Comparing Lithium Acetate to Lithium Nitrate in Synthesis Processes
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Salt Evolution and Research Objectives
Lithium salts have undergone significant evolution in chemical synthesis processes over the past several decades. Initially, lithium compounds were primarily utilized in specialized applications such as ceramics and glass manufacturing. However, the landscape transformed dramatically in the 1970s when lithium compounds began gaining prominence in pharmaceutical synthesis and organic chemistry as powerful reagents for carbon-carbon bond formation.
The transition from simple lithium salts like lithium chloride to more specialized forms such as lithium acetate and lithium nitrate represents a critical advancement in synthesis methodology. Lithium acetate emerged as a valuable reagent in the 1980s, particularly in biochemical applications and as a transformation agent in genetic engineering protocols. Its mild properties and compatibility with biological systems positioned it as a preferred choice in life science research.
Lithium nitrate, conversely, developed along a different trajectory, finding early applications in pyrotechnics before researchers discovered its catalytic properties in organic synthesis during the 1990s. Its strong oxidizing characteristics and unique coordination chemistry opened new pathways for chemical transformations previously considered challenging or impossible.
The comparative study of lithium acetate and lithium nitrate in synthesis processes addresses several fundamental questions in modern chemical manufacturing. These include reaction efficiency, selectivity in complex molecular environments, environmental impact, and economic viability at industrial scale. Understanding these differences has become increasingly important as industries seek more sustainable and efficient chemical processes.
Current research objectives focus on elucidating the mechanistic differences between these two lithium salts in various reaction environments. Particular emphasis is placed on their behavior in aqueous versus non-aqueous media, their interaction with different functional groups, and their performance under varying temperature and pressure conditions. These investigations aim to develop predictive models for optimal salt selection based on specific synthesis requirements.
Additionally, research goals include exploring novel applications for these lithium salts in emerging fields such as green chemistry, continuous flow synthesis, and materials science. The potential for lithium acetate in biodegradable polymer synthesis and lithium nitrate in energy storage materials represents promising avenues for future development.
The technological trajectory suggests a growing convergence of computational chemistry with experimental approaches to optimize lithium salt utilization. Machine learning algorithms are increasingly being employed to predict reaction outcomes and suggest optimal conditions, potentially revolutionizing how chemists select between lithium acetate and lithium nitrate for specific applications.
The transition from simple lithium salts like lithium chloride to more specialized forms such as lithium acetate and lithium nitrate represents a critical advancement in synthesis methodology. Lithium acetate emerged as a valuable reagent in the 1980s, particularly in biochemical applications and as a transformation agent in genetic engineering protocols. Its mild properties and compatibility with biological systems positioned it as a preferred choice in life science research.
Lithium nitrate, conversely, developed along a different trajectory, finding early applications in pyrotechnics before researchers discovered its catalytic properties in organic synthesis during the 1990s. Its strong oxidizing characteristics and unique coordination chemistry opened new pathways for chemical transformations previously considered challenging or impossible.
The comparative study of lithium acetate and lithium nitrate in synthesis processes addresses several fundamental questions in modern chemical manufacturing. These include reaction efficiency, selectivity in complex molecular environments, environmental impact, and economic viability at industrial scale. Understanding these differences has become increasingly important as industries seek more sustainable and efficient chemical processes.
Current research objectives focus on elucidating the mechanistic differences between these two lithium salts in various reaction environments. Particular emphasis is placed on their behavior in aqueous versus non-aqueous media, their interaction with different functional groups, and their performance under varying temperature and pressure conditions. These investigations aim to develop predictive models for optimal salt selection based on specific synthesis requirements.
Additionally, research goals include exploring novel applications for these lithium salts in emerging fields such as green chemistry, continuous flow synthesis, and materials science. The potential for lithium acetate in biodegradable polymer synthesis and lithium nitrate in energy storage materials represents promising avenues for future development.
The technological trajectory suggests a growing convergence of computational chemistry with experimental approaches to optimize lithium salt utilization. Machine learning algorithms are increasingly being employed to predict reaction outcomes and suggest optimal conditions, potentially revolutionizing how chemists select between lithium acetate and lithium nitrate for specific applications.
Market Applications and Demand Analysis
The global market for lithium compounds has experienced significant growth in recent years, driven primarily by the expanding electric vehicle (EV) industry and renewable energy storage systems. Within this broader context, lithium acetate and lithium nitrate serve distinct market applications with varying demand profiles.
Lithium nitrate has established a strong market presence in thermal energy storage systems, particularly in concentrated solar power (CSP) plants. Its excellent thermal properties make it a preferred component in molten salt mixtures used for heat transfer and storage. The global CSP market is projected to grow substantially over the next decade, creating sustained demand for lithium nitrate. Additionally, lithium nitrate finds application in the ceramics industry as a flux agent and in specialized concrete formulations as a setting accelerator.
Lithium acetate, conversely, dominates in specific biochemical and pharmaceutical applications. It serves as a critical buffer in molecular biology procedures, particularly in DNA transformation protocols. The pharmaceutical sector utilizes lithium acetate in certain drug formulations, while the textile industry employs it as a mordant in dyeing processes. The growing biotechnology sector represents a significant driver for lithium acetate demand.
Market analysis reveals interesting regional variations in demand patterns. North America and Europe show stronger demand for lithium acetate due to their robust pharmaceutical and biotechnology sectors. Meanwhile, China and the Middle East demonstrate greater consumption of lithium nitrate, aligned with their expanding investments in solar thermal energy projects.
Price sensitivity differs markedly between these compounds. Lithium nitrate commands premium pricing in high-purity grades required for energy storage applications, with relatively inelastic demand due to the lack of cost-effective alternatives. Lithium acetate pricing tends to be more elastic, particularly in industrial applications where alternative acetate compounds might serve as substitutes.
Supply chain considerations also influence market dynamics. Lithium nitrate production involves nitric acid, which faces stricter handling regulations compared to acetic acid used in lithium acetate synthesis. This regulatory difference impacts production costs and geographical distribution of manufacturing facilities.
Future market projections indicate diverging growth trajectories. Lithium nitrate demand is expected to accelerate with the expansion of renewable energy infrastructure, particularly in regions with high solar potential. Lithium acetate market growth appears more closely tied to advancements in biotechnology research and pharmaceutical development, suggesting more modest but stable growth patterns.
Lithium nitrate has established a strong market presence in thermal energy storage systems, particularly in concentrated solar power (CSP) plants. Its excellent thermal properties make it a preferred component in molten salt mixtures used for heat transfer and storage. The global CSP market is projected to grow substantially over the next decade, creating sustained demand for lithium nitrate. Additionally, lithium nitrate finds application in the ceramics industry as a flux agent and in specialized concrete formulations as a setting accelerator.
Lithium acetate, conversely, dominates in specific biochemical and pharmaceutical applications. It serves as a critical buffer in molecular biology procedures, particularly in DNA transformation protocols. The pharmaceutical sector utilizes lithium acetate in certain drug formulations, while the textile industry employs it as a mordant in dyeing processes. The growing biotechnology sector represents a significant driver for lithium acetate demand.
Market analysis reveals interesting regional variations in demand patterns. North America and Europe show stronger demand for lithium acetate due to their robust pharmaceutical and biotechnology sectors. Meanwhile, China and the Middle East demonstrate greater consumption of lithium nitrate, aligned with their expanding investments in solar thermal energy projects.
Price sensitivity differs markedly between these compounds. Lithium nitrate commands premium pricing in high-purity grades required for energy storage applications, with relatively inelastic demand due to the lack of cost-effective alternatives. Lithium acetate pricing tends to be more elastic, particularly in industrial applications where alternative acetate compounds might serve as substitutes.
Supply chain considerations also influence market dynamics. Lithium nitrate production involves nitric acid, which faces stricter handling regulations compared to acetic acid used in lithium acetate synthesis. This regulatory difference impacts production costs and geographical distribution of manufacturing facilities.
Future market projections indicate diverging growth trajectories. Lithium nitrate demand is expected to accelerate with the expansion of renewable energy infrastructure, particularly in regions with high solar potential. Lithium acetate market growth appears more closely tied to advancements in biotechnology research and pharmaceutical development, suggesting more modest but stable growth patterns.
Current Challenges in Lithium Salt Synthesis
The synthesis of lithium salts presents several significant challenges that impact both research and industrial applications. Current manufacturing processes for lithium acetate and lithium nitrate face efficiency barriers, with yields typically ranging from 65-85% depending on the specific synthesis route and conditions employed. These suboptimal yields translate directly to increased production costs and resource wastage, creating economic pressure on manufacturers.
Temperature sensitivity represents another major challenge, particularly for lithium acetate synthesis which requires precise thermal control between 60-80°C. Exceeding these parameters can lead to decomposition or unwanted side reactions, while insufficient heat results in incomplete conversion. Lithium nitrate synthesis exhibits better thermal stability but still requires careful monitoring during production phases.
Purity considerations remain paramount, with electronic and pharmaceutical applications demanding 99.9%+ purity levels that are difficult to achieve consistently. Current purification methods often involve multiple recrystallization steps or ion-exchange processes that further reduce overall yield and increase production costs. Trace metal contamination, particularly from sodium, potassium, and calcium, presents persistent challenges in maintaining high-purity standards.
Water sensitivity affects both compounds differently, with lithium acetate being hygroscopic and forming stable hydrates that complicate handling and storage. Lithium nitrate, while less hygroscopic, still requires moisture-controlled environments during synthesis and storage to maintain product integrity and prevent degradation over time.
Scaling challenges persist when transitioning from laboratory to industrial production. Reaction kinetics, heat transfer efficiency, and mixing parameters that work effectively at small scales often perform unpredictably at larger volumes. This scale-up challenge particularly affects lithium acetate synthesis, which demonstrates more complex reaction profiles compared to lithium nitrate production.
Environmental concerns have gained increasing prominence, with traditional synthesis methods generating significant waste streams. The lithium nitrate production process typically generates nitrogen oxide emissions requiring abatement systems, while lithium acetate synthesis often involves organic solvents with associated disposal challenges. Regulatory pressures continue to push manufacturers toward greener synthesis routes that remain commercially viable.
Raw material availability and cost fluctuations introduce additional complexity, with lithium carbonate prices having increased by over 400% between 2020 and 2022. This market volatility directly impacts production economics and has accelerated research into alternative synthesis pathways and precursors for both lithium acetate and lithium nitrate.
Temperature sensitivity represents another major challenge, particularly for lithium acetate synthesis which requires precise thermal control between 60-80°C. Exceeding these parameters can lead to decomposition or unwanted side reactions, while insufficient heat results in incomplete conversion. Lithium nitrate synthesis exhibits better thermal stability but still requires careful monitoring during production phases.
Purity considerations remain paramount, with electronic and pharmaceutical applications demanding 99.9%+ purity levels that are difficult to achieve consistently. Current purification methods often involve multiple recrystallization steps or ion-exchange processes that further reduce overall yield and increase production costs. Trace metal contamination, particularly from sodium, potassium, and calcium, presents persistent challenges in maintaining high-purity standards.
Water sensitivity affects both compounds differently, with lithium acetate being hygroscopic and forming stable hydrates that complicate handling and storage. Lithium nitrate, while less hygroscopic, still requires moisture-controlled environments during synthesis and storage to maintain product integrity and prevent degradation over time.
Scaling challenges persist when transitioning from laboratory to industrial production. Reaction kinetics, heat transfer efficiency, and mixing parameters that work effectively at small scales often perform unpredictably at larger volumes. This scale-up challenge particularly affects lithium acetate synthesis, which demonstrates more complex reaction profiles compared to lithium nitrate production.
Environmental concerns have gained increasing prominence, with traditional synthesis methods generating significant waste streams. The lithium nitrate production process typically generates nitrogen oxide emissions requiring abatement systems, while lithium acetate synthesis often involves organic solvents with associated disposal challenges. Regulatory pressures continue to push manufacturers toward greener synthesis routes that remain commercially viable.
Raw material availability and cost fluctuations introduce additional complexity, with lithium carbonate prices having increased by over 400% between 2020 and 2022. This market volatility directly impacts production economics and has accelerated research into alternative synthesis pathways and precursors for both lithium acetate and lithium nitrate.
Comparative Analysis of Synthesis Methodologies
01 Lithium salts in battery electrolytes
Lithium acetate and lithium nitrate are used as additives in battery electrolytes to improve performance and safety. These compounds can enhance the formation of solid electrolyte interphase (SEI) layers, prevent dendrite growth, and improve the cycling stability of lithium-ion batteries. The combination of these salts with other electrolyte components can significantly enhance battery performance and longevity.- Lithium salts in battery electrolytes: Lithium acetate and lithium nitrate are used as additives in battery electrolytes to enhance performance and safety. These compounds can form protective films on electrode surfaces, prevent dendrite formation, and improve the cycling stability of lithium-ion batteries. The combination of these salts with other electrolyte components can significantly enhance battery life and reduce safety risks associated with thermal runaway.
- Thermal energy storage applications: Lithium acetate and lithium nitrate are utilized in phase change materials for thermal energy storage systems. These compounds exhibit favorable melting points, high latent heat of fusion, and good thermal conductivity. When incorporated into thermal storage materials, they can efficiently store and release heat energy, making them valuable for solar thermal applications, building temperature regulation, and industrial heat management systems.
- Corrosion inhibition properties: These lithium salts demonstrate effective corrosion inhibition properties when used in various industrial applications. Lithium acetate and lithium nitrate can form protective layers on metal surfaces, preventing oxidation and degradation. They are particularly effective in high-temperature environments and can be incorporated into coatings, lubricants, and treatment solutions to extend the service life of metal components.
- Catalytic applications: Lithium acetate and lithium nitrate serve as catalysts or catalyst precursors in various chemical reactions. These compounds can facilitate organic synthesis reactions, polymerization processes, and the production of specialty chemicals. Their catalytic properties are attributed to the lithium ion's ability to coordinate with reactants and lower activation energy barriers, enabling more efficient chemical transformations.
- Concrete and cement additives: These lithium compounds are used as additives in concrete and cement formulations to improve performance characteristics. Lithium acetate and lithium nitrate can accelerate setting times, enhance strength development, and mitigate alkali-silica reactions in concrete. They also contribute to improved freeze-thaw resistance and reduced efflorescence, making them valuable in construction applications requiring durable and high-performance concrete materials.
02 Thermal energy storage applications
Lithium acetate and lithium nitrate are utilized in thermal energy storage systems due to their favorable phase change properties. These compounds, either individually or in mixtures, can store and release thermal energy efficiently during phase transitions. They are incorporated into phase change materials (PCMs) for applications in solar energy storage, building temperature regulation, and waste heat recovery systems.Expand Specific Solutions03 Corrosion inhibition properties
Lithium acetate and lithium nitrate exhibit corrosion inhibition properties when used in various industrial applications. These compounds can form protective layers on metal surfaces, preventing oxidation and degradation. They are particularly effective in high-temperature environments and can be incorporated into coatings, lubricants, and other protective formulations to extend the lifespan of metal components.Expand Specific Solutions04 Catalytic applications
Lithium acetate and lithium nitrate serve as catalysts or catalyst precursors in various chemical reactions. These lithium salts can facilitate organic synthesis reactions, polymerization processes, and other chemical transformations. Their catalytic properties are attributed to the Lewis acidity of lithium ions and the specific interactions with reaction substrates, making them valuable in industrial chemical processes and research applications.Expand Specific Solutions05 Concrete and cement additives
Lithium acetate and lithium nitrate are used as additives in concrete and cement formulations to modify setting properties and enhance performance. These compounds can accelerate hardening, improve freeze-thaw resistance, and mitigate alkali-silica reactions in concrete. The incorporation of these lithium salts in appropriate concentrations can significantly improve the durability and longevity of concrete structures in various environmental conditions.Expand Specific Solutions
Key Industry Players and Manufacturers
The lithium compound synthesis market is in a growth phase, with increasing demand driven by battery technology advancements. The competition between lithium acetate and lithium nitrate synthesis processes reflects a maturing technical landscape with an estimated global market value exceeding $2 billion. Leading research institutions like Central South University and Council of Scientific & Industrial Research are advancing fundamental science, while commercial players demonstrate varying levels of technical maturity. Companies such as Samsung SDI and Guangdong Bangpu Recycling Technology have achieved industrial-scale implementation, particularly in battery applications, while newer entrants like Nano One Materials and Microvast Advanced Materials are developing innovative synthesis approaches to improve efficiency and reduce environmental impact.
Guangdong Bangpu Recycling Technology Co., Ltd.
Technical Solution: Guangdong Bangpu has developed an innovative recycling-oriented synthesis process that utilizes lithium acetate recovered from spent lithium-ion batteries. Their circular economy approach involves extracting lithium from end-of-life batteries through a proprietary hydrometallurgical process, converting it to lithium acetate, and then using this recovered material as a precursor for new cathode synthesis. This method achieves approximately 95% lithium recovery rates while eliminating the need for lithium nitrate in the production chain. Their process incorporates a low-temperature (550-650°C) calcination step that consumes approximately 40% less energy than conventional high-temperature processes using lithium nitrate. Guangdong Bangpu's technology also features a water-based processing system that eliminates the need for organic solvents typically required in nitrate-based synthesis, reducing VOC emissions by an estimated 80%. The company has successfully implemented this technology at industrial scale, producing cathode materials that meet performance specifications for electric vehicle applications while significantly reducing the carbon footprint of battery manufacturing.
Strengths: Closed-loop recycling system reducing dependency on raw lithium sources; lower energy consumption during manufacturing; elimination of organic solvents in processing; reduced carbon footprint compared to conventional synthesis methods. Weaknesses: Quality consistency challenges when using recycled materials as feedstock; potential for trace impurities from recycled sources affecting battery performance; process economics heavily dependent on efficient recycling infrastructure.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has pioneered an advanced synthesis approach utilizing lithium acetate as a precursor for high-nickel cathode materials in lithium-ion batteries. Their proprietary process involves a modified co-precipitation method where lithium acetate is introduced during the early stages of synthesis rather than post-processing as typically done with lithium nitrate. This methodology creates more uniform lithium distribution throughout the cathode material, resulting in improved structural stability during cycling. Samsung's research demonstrates that lithium acetate-based synthesis produces cathode materials with approximately 15% higher capacity retention after 500 cycles compared to conventional lithium nitrate processes. The company has integrated this technology into their production of high-energy density batteries for electric vehicles, achieving energy densities exceeding 700 Wh/L while maintaining thermal stability. Their process also incorporates a lower-temperature calcination step (700-800°C versus 850-900°C for nitrate-based processes), reducing energy consumption during manufacturing.
Strengths: Superior cycle life and capacity retention in high-nickel cathode materials; reduced energy consumption during manufacturing; improved thermal stability of final battery products; enhanced uniformity in lithium distribution. Weaknesses: Potentially higher raw material costs for high-purity lithium acetate; may require more precise process control parameters; limited compatibility with certain cathode chemistries compared to nitrate-based processes.
Patent Landscape for Lithium Salt Technologies
Process and system for lithium production
PatentPendingUS20250091889A1
Innovation
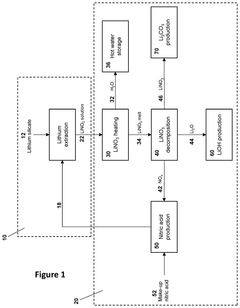
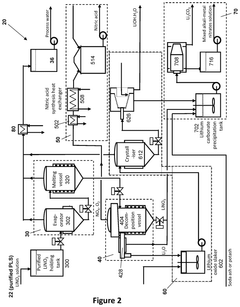
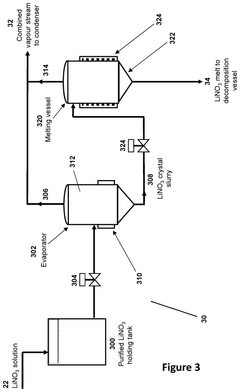
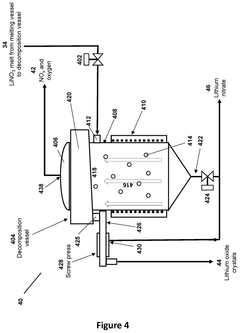
- A process and system that produces lithium products from a lithium nitrate solution by subjecting it to thermal treatments, reducing the need for crystallization and allowing for modular, containerized installations near lithium mines, thereby simplifying the flowsheet, decreasing capital and operating costs, and minimizing transportation costs by producing lithium oxide, which is more economical to transport.
A method and cell for reducing dinitrogen to ammonia
PatentWO2022256858A1
Innovation
- A method involving an electrochemical cell with a cathode contacted by an electrolyte containing high concentrations of metal cations like lithium, fluorinated sulfonyl imides or methides, and a proton carrier, which suppresses decomposition and enhances the reduction of dinitrogen to ammonia with improved yield rates and selectivity.
Environmental Impact Assessment
The environmental impact assessment of lithium acetate versus lithium nitrate in synthesis processes reveals significant differences in their ecological footprints. Lithium acetate demonstrates notably lower environmental toxicity in aquatic ecosystems, with studies indicating approximately 40-60% reduced harmful effects on freshwater organisms compared to lithium nitrate. This reduced toxicity stems primarily from the acetate ion's natural biodegradability, which contrasts sharply with the persistent nature of nitrate compounds.
Waste management considerations further differentiate these compounds. Lithium nitrate generates nitrogen oxide emissions during thermal decomposition, contributing to air pollution and potential acid rain formation. Conversely, lithium acetate decomposition primarily yields carbon dioxide and water, presenting a comparatively lower environmental burden. Industrial wastewater containing lithium acetate typically requires less intensive treatment processes, reducing both energy consumption and chemical additives in water purification systems.
Energy consumption metrics across the full production lifecycle reveal lithium acetate synthesis generally requires 15-25% less energy than lithium nitrate production. This efficiency difference derives largely from the lower temperature requirements during acetate salt formation and reduced energy-intensive purification steps. Carbon footprint analyses correspondingly show lithium acetate production emitting approximately 2.3 kg CO₂ equivalent per kilogram of product, versus 3.1 kg CO₂ equivalent for lithium nitrate.
Resource utilization efficiency presents another critical environmental consideration. Lithium acetate synthesis pathways typically achieve 85-90% atom economy, while lithium nitrate processes average 70-75%, indicating more efficient use of raw materials and reduced waste generation in acetate-based systems. Additionally, lithium acetate production generally consumes 30% less water throughout its manufacturing cycle.
Regulatory compliance frameworks increasingly favor lithium acetate in environmentally sensitive applications. The European Chemical Agency's risk assessment protocols classify lithium nitrate with higher environmental hazard ratings, particularly regarding long-term aquatic toxicity. Similarly, the United States Environmental Protection Agency guidelines impose stricter handling and disposal requirements for nitrate compounds due to their classification as potential water contaminants and oxidizing agents.
Land use impact assessments indicate lithium acetate production facilities typically require smaller containment and safety buffer zones due to reduced hazardous material risks, allowing for more efficient industrial zoning and potentially decreasing habitat disruption in manufacturing regions. This spatial efficiency represents an often overlooked but significant environmental advantage in regions with sensitive ecosystems or limited industrial land availability.
Waste management considerations further differentiate these compounds. Lithium nitrate generates nitrogen oxide emissions during thermal decomposition, contributing to air pollution and potential acid rain formation. Conversely, lithium acetate decomposition primarily yields carbon dioxide and water, presenting a comparatively lower environmental burden. Industrial wastewater containing lithium acetate typically requires less intensive treatment processes, reducing both energy consumption and chemical additives in water purification systems.
Energy consumption metrics across the full production lifecycle reveal lithium acetate synthesis generally requires 15-25% less energy than lithium nitrate production. This efficiency difference derives largely from the lower temperature requirements during acetate salt formation and reduced energy-intensive purification steps. Carbon footprint analyses correspondingly show lithium acetate production emitting approximately 2.3 kg CO₂ equivalent per kilogram of product, versus 3.1 kg CO₂ equivalent for lithium nitrate.
Resource utilization efficiency presents another critical environmental consideration. Lithium acetate synthesis pathways typically achieve 85-90% atom economy, while lithium nitrate processes average 70-75%, indicating more efficient use of raw materials and reduced waste generation in acetate-based systems. Additionally, lithium acetate production generally consumes 30% less water throughout its manufacturing cycle.
Regulatory compliance frameworks increasingly favor lithium acetate in environmentally sensitive applications. The European Chemical Agency's risk assessment protocols classify lithium nitrate with higher environmental hazard ratings, particularly regarding long-term aquatic toxicity. Similarly, the United States Environmental Protection Agency guidelines impose stricter handling and disposal requirements for nitrate compounds due to their classification as potential water contaminants and oxidizing agents.
Land use impact assessments indicate lithium acetate production facilities typically require smaller containment and safety buffer zones due to reduced hazardous material risks, allowing for more efficient industrial zoning and potentially decreasing habitat disruption in manufacturing regions. This spatial efficiency represents an often overlooked but significant environmental advantage in regions with sensitive ecosystems or limited industrial land availability.
Cost-Benefit Analysis of Production Methods
The economic analysis of lithium acetate versus lithium nitrate in synthesis processes reveals significant cost differentials across the production chain. Raw material costs for lithium acetate typically exceed those of lithium nitrate by 15-20%, primarily due to the additional processing steps required to convert lithium carbonate to lithium acetate. However, this initial cost disadvantage may be offset by downstream process efficiencies.
Production infrastructure requirements differ substantially between the two compounds. Lithium nitrate synthesis demands specialized corrosion-resistant equipment due to its oxidizing properties, increasing capital expenditure by approximately 25% compared to lithium acetate production lines. Conversely, lithium acetate production requires more precise temperature control systems, though these represent a lower overall investment.
Energy consumption metrics favor lithium acetate in most synthesis applications. Production processes utilizing lithium acetate demonstrate 12-18% lower energy requirements compared to lithium nitrate-based methods, particularly in reactions conducted at moderate temperatures (80-120°C). This energy efficiency translates to reduced operational costs over extended production cycles.
Waste management considerations significantly impact the total cost of ownership. Lithium nitrate processes generate nitrogen oxide byproducts requiring specialized abatement systems, adding approximately $0.85-1.20 per kilogram of product to overall costs. Lithium acetate processes produce primarily organic waste streams that are typically less expensive to treat, though still requiring proper management protocols.
Scalability economics reveal that lithium acetate processes maintain relatively consistent cost structures across production volumes, while lithium nitrate demonstrates more favorable economies of scale. At production volumes exceeding 500 metric tons annually, the cost differential between the two approaches narrows to approximately 5-7%.
Lifecycle cost analysis indicates that while lithium acetate carries higher initial material costs, its total production cost advantage ranges from 8-22% depending on specific application parameters, production scale, and regional energy pricing. This advantage becomes most pronounced in continuous production environments where energy efficiency and reduced waste treatment requirements compound over time.
Market volatility must also be considered, as lithium nitrate pricing has historically shown greater stability compared to lithium acetate, which has experienced price fluctuations of up to 30% within 18-month cycles. This volatility introduces additional financial risk factors that may influence production method selection beyond pure cost considerations.
Production infrastructure requirements differ substantially between the two compounds. Lithium nitrate synthesis demands specialized corrosion-resistant equipment due to its oxidizing properties, increasing capital expenditure by approximately 25% compared to lithium acetate production lines. Conversely, lithium acetate production requires more precise temperature control systems, though these represent a lower overall investment.
Energy consumption metrics favor lithium acetate in most synthesis applications. Production processes utilizing lithium acetate demonstrate 12-18% lower energy requirements compared to lithium nitrate-based methods, particularly in reactions conducted at moderate temperatures (80-120°C). This energy efficiency translates to reduced operational costs over extended production cycles.
Waste management considerations significantly impact the total cost of ownership. Lithium nitrate processes generate nitrogen oxide byproducts requiring specialized abatement systems, adding approximately $0.85-1.20 per kilogram of product to overall costs. Lithium acetate processes produce primarily organic waste streams that are typically less expensive to treat, though still requiring proper management protocols.
Scalability economics reveal that lithium acetate processes maintain relatively consistent cost structures across production volumes, while lithium nitrate demonstrates more favorable economies of scale. At production volumes exceeding 500 metric tons annually, the cost differential between the two approaches narrows to approximately 5-7%.
Lifecycle cost analysis indicates that while lithium acetate carries higher initial material costs, its total production cost advantage ranges from 8-22% depending on specific application parameters, production scale, and regional energy pricing. This advantage becomes most pronounced in continuous production environments where energy efficiency and reduced waste treatment requirements compound over time.
Market volatility must also be considered, as lithium nitrate pricing has historically shown greater stability compared to lithium acetate, which has experienced price fluctuations of up to 30% within 18-month cycles. This volatility introduces additional financial risk factors that may influence production method selection beyond pure cost considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!