment, while GC-MS provides cost-effective molecular identification with widespread accessibility. Key industry players demonstrate distinct technological focuses: Bruker, JEOL, and Agilent lead in high-field NMR innovation; Waters, Shimadzu, and Agilent dominate the GC-MS sector with enhanced sensitivity and resolution capabilities. Academic institutions like EPFL and IIT Bombay collaborate with pharmaceutical companies (Momenta, Boehringer Ingelheim) to advance hybrid analytical approaches, indicating a trend toward complementary rather than competitive technology utilization for complex structural challenges.
JEOL Ltd.
Technical Solution: JEOL has pioneered advanced NMR technologies for structure elucidation with their JNM-ECZ series spectrometers operating at field strengths up to 930 MHz, providing exceptional resolution for complex molecular structures[2]. Their Delta software platform integrates sophisticated pulse sequence programming with automated structure verification tools that can process multiple 2D experiments simultaneously. JEOL's proprietary ROYAL probe technology achieves superior sensitivity through cryogenically cooled components, reducing experiment times by up to 75% compared to conventional probes[4]. Their systems feature automated tuning and matching capabilities that optimize performance for different nuclei without manual intervention. JEOL has also developed specialized solid-state NMR solutions with ultra-fast magic angle spinning (MAS) exceeding 100 kHz for analyzing insoluble compounds and materials. Their latest innovations include non-uniform sampling (NUS) techniques that dramatically reduce acquisition times for multidimensional experiments while maintaining spectral quality, and integrated quantum computing algorithms that enhance signal processing for complex mixtures[5].
Strengths: Direct observation of atomic connectivity through coupling patterns; non-destructive analysis preserving sample integrity; exceptional structural detail including stereochemistry; quantitative analysis without calibration standards. Weaknesses: Lower sensitivity compared to mass spectrometry techniques; requires relatively large sample amounts (typically micrograms to milligrams); expensive instrumentation and maintenance; complex data interpretation requiring specialized expertise.
Bruker Switzerland AG
Technical Solution: Bruker has developed cutting-edge NMR technology with their AVANCE NEO platform, featuring multi-receive architecture that enables parallel acquisition from multiple samples, increasing throughput by up to 400%[1]. Their CryoProbe technology reduces thermal noise by cooling detection coils to near absolute zero temperatures, enhancing sensitivity by factors of 4-5 for 1H and up to 10 for 13C compared to conventional probes[3]. Bruker's TopSpin software incorporates advanced pulse sequence programming with automated structure verification tools and machine learning algorithms that can predict chemical shifts based on proposed structures. Their systems feature automated sample changers handling up to 600 samples for high-throughput screening applications. Bruker has pioneered hyphenated techniques like LC-NMR-MS that combine the separation power of chromatography with the structural elucidation capabilities of both NMR and MS in a single analytical platform. Their latest innovations include compact benchtop NMR systems (80 MHz) with permanent magnets that require no cryogens, making NMR technology more accessible for routine structure confirmation applications[5].
Strengths: Unparalleled structural information including atom connectivity, stereochemistry, and conformational details; non-destructive analysis allowing sample recovery; excellent reproducibility and quantitative capabilities without standards; minimal sample preparation requirements. Weaknesses: Lower sensitivity compared to MS techniques requiring larger sample amounts; higher initial investment and maintenance costs; longer analysis times for complex experiments; limited applicability for very large molecules due to spectral complexity.
Key Technical Principles and Innovations in Spectroscopic Analysis
Metabolomics analysis of renal cell carcinoma
PatentWO2017165956A1
Innovation
- The use of metabolomics analysis involving nuclear magnetic resonance (NMR) spectroscopy and gas chromatography-mass spectrometry (GCMS) to measure specific metabolites in serum and urine samples, combined with multivariate statistical analysis, to establish metabolic signatures that differentiate RCC from benign conditions and assess disease severity.
Phospholipid containing garlic, curry leaves and turmeric extracts for treatment of adipogenesis
PatentPendingIN202141048482A
Innovation
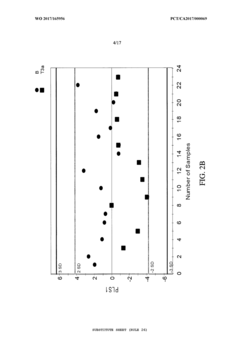
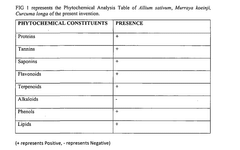
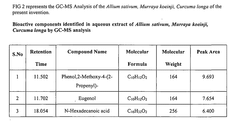
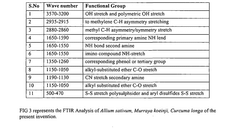
- A synergistic extract derived from Allium sativum, Murraya koenji, and Curcuma longa, combined with phospholipid as a Phytosome complex, is developed for enhanced bioavailability and therapeutic potential, involving a method of extraction, purification, and characterization using GC-MS, FTIR, and SEM, demonstrating the presence of bioactive compounds and antioxidant activity.
Cost-Benefit Analysis of GC-MS vs NMR Implementation
Implementing GC-MS or NMR technologies for structure elucidation requires significant financial investment and operational considerations. The initial acquisition costs for GC-MS systems typically range from $50,000 to $150,000, while NMR spectrometers represent substantially higher capital expenditure, ranging from $300,000 for basic models to over $1.5 million for high-field instruments. This considerable difference in initial investment must be carefully weighed against long-term benefits.
Operational expenses also differ markedly between these technologies. GC-MS systems consume relatively inexpensive carrier gases and require moderate maintenance, with annual operational costs typically between $10,000-$25,000. Conversely, NMR systems demand costly cryogens (liquid helium and nitrogen) for superconducting magnets, with annual operational expenses potentially exceeding $50,000 for high-field instruments, though newer shielded magnets have reduced these costs somewhat.
Personnel requirements represent another significant cost factor. GC-MS operation can be mastered by technicians with moderate training, whereas NMR typically requires specialists with advanced degrees and extensive experience, commanding higher salaries. This expertise gap translates to higher ongoing personnel costs for NMR facilities.
Sample throughput considerations reveal that GC-MS offers advantages for high-volume routine analyses, processing 20-30 samples daily with minimal preparation. NMR provides lower throughput but delivers comprehensive structural information in a single experiment, potentially reducing the need for multiple analytical techniques. This efficiency in information gathering may offset lower sample throughput in certain research contexts.
Return on investment calculations indicate that GC-MS systems typically achieve ROI within 2-3 years in high-throughput environments, while NMR systems require 5-7 years to reach ROI, primarily justified through their versatility and comprehensive analytical capabilities. Organizations must consider their specific analytical needs, sample volumes, and available expertise when making implementation decisions.
Facility requirements add another dimension to cost considerations. GC-MS systems occupy minimal laboratory space with standard ventilation requirements, while NMR instruments demand specialized facilities with reinforced floors, electromagnetic shielding, and often dedicated rooms, adding substantial infrastructure costs that may not be immediately apparent in equipment pricing.
Operational expenses also differ markedly between these technologies. GC-MS systems consume relatively inexpensive carrier gases and require moderate maintenance, with annual operational costs typically between $10,000-$25,000. Conversely, NMR systems demand costly cryogens (liquid helium and nitrogen) for superconducting magnets, with annual operational expenses potentially exceeding $50,000 for high-field instruments, though newer shielded magnets have reduced these costs somewhat.
Personnel requirements represent another significant cost factor. GC-MS operation can be mastered by technicians with moderate training, whereas NMR typically requires specialists with advanced degrees and extensive experience, commanding higher salaries. This expertise gap translates to higher ongoing personnel costs for NMR facilities.
Sample throughput considerations reveal that GC-MS offers advantages for high-volume routine analyses, processing 20-30 samples daily with minimal preparation. NMR provides lower throughput but delivers comprehensive structural information in a single experiment, potentially reducing the need for multiple analytical techniques. This efficiency in information gathering may offset lower sample throughput in certain research contexts.
Return on investment calculations indicate that GC-MS systems typically achieve ROI within 2-3 years in high-throughput environments, while NMR systems require 5-7 years to reach ROI, primarily justified through their versatility and comprehensive analytical capabilities. Organizations must consider their specific analytical needs, sample volumes, and available expertise when making implementation decisions.
Facility requirements add another dimension to cost considerations. GC-MS systems occupy minimal laboratory space with standard ventilation requirements, while NMR instruments demand specialized facilities with reinforced floors, electromagnetic shielding, and often dedicated rooms, adding substantial infrastructure costs that may not be immediately apparent in equipment pricing.
Sample Preparation Requirements and Limitations
Sample preparation represents a critical determinant in the success of both GC-MS and NMR analyses for structure elucidation, with each technique imposing distinct requirements and limitations that significantly impact analytical outcomes.
GC-MS sample preparation demands rigorous processing to ensure volatility and thermal stability. Samples must typically undergo derivatization procedures, such as silylation, acylation, or alkylation, to enhance volatility of polar compounds containing hydroxyl, carboxyl, or amino groups. This chemical modification step adds complexity and potential for introduction of artifacts. Additionally, GC-MS requires samples to be completely soluble in volatile organic solvents, with water-based samples necessitating liquid-liquid extraction or solid-phase extraction prior to analysis.
Temperature sensitivity presents another significant limitation for GC-MS, as thermally labile compounds may decompose during the high-temperature separation process, potentially leading to misidentification or incomplete structural characterization. Sample concentration requirements typically fall in the microgram to nanogram range, demanding precise quantification techniques.
In contrast, NMR sample preparation follows a more straightforward protocol with fewer manipulation steps. Samples generally require dissolution in deuterated solvents to minimize interfering proton signals, with common choices including chloroform-d, dimethyl sulfoxide-d6, or deuterium oxide depending on compound polarity. This simplicity reduces the risk of sample alteration during preparation.
NMR exhibits greater flexibility regarding sample state, accommodating analysis of liquids, semi-solids, and even solids with specialized techniques. However, NMR's primary limitation lies in its sensitivity constraints, typically requiring milligram quantities of sample—approximately 1000-fold more material than GC-MS. This requirement can prove prohibitive when analyzing trace components or working with limited sample availability.
Sample homogeneity represents another critical consideration, particularly for NMR, where magnetic field inhomogeneities caused by particulates or concentration gradients can severely compromise spectral resolution. GC-MS demonstrates greater tolerance for minor sample impurities, as chromatographic separation often resolves interfering components prior to mass analysis.
Recovery of intact samples presents a distinct advantage for NMR, as the non-destructive nature of the technique allows for sample retrieval post-analysis. Conversely, GC-MS inherently destroys the sample during the ionization process, precluding additional analyses on the same material.
GC-MS sample preparation demands rigorous processing to ensure volatility and thermal stability. Samples must typically undergo derivatization procedures, such as silylation, acylation, or alkylation, to enhance volatility of polar compounds containing hydroxyl, carboxyl, or amino groups. This chemical modification step adds complexity and potential for introduction of artifacts. Additionally, GC-MS requires samples to be completely soluble in volatile organic solvents, with water-based samples necessitating liquid-liquid extraction or solid-phase extraction prior to analysis.
Temperature sensitivity presents another significant limitation for GC-MS, as thermally labile compounds may decompose during the high-temperature separation process, potentially leading to misidentification or incomplete structural characterization. Sample concentration requirements typically fall in the microgram to nanogram range, demanding precise quantification techniques.
In contrast, NMR sample preparation follows a more straightforward protocol with fewer manipulation steps. Samples generally require dissolution in deuterated solvents to minimize interfering proton signals, with common choices including chloroform-d, dimethyl sulfoxide-d6, or deuterium oxide depending on compound polarity. This simplicity reduces the risk of sample alteration during preparation.
NMR exhibits greater flexibility regarding sample state, accommodating analysis of liquids, semi-solids, and even solids with specialized techniques. However, NMR's primary limitation lies in its sensitivity constraints, typically requiring milligram quantities of sample—approximately 1000-fold more material than GC-MS. This requirement can prove prohibitive when analyzing trace components or working with limited sample availability.
Sample homogeneity represents another critical consideration, particularly for NMR, where magnetic field inhomogeneities caused by particulates or concentration gradients can severely compromise spectral resolution. GC-MS demonstrates greater tolerance for minor sample impurities, as chromatographic separation often resolves interfering components prior to mass analysis.
Recovery of intact samples presents a distinct advantage for NMR, as the non-destructive nature of the technique allows for sample retrieval post-analysis. Conversely, GC-MS inherently destroys the sample during the ionization process, precluding additional analyses on the same material.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!